Changes in Maxillary Sinus Structure Due to Tooth Loss and the Effects of Sex and Aging on CBCT Before Maxillary Sinus Augmentation: A Cross-Sectional Study of 120 Patients
Abstract
1. Introduction
2. Subjects and Methods
2.1. Study Design
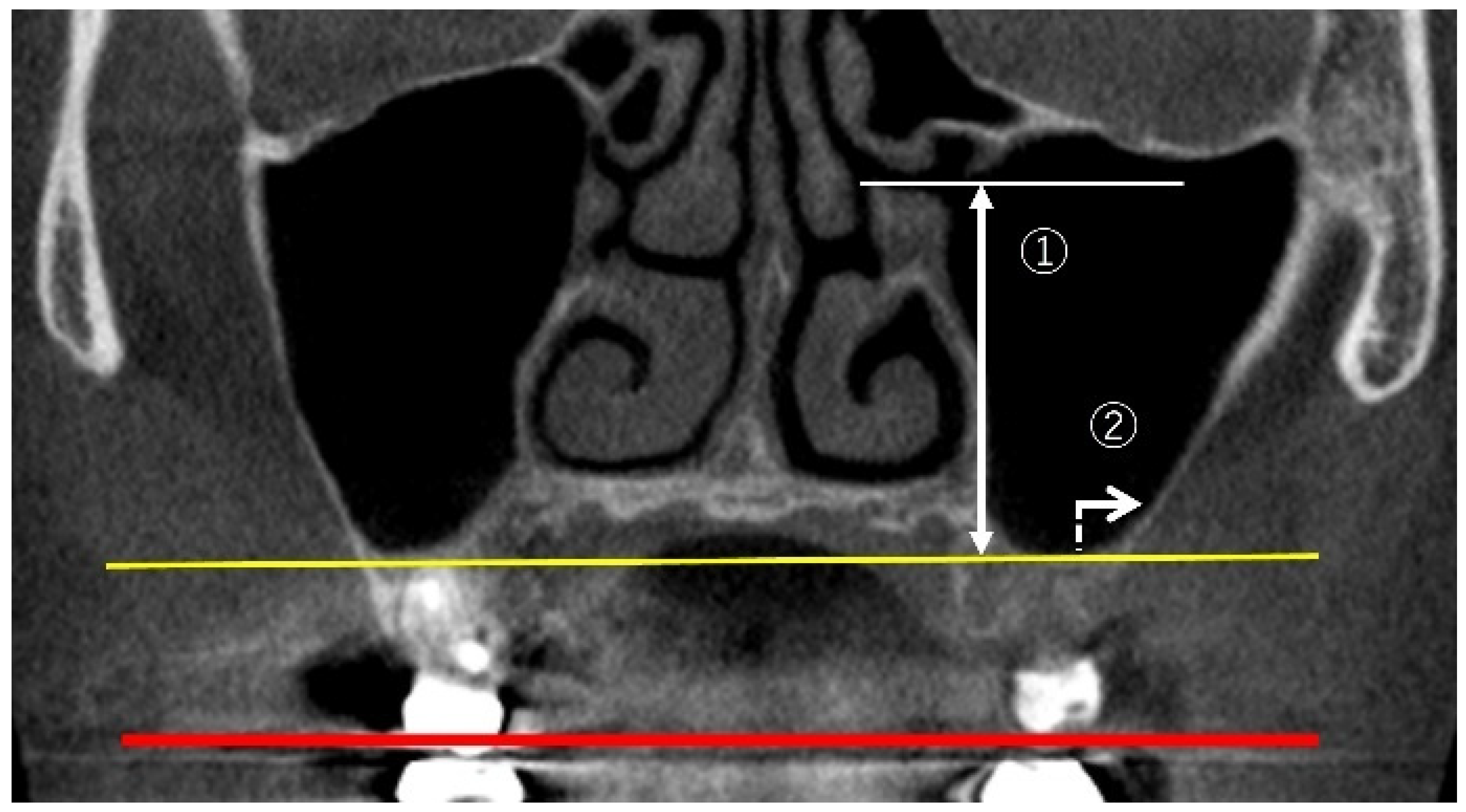
2.2. Measurement Method Using CBCT Images
2.3. Measurement Items
2.3.1. Linear Measurements of Maxillary Sinus Height
2.3.2. Measurement of PNR Angle and Maxillary Sinus Angle (MSA)
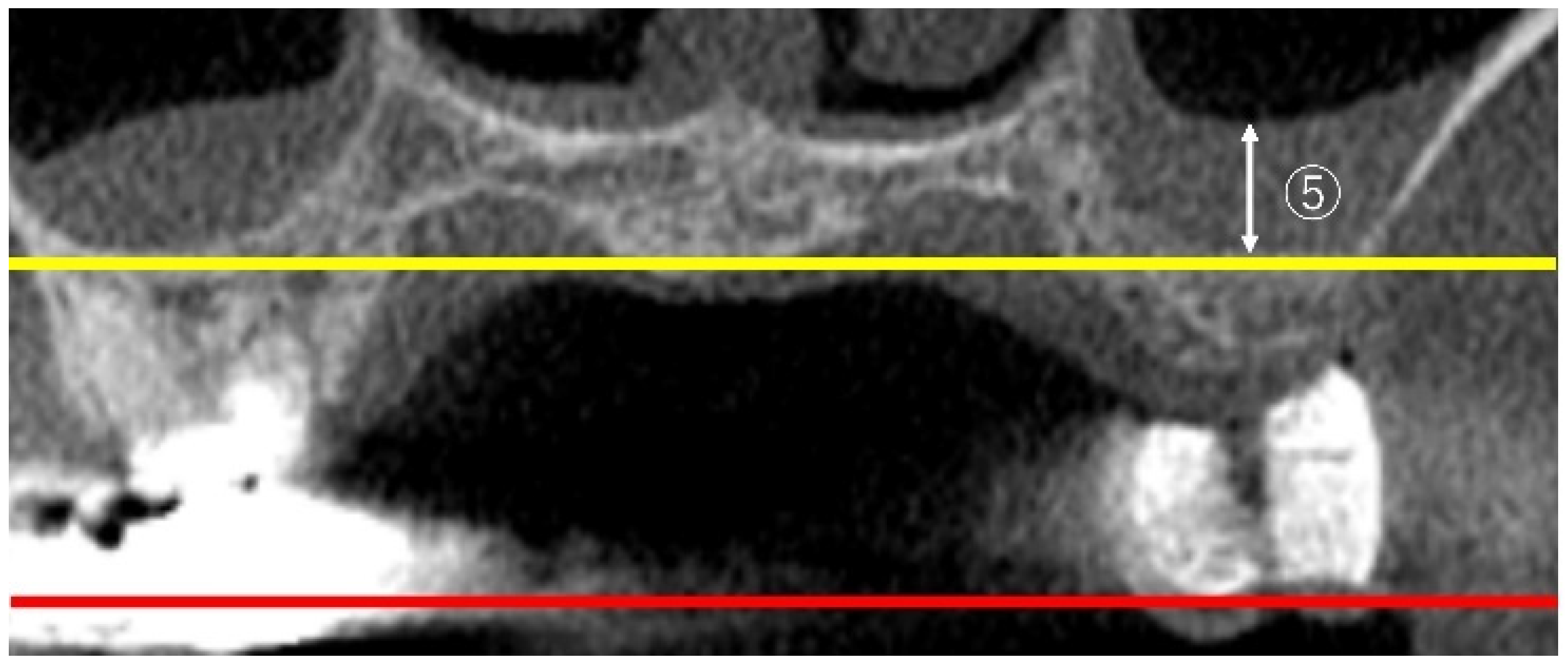
2.3.3. Measurement of Sinus Membrane Thickness (SMT)
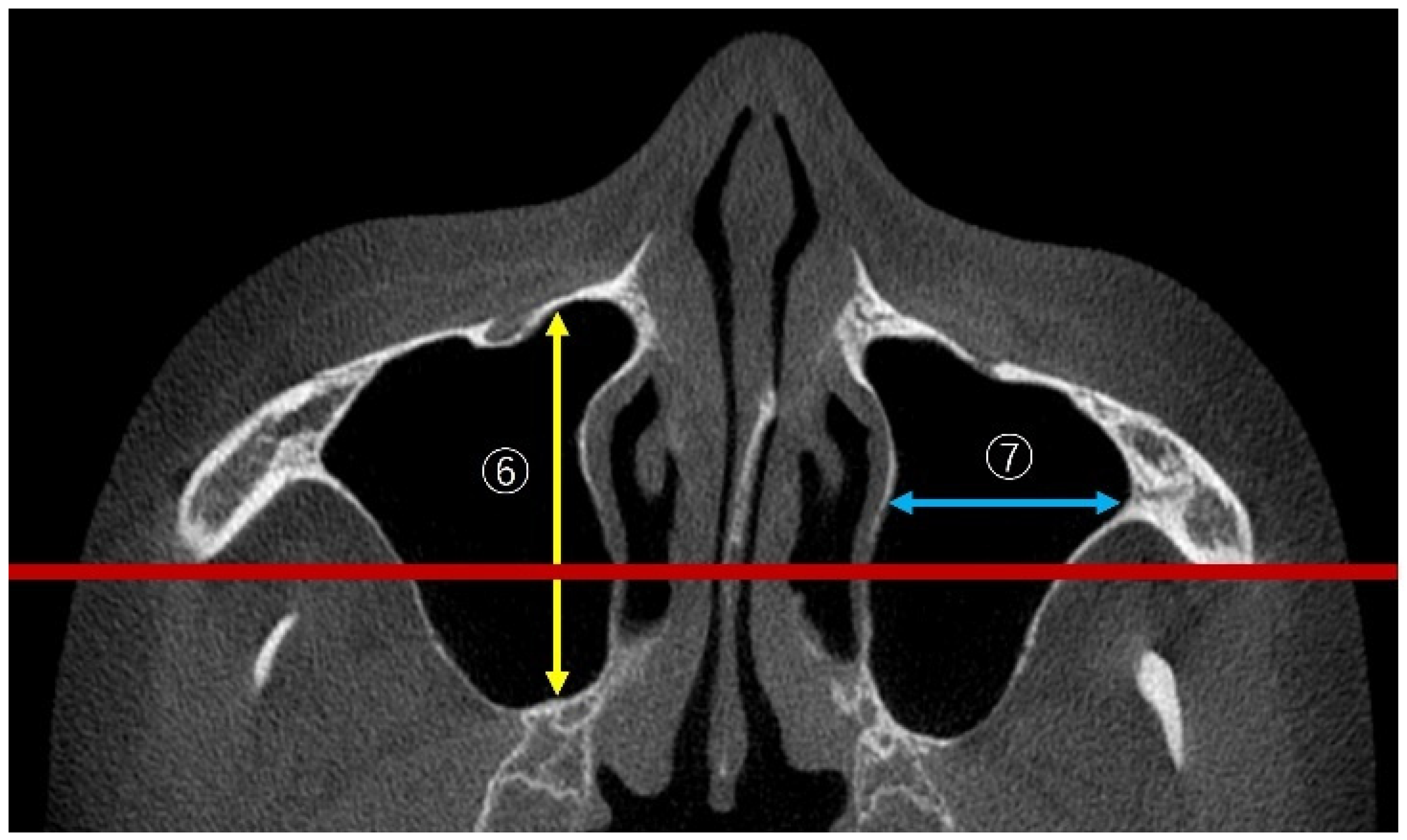
2.3.4. Linear Measurements of Maxillary Sinus Length
