Third Body Wear of an All-Polymer, PEEK-OPTIMA™ on Ultra-High-Molecular-Weight Polyethylene Total Knee Replacement
Abstract
1. Introduction
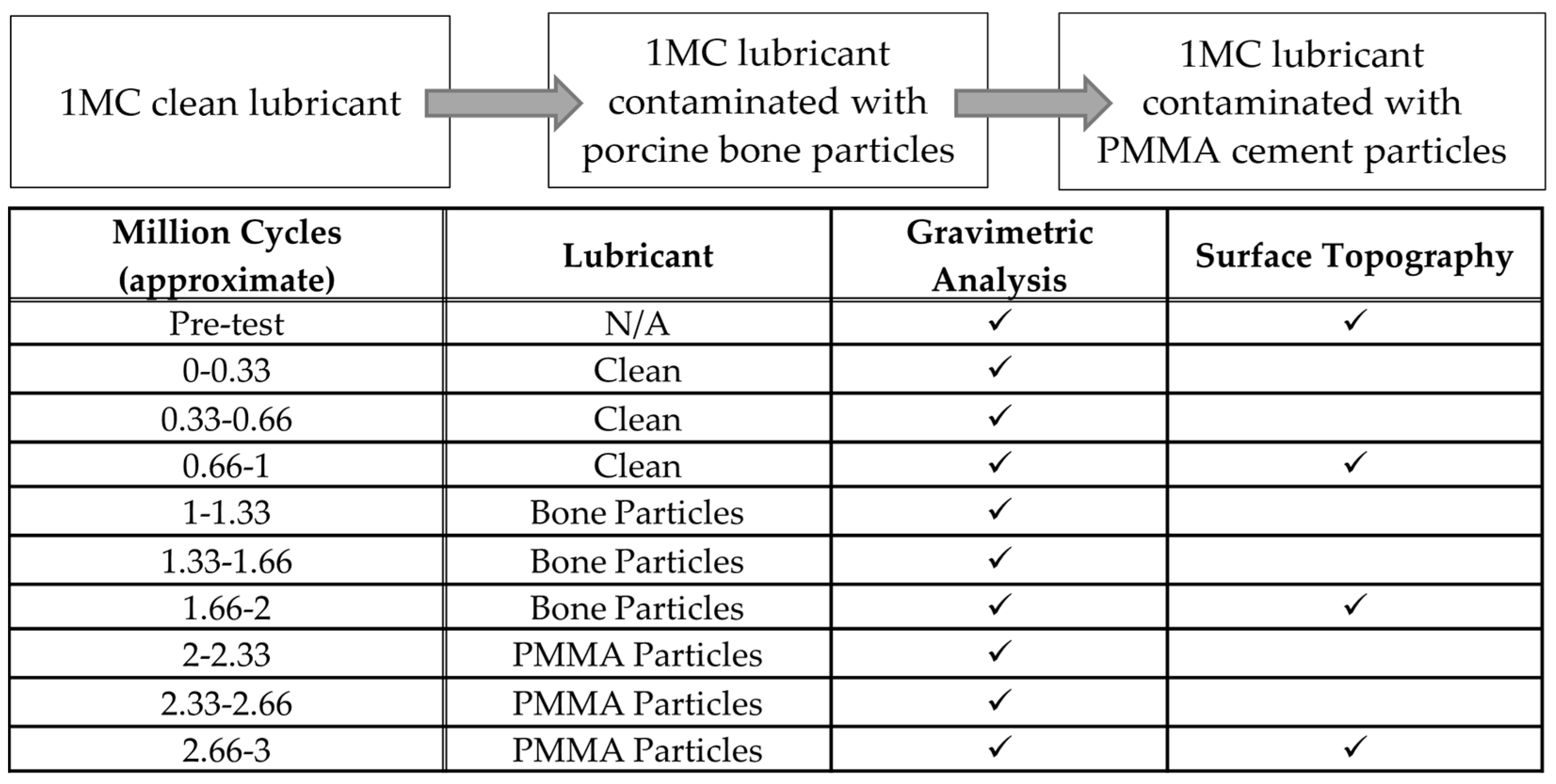
2. Materials and Methods
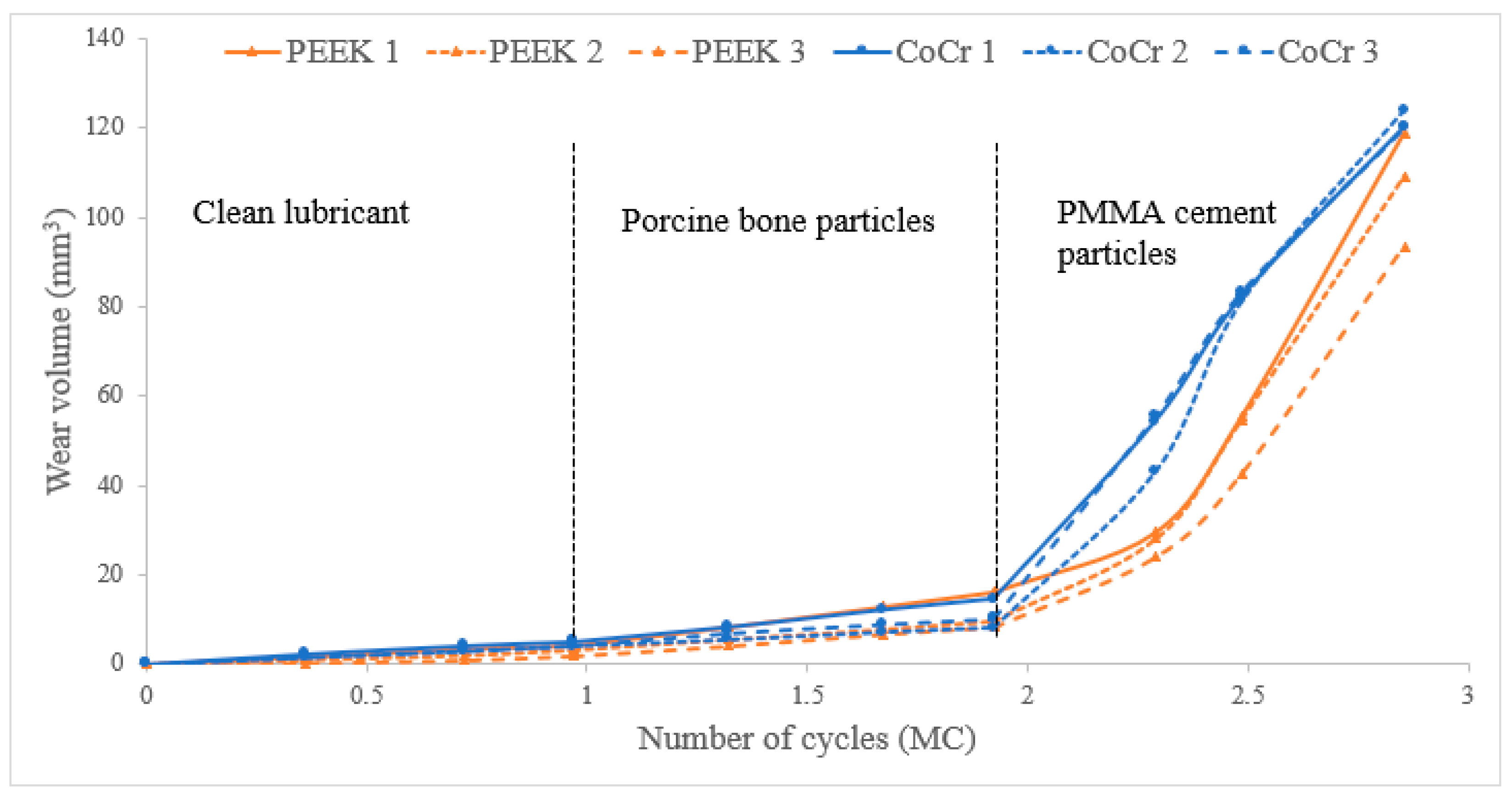
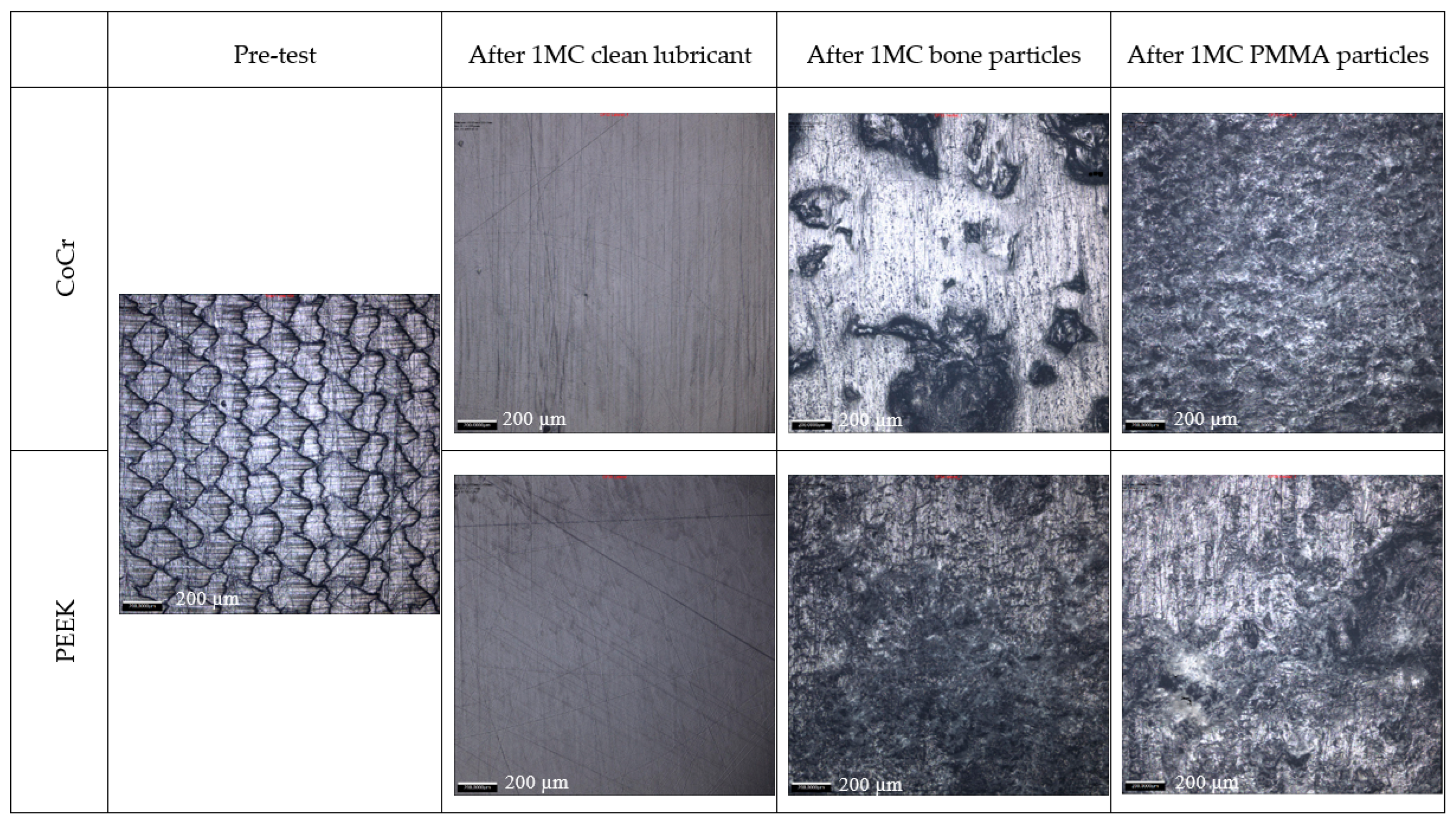
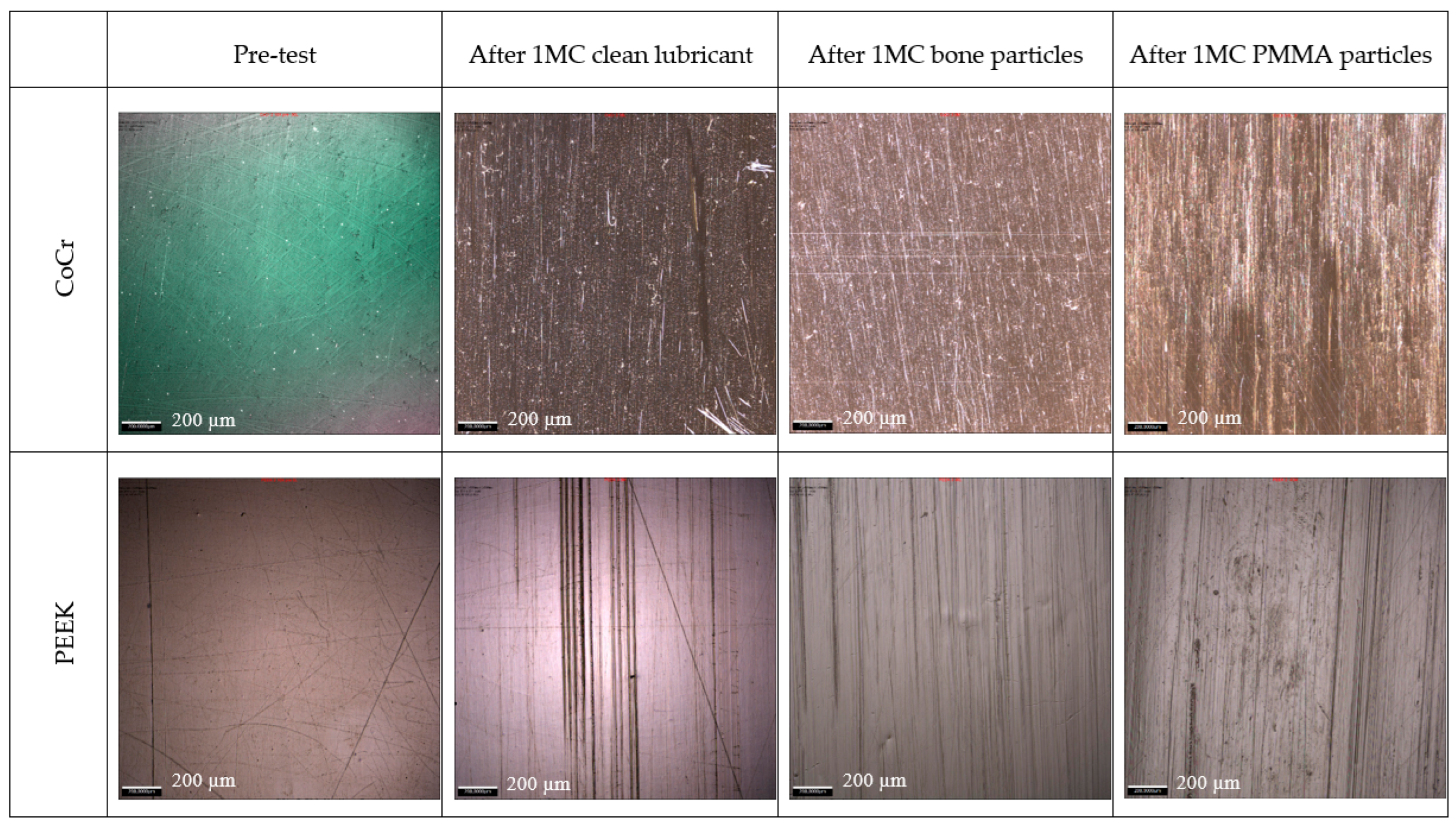
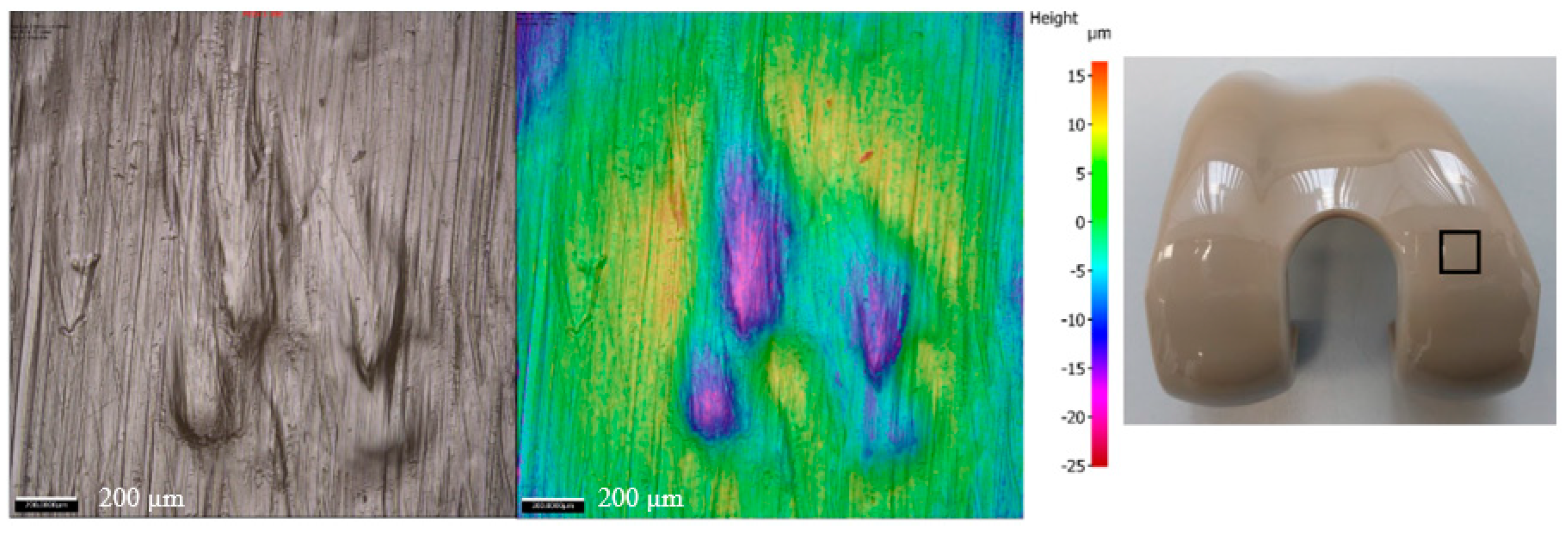
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Joint Registry England, Wales and Northern Ireland 20th Annual Report. 2023. Available online: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2020th%20Annual%20Report%202023 (accessed on 3 February 2025).
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.M.; Briscoe, A.; Fisher, J.; Jennings, L.M. PEEK-OPTIMA™ as an alternative to cobalt chrome in the femoral component of total knee replacement: A preliminary study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A. Clinical results of revision TKA in patients with presumed metal and cement allergy. J. Arthroplast. 