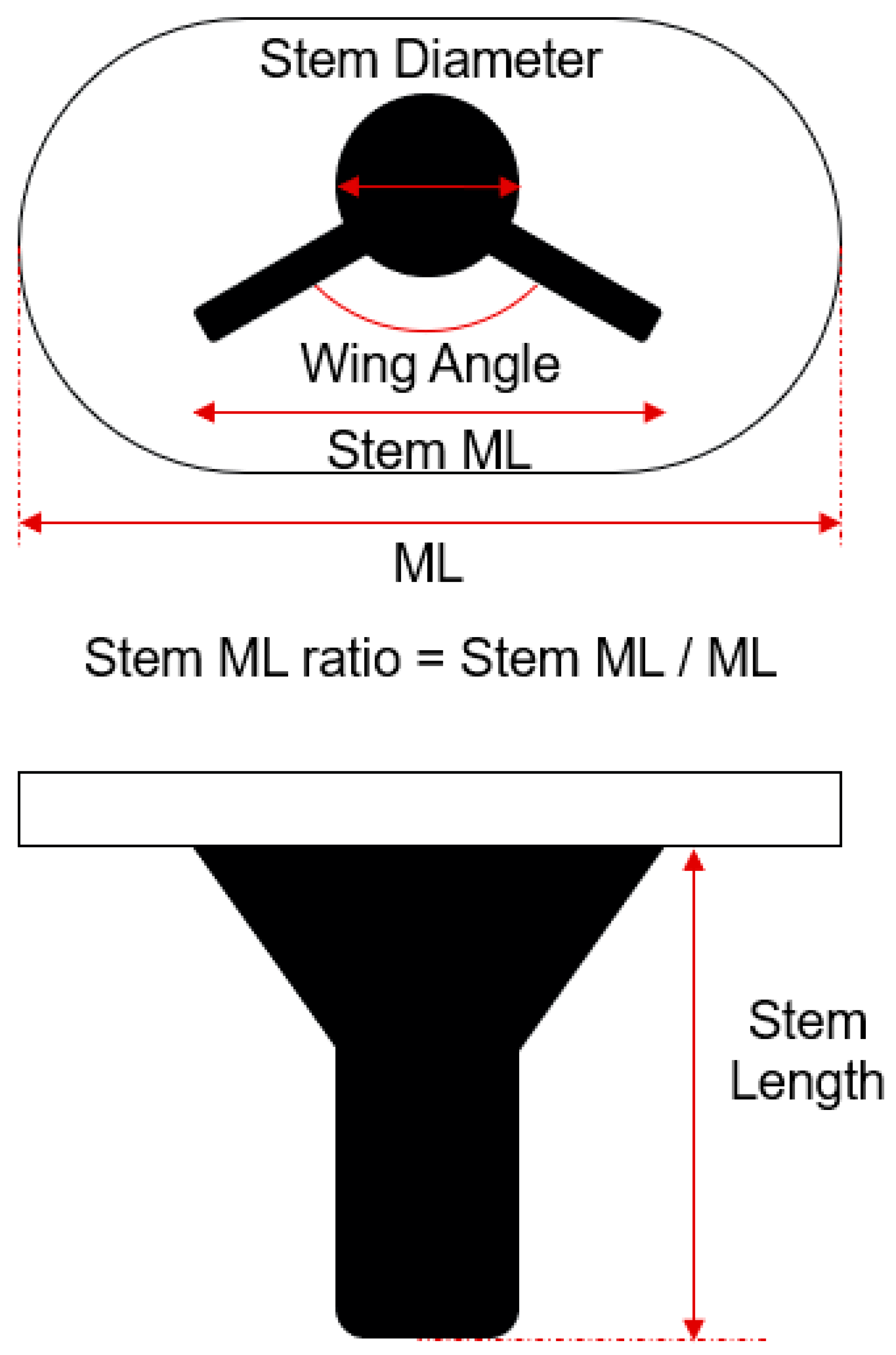
1. Introduction
Total Knee Arthroplasty (TKA) is one of the most widely performed surgical procedures, primarily aimed at alleviating pain and restoring functionality in patients suffering from severe knee joint disorders such as osteoarthritis [
1]. Despite its clinical success, the long-term outcomes of TKA are heavily dependent on the stability and durability of the tibial component, especially the tibial stem [
2]. The tibial stem plays a critical role in enhancing the fixation of the tibial baseplate to the bone, thereby reducing the risk of aseptic loosening—a leading cause of implant failure [
3]. However, clinical cases have reported complications such as non-uniform stress distribution, malalignment, malunion, and instability, which highlight the need for improved designs that minimize such failures while enhancing long-term patient outcomes.
Various tibial stem designs have been employed to address the challenges associated with TKA, with the two most common being the “cross-shaped” and “cylinder with wing” designs. While these designs have been successful in improving implant stability, there remains a significant need to optimize these designs further to minimize stress shielding and bone resorption while ensuring the long-term stability of the implant. In particular, the “cylinder with wing” design has gained widespread clinical use due to its balance of mechanical stability and ease of implantation. The biomechanical implications of design parameters such as stem length, diameter, wing angle, and anterior wing inclusion have not been thoroughly investigated, leading to gaps in understanding their influence on clinical outcomes.