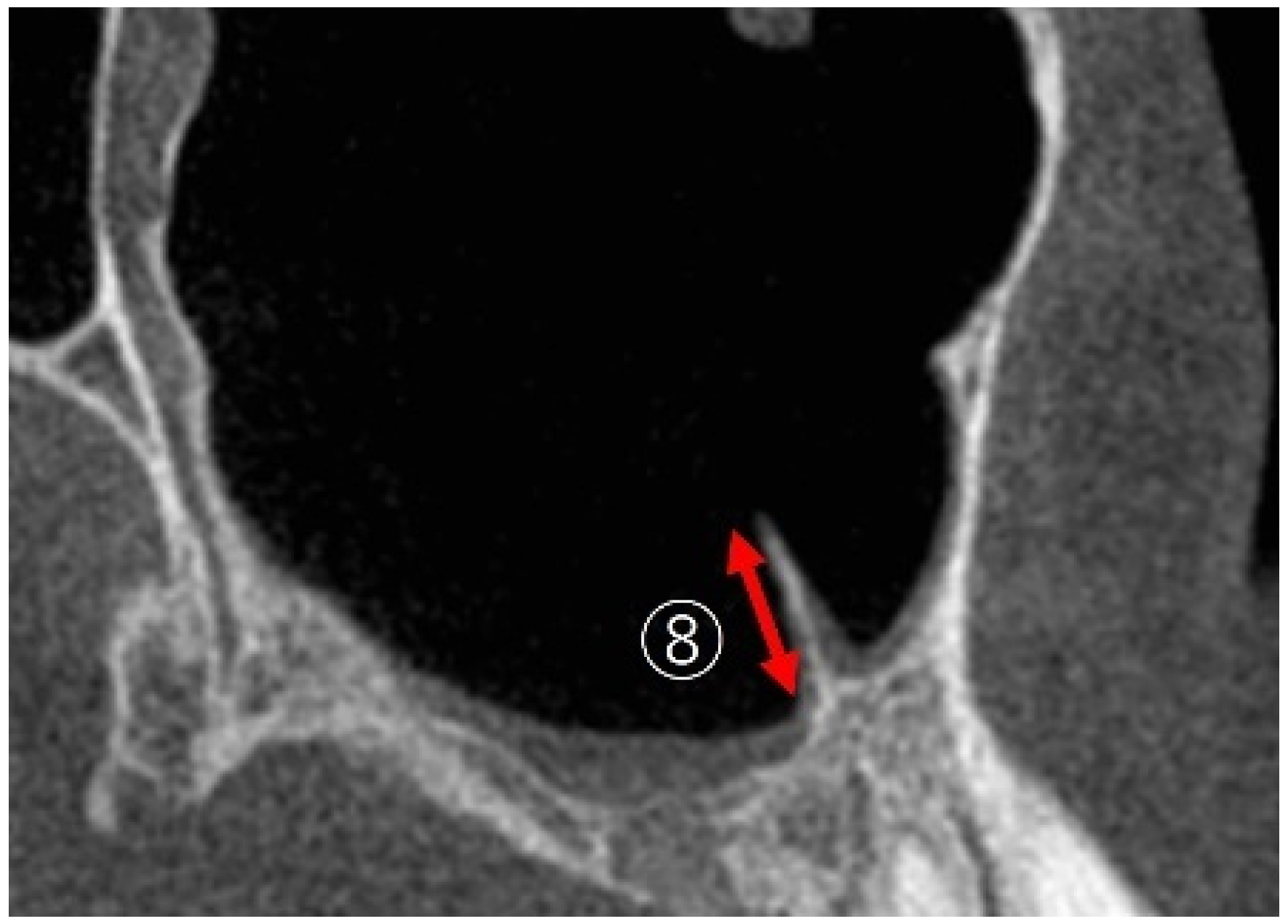
2.3.5. Maxillary Sinus Septa
2.4. Statistical Analysis
3. Results
3.1. Effects of Tooth Loss on the Maxillary Sinus (Table 1)
3.1.1. OH (Sinus Height)
| Missing Teeth | Non-Missing Teeth | p-Value | |
|---|---|---|---|
| OH (mm) | |||
| Total | 33.2 ± 4.5 | 33.4 ± 5.1 | 0.25 |
| Male | 34.3 ± 7.1 | 35.1 ± 8.1 | 0.046 * |
| Female | 32.7 ± 5.8 | 32.7 ± 5.4 | 0.43 |
| LWT (mm) | |||
| Total | 1.90 ± 0.9 | 2.40 ± 1.0 | 2.67 × 10−7 ** |
| Male | 1.87 ± 1.1 | 2.36 ± 1.3 | 0.00042 ** |
| Female | 1.93 ± 1.2 | 2.42 ± 1.3 | 7.12 × 10−5 ** |
| PNR (°) | |||
| Total | 120.5 ± 19.4 | 119.0 ± 17.9 | 0.20 |
| Male | 120.0 ± 28.2 | 117.3 ± 32.8 | 0.26 |