2022, 37, S250–S257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Chen, K.; Xu, H.; Feng, C.; Zhang, D. Wear mechanism and debris analysis of PEEK as an alternative to CoCrMo in the femoral component of total knee replacement. Friction 2023, 11, 1845–1861. [Google Scholar] [CrossRef]
- Cowie, R.M.; Briscoe, A.; Jennings, L.M. The influence of lubricant temperature on the wear of total knee replacements. Biosurf. Biotribol. 2023, 9, 71–77. [Google Scholar] [CrossRef]
- Cowie, R.M.; Briscoe, A.; Fisher, J.; Jennings, L.M. Wear and Friction of UHMWPE-on-PEEK OPTIMA™. J. Mech. Behav. Biomed. Mater. 2019, 89, 65–71. [Google Scholar] [CrossRef]
- Cowie, R.M.; Pallem, N.M.; Briscoe, A.; Jennings, L.M. Third body wear of UHMWPE-on-PEEK-OPTIMA™. Materials 2020, 13, 1264. [Google Scholar] [CrossRef]
- Heuberger, R.; Stöck, C.; Sahin, J.; Eschbach, L. PEEK as a replacement for CoCrMo in knee prostheses: Pin-on-disc wear test of PEEK-on-polyethylene articulations. Biotribology 2021, 27, 100189. [Google Scholar] [CrossRef]