Previous studies have explored various tibial stem designs and their effects on implant performance; however, several limitations remain. Many studies have relied on simplified models that fail to capture the complexities of actual clinical scenarios, such as variability in patient anatomy, implant alignment, and loading conditions [
4,
5]. Additionally, these studies have not sufficiently addressed the trade-offs between minimizing stress shielding and reducing the risk of aseptic loosening, leaving a significant research gap in optimizing tibial stem designs.
In this study, we employ DOE methodology to systematically investigate the effects of key design parameters on the biomechanical performance of the tibial stem in TKA. The parameters analyzed include stem length, stem diameter, wing angle, and the addition of an anterior wing, which is a novel design feature inspired by the cross-shaped design. By conducting a series of simulations and analyses, we aim to identify an optimized tibial stem design that balances mechanical stability, stress distribution, and bone preservation.
The findings from this research are expected to contribute to the development of more effective tibial components for TKA, potentially improving patient outcomes and extending the lifespan of the prosthesis. Ultimately, the optimized tibial stem design derived from this study could lead to a significant advancement in the field of orthopedic implant design. By advancing the understanding of tibial stem design, this study has the potential to contribute significantly to the field of orthopedic implant design and TKA outcomes.
4. Discussion
The most important finding of this study was that it could show the way for enhancing implant stability, reducing stress shielding, and minimizing the risk of aseptic loosening by the optimization of the tibial stem design in TKA. This study systematically investigated the effects of various design parameters using the DOE methodology to identify an optimized tibial stem configuration.
During knee movement, flexion and extension occur within the sagittal plane, accompanied by additional femoral external rotation and roll-back on the tibia during flexion [
15,
16,
17]. These movements generate forces such as compression, tension, axial torque, varus/valgus moments, and shear, all of which must be resisted by the components of a TKA to ensure stability [
18]. To mitigate these forces at the interface between the tibial component and the proximal tibia, projections like stems, pegs, or keels/wings can be integrated into the underside of the tibial component (
Figure 1). These projections help reduce shear forces and axial displacement (lift-off) caused by varus-valgus moments [
19]. Stems also limit micromotion at the bone/cement interface, thereby reducing the risk of aseptic loosening [
20]. However, the presence of a stem introduces new shear forces between the stem and the proximal tibia. In this study, to implement the optimized model, four parameters were screened in DOE1 to measure their responses in terms of minimum principal stress and strain energy. The results from DOE 1 indicated that stem diameter and length are dominant factors influencing both the minimum principal stress and strain energy. The optimization process in DOE 2 showed that a smaller stem diameter (12 mm) combined with a stem length (40 mm) with the minimum amount of bone resection produced favorable outcomes in terms of both stress shielding and aseptic loosening. The regression analysis further validated these findings, demonstrating that the optimized model significantly outperforms the base model in multi-objective responses, with improvements in stress distribution and strain energy. As a key difference from previous studies [
7], in DOE 2 there was no significant effect of stem length on response that a value was determined in case of minimum responses and there was significant effect of stem diameter on response that we decided further DOE. This is likely because, under the optimized model conditions, variations in stem diameter have a more significant impact on stress shielding and other related responses compared to changes in stem length.
Stems enhance the stiffness of the tibial construct and offer resistance to bending [
21,
22]. When tibial stems are sufficiently long, they engage the cortical bone as the metaphyseal flare tapers into the diaphysis. This engagement is more pronounced in press-fit stems, which are designed for bone ongrowth or ingrowth, compared to long stems with a smooth or polished surface. This configuration directs load directly from the stem to the cortical bone, causing stress to concentrate in this area and leading to stress shielding of the proximal metaphysis [
22]. Even without direct cortical contact, the length of the stem correlates with the extent of stress shielding that occurs [
23]. This stress shielding reduces bone density in the unloaded regions, increasing the risk of implant subsidence (tibial migration), loosening, and periprosthetic fractures. Another potential downside of longer stems is pain at the implant tip, where stress concentration happens [
24]. In primary TKA with short-stem designs, load transfer and stress shielding are influenced by the implant’s geometry, material, tibial coverage, and the use of cement. Previous studies have predominantly focused on analyzing the impact of stem length on the outcomes of tibial constructs, as mentioned above. However, in this study, we developed a new response model by assigning different weight values to key factors influencing early and late failures after TKA. Specifically, minimum principal stress was identified as a critical factor in early failure, while strain energy was linked to late failure. This approach provided a new perspective, suggesting that in addition to stem length, stem diameter should also be considered when evaluating tibial geometry.
Contrary to initial expectations, the wing angle had a minimal impact on biomechanical responses, leading to its exclusion from further optimization stages. Similarly, the anterior wing design, inspired by the cross-shaped configuration, did not significantly reduce the minimum principal stress, indicating that its inclusion may not be necessary in the final design.