| Female | 120.8 ± 24.2 | 120.0 ± 23.3 | 0.29 |
| MSA (°) | |||
| Total | 102.2 ± 25.4 | 88.7 ± 15.5 | 0.00025 ** |
| Male | 104.0 ± 42.7 | 88.5 ± 21.2 | 0.014 * |
| Female | 101.1 ± 31.7 | 88.7 ± 21.4 | 0.0035 ** |
| SMT (mm) | |||
| Total | 0.78 ± 1.3 | 0.37 ± 0.8 | 0.009 ** |
| Male | 1.01 ± 2.4 | 0.46 ± 1.8 | 0.017 * |
| Female | 0.49 ± 1.4 | 0.30 ± 1.3 | 0.17 |
| AP (mm) | |||
| Total | 34.9 ± 4.5 | 36.5 ± 3.7 | 1.95 × 10−5 ** |
| Male | 35.4 ± 7.9 | 36.9 ± 6.4 | 0.0078 * |
| Female | 34.5 ± 4.6 | 34.5 ± 5.3 | 0.00044 ** |
| ML (mm) | |||
| Total | 25.2 ± 4.2 | 26.9 ± 3.7 | 1.78 × 10−7 ** |
| Male | 25.2 ± 7.7 | 27.5 ± 6.7 | 3.14 × 10−6 ** |
| Female | 25.3 ± 4.9 | 26.6 ± 4.2 | 0.00021 ** |
| Septa % (n) | |||
| Total | 43.3 (51/120) | 25.8 (30/120) | 0.0043 ** |
| Male | 19.6 (16/46) | 17.3 (8/46) | 0.039 * |
| Female | 48.6 (36/74) | 18.9 (14/74) | 0.042 * |
3.1.2. LWT
3.1.3. PNR
3.1.4. MSA
3.1.5. SMT
3.1.6. AP and ML (Sinus Length and Width)
3.1.7. Septa
3.2. Effects of Sex on the Maxillary Sinus
3.2.1. Missing Teeth
3.2.2. Non-Missing Teeth
3.3. Effects of Aging on the Maxillary Sinus
4. Discussion
5. Conclusions
- MSV, particularly sinus length and width, significantly decreased with tooth loss. Additionally, LWT decreased, MSA significantly increased, and the proportion of the sinus septa increased.