- Cowie, R.M.; Briscoe, A.; Jennings, L.M. The influence of cross shear and contact pressure on the wear of UHMWPE-on-PEEK-OPTIMA™ for use in total knee replacement. J. Mech. Behav. Biomed. Mater. 2023, 148, 106196. [Google Scholar] [CrossRef]
- Jennings, L.M.; Al-Hajjar, M.; Brockett, C.L.; Williams, S.; Tipper, J.L.; Ingham, E.; Fisher, J. (iv) Enhancing the safety and reliability of joint replacement implants. Orthop. Trauma 2012, 26, 246–252. [Google Scholar] [CrossRef]
- Zietz, C.; Bergschmidt, P.; Lange, R.; Mittelmeier, W.; Bader, R. Third-body abrasive wear of tibial polyethylene inserts combined with metallic and ceramic femoral components in a knee simulator study. Int. J. Artif. Organs 2013, 36, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.; Aiken, S.; Cooper, J.; Jennings, L. The influence of a calcium sulphate bone void filler on the third-body damage and polyethylene wear of total knee arthroplasty. Bone Jt. Res. 2019, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Grupp, T.M.; Fritz, B.; Schilling, C.; Chevalier, Y.; Utzschneider, S.; Jansson, V. The influence of third-body particles on wear rate in unicondylar knee arthroplasty: A wear simulator study with bone and cement debris. J. Mater. Sci. Mater. Med. 2013, 24, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R.M.; Carbone, S.; Aiken, S.; Cooper, J.J.; Jennings, L.M. Influence of third-body particles originating from bone void fillers on the wear of ultra-high-molecular-weight polyethylene. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 775–783. [Google Scholar] [CrossRef]
- Cowie, R.M.; Jennings, L.M. Third body damage and wear in arthroplasty bearing materials: A review of laboratory methods. Biomater. Biosyst. 2021, 4, 100028. [Google Scholar] [CrossRef]
- Atkinson, J.; Dowson, D.; Isaac, G.; Wroblewski, B. Laboratory wear tests and clinical observations of the penetration of femoral heads into acetabular cups in total replacement