The DOE 3 analysis focused on minimizing bone resection by varying the M/L ratio and stem length. The findings revealed that there were no significant effect of stem length and M/L ratio on response. The final model, with a stem length of 40 mm and an M/L ratio of 0.61, was determined to achieve minimal bone resection while maintaining mechanical stability. This configuration effectively balances the need to preserve bone stock during implantation with the necessity of ensuring sufficient implant stability to prevent complications like aseptic loosening and stress shielding. The study found that reducing both the M/L ratio and stem length minimized bone resection without compromising the mechanical integrity of the implant, making it an ideal choice for enhancing long-term outcomes in TKA. Research on the M/L ratio of tibial stems in knee replacement surgery is somewhat limited, but it is a relevant factor in optimizing implant design and stability. The M/L ratio, which refers to the width of the stem relative to the width of the tibial component, plays a significant role in ensuring proper load distribution and reducing the risk of stress shielding or implant migration [
7]. Several studies have touched on aspects of tibial stem design, including length, diameter, and surface finish, which all interact with the M/L ratio to influence the biomechanics of the implant. A balanced M/L ratio is important for maintaining stability, especially in cases where the tibial bone structure is compromised, such as in patients with osteoporosis or large bone defects. A well-chosen M/L ratio can help in achieving better alignment and fixation, thereby reducing the risks of loosening or subsidence [
25]. While specific studies on the M/L ratio alone are scarce, related research indicates that this parameter, along with stem length and diameter, needs careful consideration during the design and selection of implants to ensure long-term success in TKA.
Additionally, while this study primarily focused on primary TKA, it is important to consider its implications for revision surgeries. Revision TKA presents unique challenges due to bone loss and the need for more robust fixation. The findings from this study, particularly regarding the significance of stem diameter in stress distribution, could inform future research aimed at optimizing tibial stems specifically for revision procedures. Further studies are warranted to evaluate how the optimized design might perform under the more demanding conditions of revision TKA, where additional fixation methods and stem modifications may be required to address the complexities of bone quality and implant stability.
This study also has several limitations. First, the exclusion of bone-cement interface from this study represents a significant limitation, as the interaction between bone and cement plays a critical role in the overall stability and long-term success of implants. Additionally, the TKA prosthesis was analyzed and implanted in a previously validated normal knee joint model derived from a 62-year-old Asian female. Including knee models representing end-stage osteoarthritis or other deformities in the finite element analysis could have provided insights into a broader range of clinical situations. The lack of consideration for this factor may lead to an incomplete understanding of the biomechanical behavior of the tibial stem, potentially affecting the generalizability of the findings to clinical settings where cemented fixation is commonly used [
7]. Future research should incorporate bone-cement interference to provide a more comprehensive analysis of implant performance under realistic conditions. Second, significant limitation of this study is the use of isotropic, homogeneous, and linear material properties for cortical and cancellous bone. In reality, bone exhibits anisotropic and heterogeneous behavior, which significantly affects the mechanical response under various loading conditions [
26]. The assumption of linear elasticity may oversimplify the complex nonlinear behavior of bone under physiological conditions, potentially leading to inaccuracies in stress and strain predictions. Third, the exclusive use of tetrahedral elements for meshing the entire model is another limitation. While tetrahedral elements provide flexibility in meshing complex geometries, they are less accurate in stress analysis compared to hexahedral elements, particularly in areas of high stress gradients [
27,
28]. This choice may lead to localized inaccuracies in the predicted stress and strain distributions. Employing a combination of hexahedral and tetrahedral elements, or conducting a detailed mesh convergence study, would enhance the accuracy and reliability of the FEA results. Fourth, this study does not include experimental or clinical validation of the FEA results, which is a critical limitation. Without validation, the accuracy of the predicted outcomes, such as stress shielding and aseptic loosening, remains uncertain [
29]. The absence of validation may result in overestimation or underestimation of the model’s performance. Future work should incorporate validation through experimental testing or in vivo data to ensure the reliability of the findings and to bridge the gap between simulation and clinical application. Lastly, the most important aspect to consider in this study is the subjectivity of the weight setting. The weight factors (0.7 for minimum principal stress in early failure and 0.3 for strain energy in late failure) are determined based on the researcher’s subjective judgment of the importance of each factor. If the weight is not appropriately balanced, it may lead to suboptimal results in other criteria, such as long-term stress shielding. Additionally, trade-off management in response formula combines both objectives into one, which can oversimplify the relationship between the conflicting goals. For instance, improving one objective, such as reducing aseptic loosening, could inadvertently exacerbate another, such as increasing stress shielding. This interplay between objectives is not always accurately represented in a simple weighted approach. In conclusion, while the formula effectively captures the trade-off between early failure and long-term implant stability, the limitations regarding subjectivity in weighting and potential oversimplification of complex relationships should be carefully considered.
Future investigations should focus on understanding the effects of implant geometry on long-term outcomes such as bone remodeling and implant migration. Recent studies provide valuable insights into these areas. Giorgio et al. proposed an orthotropic continuum model with substructure evolution to explain bone remodeling mechanisms, aligning with Wolff’s law [
30], while Allena and Rémond explored innovative approaches to medical treatments through mechanical interventions [
31]. Integrating these perspectives into future analyses could significantly improve the understanding of long-term implant performance and their interaction with complex biomechanical environments.