- Regarding sex differences in the maxillary sinus, sinus height (OH) was significantly greater in males on the non-missing side, and LWT (SMT) was significantly thicker in males on the missing side.
- Regarding the effects of aging on the maxillary sinus, in males, PNR increased and ML decreased on the missing side with age, while AP and ML decreased on the non-missing side with age. However, no effects of aging were observed in females.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBCT | Cone-beam computed tomography |
| AP | Anteroposterior dimension |
| ML | Mediolateral dimension |
| MSA | Maxillary sinus angle |
| MDPI | Multidisciplinary Digital Publishing Institute |
| LWT | Lateral wall thickness |
| OH | Ostium height |
| OMC | Ostiomeatal complex |
| PNR | Palatal–nasal recess |
| SMT | Sinus membrane thickness |
| ZMB | Zygomaticomaxillary buttress |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Karacayli, U.; Dikicier, E.; Dikicier, S. Dental implant placement in inadequate posterior maxilla. In Current Concepts in Dental Implantology; Turkyilmaz, I., Ed.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Sharan, A.; Madjar, D. Maxillary sinus pneumatization following extractions: A radiographic study. Int. J. Oral Maxillofac. Implant. 2008, 23, 48–56. [Google Scholar]
- Kalabalık, F.; Tarım Ertaş, E. Investigation of maxillary sinus volume relationships with nasal septal deviation, concha bullosa, and impacted or missing teeth using cone-beam computed tomography. Oral Radiol. 2019, 35, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.C.; Guirado, T.E.; Sapata, V.M.; Costa, C.; Pannuti, C.M.; Jung, R.E.; Neto, J.B.C. Maxillary sinus floor pneumatization and alveolar ridge resorption after tooth loss: A cross-sectional study. Braz. Oral Res. 2018, 32, e64. [Google Scholar] [CrossRef]
- Velasco-Torres, M.; Padial-Molina, M.; Alarcón, J.A.; OʼValle, F.; Catena, A.; Galindo-Moreno, P. Maxillary sinus dimensions with respect to the posterior superior alveolar artery decrease with tooth loss. Implant. Dent. 2016, 25, 464–470. [Google Scholar] [CrossRef]
- Sharan, A.; Madjar, D. Correlation between maxillary sinus floor topography and related rootposition of posterior teeth using panoramic and cross-sectional computed tomography imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 375–381. [Google Scholar] [CrossRef]
- Velasco-Torres, M.; Padial-Molina, M.; Avila-Ortiz, G.; García-Delgado, R.; OʼValle, F.; Catena, A.; Galindo-Moreno, P. Maxillary sinus dimensions decrease as age and tooth loss increase. Implant. Dent. 2017, 26, 288–295. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Heussen, N.; Peters, F.; Steiner, T.; Hölzle, F.; Modabber, A. Is the maxillary sinus really suitable in sex determination? A three-dimensional analysis of maxillary sinus volume and surface depending on sex and dentition. J. Craniofac Surg. 2015, 26, e723–e726. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Munakata, M.; Kataoka, Y.; Uesugi, T.; Shimoo, Y. Effects of missing teeth and nasal septal deviation on maxillary sinus volume: A pilot study. Int. J. Implant. Dent. 2022, 8, 19. [Google Scholar] [CrossRef]
- Luz, J.; Greutmann, D.; Wiedemeier, D.; Rostetter, C.; Rücker, M.; Stadlinger, B. 3D-evaluation of the maxillary sinus in cone-beam computed tomography. Int. J. Implant. Dent. 2018, 4, 17. [Google Scholar] [CrossRef]