hip joints: II: A microscopical study of the surfaces of Charnley polyethylene acetabular sockets. Wear 1985, 104, 217–224. [Google Scholar] [CrossRef]
- Jones, V.; Williams, I.; Auger, D.; Walsh, W.; Barton, D.; Stone, M.; Fisher, J. Quantification of third body damage to the tibial counterface in mobile bearing knees. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 171–179. [Google Scholar] [CrossRef]
- De Baets, T.; Waelput, W.; Bellemans, J. Analysis of third body particles generated during total knee arthroplasty: Is metal debris an issue? Knee 2008, 15, 95–97. [Google Scholar] [CrossRef]
- De Ruiter, L.; Cowie, R.M.; Jennings, L.M.; Briscoe, A.; Janssen, D.; Verdonschot, N. The effects of cyclic loading and motion on the implant–cement interface and cement mantle of PEEK and cobalt–chromium femoral total knee arthroplasty implants: A preliminary study. Materials 2020, 13, 3323. [Google Scholar] [CrossRef]
- Caravia, L.; Dowson, D.; Fisher, J.; Jobbins, B. The influence of bone and bone cement debris on counterface roughness in sliding wear tests of ultra-high molecular weight polyethylene on stainless steel. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1990, 204, 65–70. [Google Scholar] [CrossRef]
- Isaac, G.; Atkinson, J.; Dowson, D.; Kennedy, P.; Smith, M. The causes of femoral head roughening in explanted Charnley hip prostheses. Eng. Med. 1987, 16, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, H.; Stone, M.; Wroblewski, B.; Porter, M.; Ingham, E.; Fisher, J. Comparison of third body damage on the femoral head caused by bone and bone cement. In Transactions of the Annual Meeting-Orthopaedic Research Society; Orthopaedic Research Scoiety: Rosemont, IL, USA, 1998; p. 779. [Google Scholar]
- Metcalfe, A.; Dressler, M.; Hardaker, C. Recreating Third Body Damage on RP Knee Inserts; Orthopedic Research Society: San Antonio, TX, USA, 2013; Available online: https://www.ors.org/transactions/59/PS2--102/1805.html (accessed on 3 February 2025).
- Minakawa, H.; Stone, M.; Wroblewski, B.; Lancaster, J.; Ingham, E.; Fisher, J. Quantification of third-body damage and its effect on UHMWPE wear with different types of femoral head. J. Bone Jt. Surg. Br. Vol. 1998, 80, 894–899. [Google Scholar] [CrossRef]
- Poggie, R.; Mishra, A.; Davidson, J. Three-body abrasive wear behaviour of orthopaedic implant bearing surfaces from titanium debris. J. Mater. Sci. Mater. Med. 1994, 5, 387–392. [Google Scholar] [CrossRef]
- Reich, J.; Hovy, L.; Lindenmaier, H.L.; Zeller, R.; Schwiesau, J.; Thomas, P.; Grupp, T.M. Preclinical evaluation of coated knee implants for allergic patients. Orthopade 2010, 39, 495–502. [Google Scholar] [CrossRef]
- Bowland, P.; Cowie, R.M.; Ingham, E.; Fisher, J.; Jennings, L.M. Biomechanical assessment of the stability of osteochondral grafts implanted in porcine and bovine femoral condyles. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 163–170. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Vena, P.; Pietrabissa, R. Quantitative evaluation of the prosthetic head damage induced by microscopic third-body particles in total hip replacement. J. Biomed. Mater. Res. 2001, 58, 436–448. [Google Scholar] [CrossRef]
- Paulus, A.C.; Franke, M.; Kraxenberger, M.; Schröder, C.; Jansson, V.; Utzschneider, S. PMMA third-body wear after unicondylar knee arthroplasty decuples the UHMWPE wear particle generation in vitro. BioMed Res. Int. 2015, 2015, 575849. [Google Scholar] [CrossRef]
- McEwen, H.; Barnett, P.; Bell, C.; Farrar, R.; Auger, D.; Stone, M.; Fisher, J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J. Biomech. 2005, 38, 357–365. [Google Scholar] [CrossRef]
- ISO 14243-3:2014/AMD 1:2020; Implants for Surgery-Wear of Total Knee-Joint Prostheses-Part 3: Loading and Displacement Parameters for Wear-Testing Machines with Displacement Control and Corresponding Environmental Conditions for Test-Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 14243-1:2009/AMD 1:2020; Implants for Surgery-Wear of Total Knee-Joint Prostheses-Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test-Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2020.
- Abdelgaied, A.; Fisher, J.; Jennings, L. Understanding the differences in wear testing method standards for total knee replacement. J. Mech. Behav. Biomed. Mater. 2022, 132, 105258. [Google Scholar] [CrossRef]
- Cowie, R.M.; Jennings, L. Dataset Associated with “Third Body Wear of an All-Polymer, PEEK-OPTIMA™-on-UHMWPE Total Knee Replacement”; University of Leeds: Leeds, UK, 2025. [Google Scholar]
- Manero, J.; Gil, F.; Ginebra, M.; Planell, J.; Artola, A.; Goñ, I.; Gurruchaga, M. Wear Behaviour of the Pair Ti–6Al–4V–UHMWPE of acrylic bone cements containing different radiopaque agents. J. Biomater. Appl. 2004, 18, 305–319. [Google Scholar] [CrossRef]
- Halim, T.; Clarke, I.; Burgett-Moreno, M.; Donaldson, T.; Savisaar, C.; Bowsher, J. A simulator study of adverse wear with metal and cement debris contamination in metal-on-metal hip bearings. Bone Jt. Res. 2015, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kretzer, J.P.; Reinders, J.; Sonntag, R.; Hagmann, S.; Streit, M.; Jeager, S.; Moradi, B. Wear in total knee arthroplasty—Just a question of polyethylene? Int. Orthop. 2014, 38, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Panni, A.S.; Vasso, M.; Cerciello, S.; Maccauro, G. Metallosis following knee arthroplasty: A histological and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 2011, 24, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Willis-Owen, C.; Keene, G.; Oakeshott, R. Early metallosis-related failure after total knee replacement: A report of 15 cases. J. Bone Jt. Surg. Br. Vol. 2011, 93, 205–209. [Google Scholar] [CrossRef]
- King, S.W.; Royeca, J.M.; Cunningham, C.M.; Madegowda, R.; Sha, S.; Pandit, H. Metal hypersensitivity in total knee arthroplasty. J. Arthrosc. Jt. Surg. 2020, 7, 184–188. [Google Scholar] [CrossRef]