- Schriber, M.; Bornstein, M.M.; Suter, V.G.A. Is the pneumatisation of the maxillary sinus following tooth loss a reality? A retrospective analysis using cone beam computed tomography and a customised software program. Clin. Oral Investig. 2019, 23, 1349–1358. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Aludden, H.; Dahlin, C.; Christensen, A.E.; Mordenfeld, A. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int. J. Oral Maxillofac. Surg. 2018, 47, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 21, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Taschieri, S.; Wallace, S.S. Risk factors in lateral window sinus elevation surgery. Periodontol. 2000 2019, 81, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.S. Lateral window sinus augmentation: Complications and outcomes of 101 consecutive procedures. Implant. Dent. 2015, 24, 354–361. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Munakata, M.; Sato, D.; Kataoka, Y.; Kawamata, R. The effectiveness and practicality of a novel barrier membrane for the open window in maxillary sinus augmentation with a lateral approach, with risk indicators for bone graft displacement and bone height decrease: A prospective study in humans. Bioengineering 2023, 10, 1110. [Google Scholar] [CrossRef]
- Benjaphalakron, N.; Jansisyanont, P.; Chuenchompoonut, V.; Kiattavorncharoen, S. Evaluation of the maxillary sinus anatomical variations related to maxillary sinus augmentation using cone beam computed tomography images. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 18–25. [Google Scholar] [CrossRef]
- Chan, H.L.; Monje, A.; Suarez, F.; Benavides, E.; Wang, H.L. Palatonasal recess on medial wall of the maxillary sinus and clinical implications for sinus augmentation via lateral window approach. J. Periodontol. 2013, 84, 1087–1093. [Google Scholar] [CrossRef]
- Hettiarachchi, P.V.K.S.; Gunathilake, P.M.P.C.; Jayasinghe, R.M.; Fonseka, M.C.; Bandara, R.M.W.R.; Nanayakkara, C.D.; Jayasinghe, R.D. Linear and volumetric analysis of maxillary sinus pneumatization in a Sri Lankan population using cone beam computer tomography. Biomed. Res. Int. 2021, 2021, 6659085. [Google Scholar] [CrossRef]
- Lyu, M.; Xu, D.; Zhang, X.; Yuan, Q. Maxillary sinus floor augmentation: A review of current evidence on anatomical factors and a decision tree. Int. J. Oral Sci. 2023, 15, 41. [Google Scholar] [CrossRef]
- Khehra, A.; Levin, L. Maxillary sinus augmentation procedures: A narrative clinical review. Quintessence Int. 2020, 51, 578–584. [Google Scholar] [CrossRef]
- Testori, T.; Yu, S.H.; Tavelli, L.; Wang, H. Perforation risk assessment in maxillary sinus augmentation with lateral wall technique. Int. J. Periodontics Restor. Dent. 2020, 40, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Watanabe, T.; Iimura, A.; Takahashi, O. A study of the maxillary sinus volume in elderly persons using Japanese cadavers. Okajimas Folia Anat. Jpn. 2016, 93, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Fernandes, C.M.S.; Kuhnen, B.; Scarso Filho, J.; Gonçalves, M.; Serra, M.D.C. Maxillary sinuses’ height/width/depth of Brazilian subjects and influence of sex, age, skin color, and nutritional status: A CBCT study. Forensic Imaging 2022, 31, 200522. [Google Scholar] [CrossRef]
- Teixeira, L.C.; Walewski, L.; Tolentino, E.S.; Iwaki, L.; Silva, M.C. Three-dimensional analysis of the maxillary sinus for determining sex and age in human identification. Forensic Imaging 2020, 22, 200395. [Google Scholar] [CrossRef]
- Mathew, A.; Jacob, L.E. 3D evaluation of maxillary sinus in gender determination: A cone beam computed tomography study. J. Indian. Acad. Oral Med. Radiol. 2020, 32, 384–389. [Google Scholar] [CrossRef]
- Paknahad, M.; Shahidi, S.; Zarei, Z. Sexual dimorphism of maxillary sinus dimensions using cone-beam computed tomography. J. Forensic Sci. 2017, 62, 395–398. [Google Scholar] [CrossRef]