- Salem, K.H.; Lindner, N.; Tingart, M.; Elmoghazy, A.D. Severe metallosis-related osteolysis as a cause of failure after total knee replacement. J. Clin. Orthop. Trauma 2020, 11, 165–170. [Google Scholar] [CrossRef]
- Bara, A.; Singh, A.; Patel, K.; Herlekar, D. Extensive Metallosis in a Primary Knee Arthroplasty as a Result of Polyethylene Wear: Is It Avoidable? Cureus 2024, 16, e57888. [Google Scholar] [CrossRef]
- Thomas, P.; Arenberger, P.; Bader, R.; Bircher, A.J.; Bruze, M.; de Graaf, N.; Hartmann, D.; Johansen, J.D.; Jonitz-Heincke, A.; Krenn, V.; et al. A literature review and expert consensus statement on diagnostics in suspected metal implant allergy. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1471–1477. [Google Scholar] [CrossRef]
- Xie, F.; Sheng, S.; Ram, V.; Pandit, H. Hypoallergenic Knee Implant Usage and Clinical Outcomes: Are They Safe and Effective? Arthroplast. Today 2024, 28, 101399. [Google Scholar] [CrossRef]
- Campbell, P.; Takamura, K. Local and systemic consequences of metal-on-metal hip resurfacing implants. Ann. Jt. 2019, 5, 5. [Google Scholar] [CrossRef]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J. Biomed. Mat. Res. 2002, 60, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mat. Res. Part. B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; McAllister, K.; Brady, M.; Jarman-Smith, M. Macrophage reactivity to different polymers demonstrates particle size- and material-specific reactivity: PEEK-OPTIMA® particles versus UHMWPE particles in the submicron, micron, and 10 micron size ranges. J. Biomed. Mat. Res. Part. B. 2012, 100, 480–492. [Google Scholar] [CrossRef] [PubMed]

| Porcine Bone Particles | PMMA Cement Particles | |
|---|---|---|
| Mean diameter (µm) | 696.3 ± 257.0 | 988.3 ± 242.5 |
| Mean aspect ratio | 1.68 ± 0.70 | 1.30 ± 0.34 |
| Test Condition | Femoral Components | Tibial Components | ||||
|---|---|---|---|---|---|---|
| CoCr | PEEK | Significance | CoCr | PEEK | Significance | |
| Pre-test | 0.022 ± 0.009 | 0.021 ± 0.004 | p = 0.65 | 1.062 ± 0.057 | 1.091 ± 0.028 | p = 0.124 |
| Clean lubricant | 0.039 ± 0.075 | 0.131 ± 0.054 | p = 0.01 | 0.524 ± 0.275 | 0.660 ± 0.142 | p = 0.131 |
| Porcine bone particles | 0.027 ± 0.006 | 0.398 ± 0.131 | p < 0.001 | 8.442 ± 5.449 | 6.645 ± 1.182 | p = 0.238 |
| PMMA cement particles | 0.124 ± 0.001 | 0.234 ± 0.179 | p = 0.058 | 12.569 ± 8.814 | 9.286 ± 10.045 | p = 0.350 |
| Test Condition | Bulk Lubricant Temperature (°C) | |||
|---|---|---|---|---|
| Unloaded Soak Control | CoCr Femoral Components | PEEK Femoral Components | Significance | |
| Clean lubricant | 25.8 | 26.2 ± 1.5 | 29.2 ± 0.2 | p = 0.001 |
| Porcine bone particles | 26.1 | 26.4 ± 1.5 | 30.6 ± 1.0 | p < 0.001 |
| PMMA cement particles | 26.1 | 30.7 ± 1.6 | 30.5 ± 1.2 | p = 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowie, R.M.; Schwiesau, J.; Grupp, T.M.; Briscoe, A.; Jennings, L.M. Third Body Wear of an All-Polymer, PEEK-OPTIMA™ on Ultra-High-Molecular-Weight Polyethylene Total Knee Replacement. Bioengineering 2025, 12, 261. https://doi.org/10.3390/bioengineering12030261
Cowie RM, Schwiesau J, Grupp TM, Briscoe A, Jennings LM. Third Body Wear of an All-Polymer, PEEK-OPTIMA™ on Ultra-High-Molecular-Weight Polyethylene Total Knee Replacement. Bioengineering. 2025; 12(3):261. https://doi.org/10.3390/bioengineering12030261
Chicago/Turabian StyleCowie, Raelene M., Jens Schwiesau, Thomas M. Grupp, Adam Briscoe, and Louise M. Jennings. 2025. "Third Body Wear of an All-Polymer, PEEK-OPTIMA™ on Ultra-High-Molecular-Weight Polyethylene Total Knee Replacement" Bioengineering 12, no. 3: 261. https://doi.org/10.3390/bioengineering12030261
APA StyleCowie, R. M., Schwiesau, J., Grupp, T. M., Briscoe, A., & Jennings, L. M. (2025). Third Body Wear of an All-Polymer, PEEK-OPTIMA™ on Ultra-High-Molecular-Weight Polyethylene Total Knee Replacement. Bioengineering, 12(3), 261. https://doi.org/10.3390/bioengineering12030261