- Belgin, C.A.; Colak, M.; Adiguzel, O.; Akkus, Z.; Orhan, K. Three-dimensional evaluation of maxillary sinus volume in different age sex groups using CBCT. Eur. Arch. Otorhinolaryngol. 2019, 276, 1493–1499. [Google Scholar] [CrossRef]
- Boreak, N.; Maketone, P.; Mourlaas, J.; Wang, W.; Yu, Y. Decision tree to minimize intra-operative complications during maxillary sinus augmentation procedures. J. Oral Biol. 2018, 5, 8. [Google Scholar]
- Ella, B.; Sédarat, C.; Da Costa Noble, R.; Normand, E.; Lauverjat, Y.; Siberchicot, F.; Caix, P.; Zwetyenga, N. Vascular connections of the lateral wall of the sinus: Surgical effect in sinus augmentation. Int. J. Oral Maxillofac. Implant. 2008, 23, 1047–1052. [Google Scholar]
- Lim, E.L.; Ngeow, W.C.; Lim, D. The implications of different lateral wall thicknesses on surgical access to the maxillary sinus. Braz. Oral Res. 2017, 31, e97. [Google Scholar] [CrossRef]
- Rosano, G.; Taschieri, S.; Gaudy, J.F.; Weinstein, T.; Del Fabbro, M. Maxillary sinus vascular anatomy and its relation to sinus lift surgery. Clin. Oral Implant. Res. 2011, 22, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative complications during sinus floor elevation with lateral approach: A systematic review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef] [PubMed]
- Basma, H.; Saleh, I.; Abou-Arraj, R.; Li, P.; Benavides, E.; Wang, H.L.; Chang, H.L. Association between lateral wall thickness and sinus membrane perforation during lateral sinus elevation: A retrospective study. Int. J. Oral Implant. 2021, 14, 77–85. [Google Scholar]
- Monje, A.; Catena, A.; Monje, F.; Gonzalez-García, R.; Galindo-Moreno, P.; Suarez, F.; Wang, H.L. Maxillary sinus lateral wall thickness and morphologic patterns in the atrophic posterior maxilla. J. Periodontol. 2014, 85, 676–682. [Google Scholar] [CrossRef]
- Khajehahmadi, S.; Rahpeyma, A.; Hoseini Zarch, S.H. Association between the lateral wall thickness of the maxillary sinus and the dental status: Cone beam computed tomography evaluation. Iran. J. Radiol. 2014, 11, e6675. [Google Scholar] [CrossRef]
- Talo Yildirim, T.; Güncü, G.N.; Colak, M.; Tözüm, T.F. The relationship between maxillary sinus lateral wall thickness, alveolar bone loss, and demographic variables: A cross-sectional cone-beam computerized tomography study. Med. Princ. Pract. 2019, 28, 109–114. [Google Scholar] [CrossRef]
- Pizzini, A.; Basma, H.S.; Li, P.; Geurs, N.C.; Abou-Arraj, R.V. The impact of anatomic, patient and surgical factors on membrane perforation during lateral wall sinus floor elevation. Clin. Oral Implant. Res. 2021, 32, 274–284. [Google Scholar] [CrossRef]
- Henriques, I.; Caramês, J.; Francisco, H.; Caramês, G.; Hernández-Alfaro, F.; Marques, D. Prevalence of maxillary sinus septa: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 823–831. [Google Scholar] [CrossRef]
- Hungerbuhler, A.; Rostetter, C.; Lubbers, H.T.; Rucker, M.; Stadlinger, B. Anatomical characteristics of maxillary sinus septa visualized by cone beam computed tomography. Int. J. Oral Maxillofac. Surg. 2019, 48, 382–387. [Google Scholar] [CrossRef]
- Nemati, M.; Khodaverdi, N.; Hosn Centenero, S.A.; Tabrizi, R. Which factors affect the risk of membrane perforation in lateral window maxillary sinus elevation? A prospective cohort study. J. Craniomaxillofac Surg. 2023, 51, 427–432. [Google Scholar] [CrossRef]
- Shao, Q.; Li, J.; Pu, R.; Feng, Y.; Jiang, Z.; Yang, G. Risk factors for sinus membrane perforation during lateral window maxillary sinus floor elevation surgery: A retrospective study. Clin. Implant. Dent. Relat. Res. 2021, 23, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Tassoker, M. What are the risk factors for maxillary sinus pathologies? A CBCT study. Oral Radiol. 2020, 36, 80–84. [Google Scholar] [CrossRef]
- Al-Dajani, M. Incidence, risk factors, and complications of Schneiderian membrane perforation in sinus lift surgery: A meta-analysis. Implant. Dent. 2016, 25, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Dandekeri, S.S.; Hegde, C.; Kavassery, P.; Sowmya, M.K.; Shetty, B. CBCT study of morphologic variations of maxillary sinus septa in relevance to sinus augmentation procedures. Ann. Maxillofac. Surg. 2020, 10, 51–56. [Google Scholar] [CrossRef] [PubMed]

| Males | Females | p-Value | ||
|---|---|---|---|---|
| Missing teeth | OH (mm) | 34.4 ± 4.8 | 32.6 ± 4.9 | 0.029 * |
| LWT (mm) | 1.87 ± 0.7 | 1.93 ± 1.1 | 0.36 | |
| PNR (°) | 120.0 ± 22.2 | 120.8 ± 20.8 | 0.47 | |
| MSA (°) | 104.0 ± 29.0 | 101.1 ± 27.3 | 0.396 | |
| SMT (mm) | 1.18 ± 1.6 | 0.68 ± 1.5 | 0.045 * | |
| AP (mm) | 35.4 ± 5.3 | 34.5 ± 4.6 | 0.146 | |
| ML (mm) | 25.2 ± 5.2 | 25.3 ± 4.2 | 0.356 | |
| Septa (%) | 39.1 | 45.9 | 0.46 | |
| Non-missing teeth | OH (mm) | 35.1 ± 5.4 | 32.3 ± 5.4 | 0.0039 ** |
| LWT (mm) | 2.36 ± 0.9 | 2.42 ± 1.1 | 0.50 | |
| PNR (°) | 117.3 ± 19.1 | 120.0 ± 20.0 | 0.25 | |
| MSA (°) | 88.5 ± 14.4 | 88.7 ± 18.4 | 0.396 | |
| SMT (mm) | 0.68 ± 1.5 | 0.50 ± 1.4 | 0.291 | |
| AP (mm) | 37.0 ± 4.4 | 36.2 ± 3.9 | 0.162 | |
| ML (mm) | 27.5 ± 4.5 | 26.6 ± 3.6 | 0.044 * | |
| Septa (%) | 19.6 | 29.7 | 0.31 |
| r | p | ||
|---|---|---|---|
| Missing teeth | OH | ||
| Male | −0.089 | 0.28 | |
| Female | 0.014 | 0.45 | |
| LWT | |||
| Male | 0.058 | 0.35 | |
| Female | −0.074 | 0.26 | |
| PNR | |||
| Male | 0.36 | 0.0068 * | |
| Female | −0.12 | 0.15 | |
| MSA | |||
| Male | −0.16 | 0.15 | |
| Female | −0.09 | 0.22 | |
| AP | |||
| Male | −0.066 | 0.48 | |
| Female | −0.06 | 0.303 | |
| ML | |||
| Male | −0.348 | 0.0089 * | |
| Female | 0.053 | 0.32 | |
| Non-missing teeth | OH | ||
| Male | −0.13 | 0.19 | |
| Female | 0.037 | 0.38 | |
| LWT | |||
| Male: | 0.064 | 0.34 | |
| Female | −0.014 | 0.45 | |
| PNR | |||
| Male | 0.11 | 0.23 | |
| Female | −0.038 | 0.37 | |
| MSA | |||
| Male | 0.118 | 0.22 | |
| Female | −0.099 | 0.20 | |
| AP | |||
| Male | −0.243 | 0.049 * | |
| Female | 0.12 | 0.15 | |
| ML | |||
| Male | −0.38 | 0.0044 ** | |
| Female | 0.029 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itokawa, T.; Yamaguchi, K.; Yagi, K.; Araki, K.; Sato, D.; Munakata, M. Changes in Maxillary Sinus Structure Due to Tooth Loss and the Effects of Sex and Aging on CBCT Before Maxillary Sinus Augmentation: A Cross-Sectional Study of 120 Patients. Bioengineering 2025, 12, 240. https://doi.org/10.3390/bioengineering12030240
Itokawa T, Yamaguchi K, Yagi K, Araki K, Sato D, Munakata M. Changes in Maxillary Sinus Structure Due to Tooth Loss and the Effects of Sex and Aging on CBCT Before Maxillary Sinus Augmentation: A Cross-Sectional Study of 120 Patients. Bioengineering. 2025; 12(3):240. https://doi.org/10.3390/bioengineering12030240
Chicago/Turabian StyleItokawa, Takumi, Kikue Yamaguchi, Kotaro Yagi, Kazuyuki Araki, Daisuke Sato, and Motohiro Munakata. 2025. "Changes in Maxillary Sinus Structure Due to Tooth Loss and the Effects of Sex and Aging on CBCT Before Maxillary Sinus Augmentation: A Cross-Sectional Study of 120 Patients" Bioengineering 12, no. 3: 240. https://doi.org/10.3390/bioengineering12030240
APA StyleItokawa, T., Yamaguchi, K., Yagi, K., Araki, K., Sato, D., & Munakata, M. (2025). Changes in Maxillary Sinus Structure Due to Tooth Loss and the Effects of Sex and Aging on CBCT Before Maxillary Sinus Augmentation: A Cross-Sectional Study of 120 Patients. Bioengineering, 12(3), 240. https://doi.org/10.3390/bioengineering12030240







