Digging into the Cause of Abnormal Patellar Kinematics After Open-Wedge High Tibial Osteotomy via a Quantitative Study on In Vivo Soft Tissue Functional Changes
Abstract
:1. Introduction
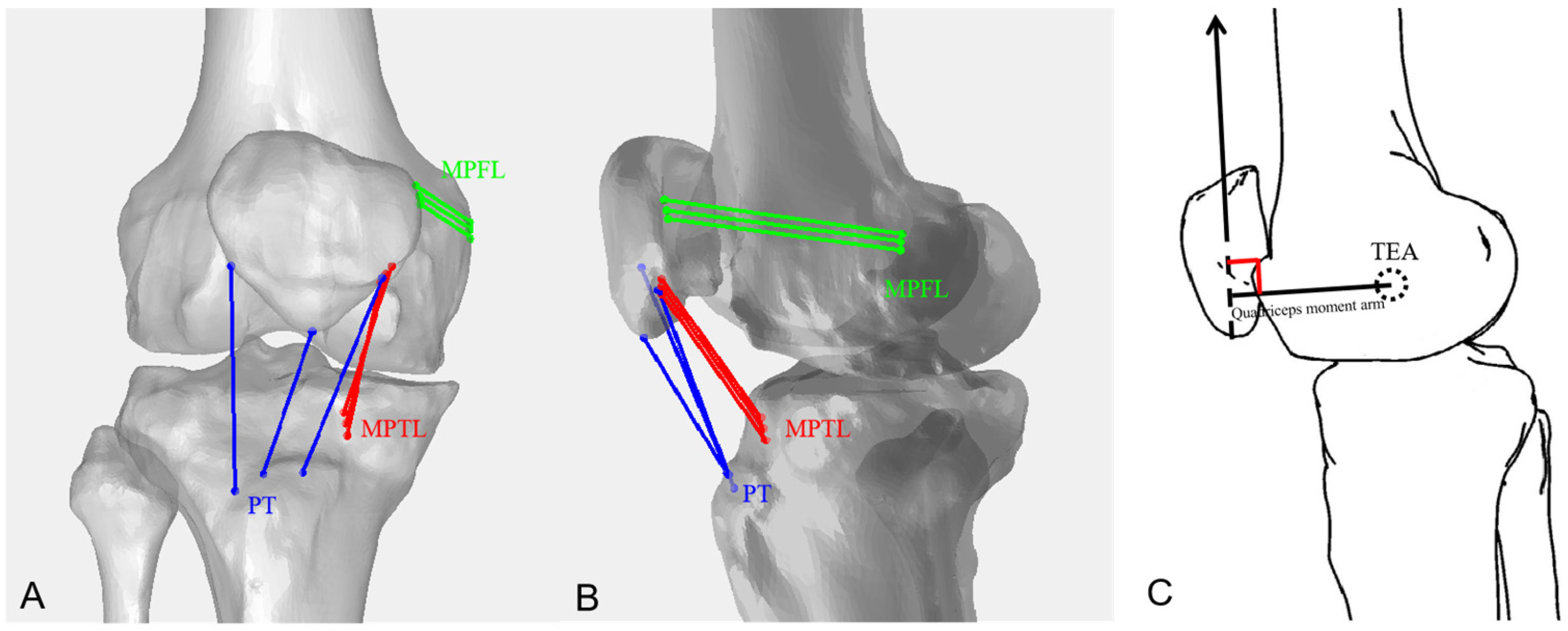
2. Materials and Methods
3. Results
3.1. Ligaments and Tendons
Length Comparisons of Each PT, MPTL, and MPFL Bundle
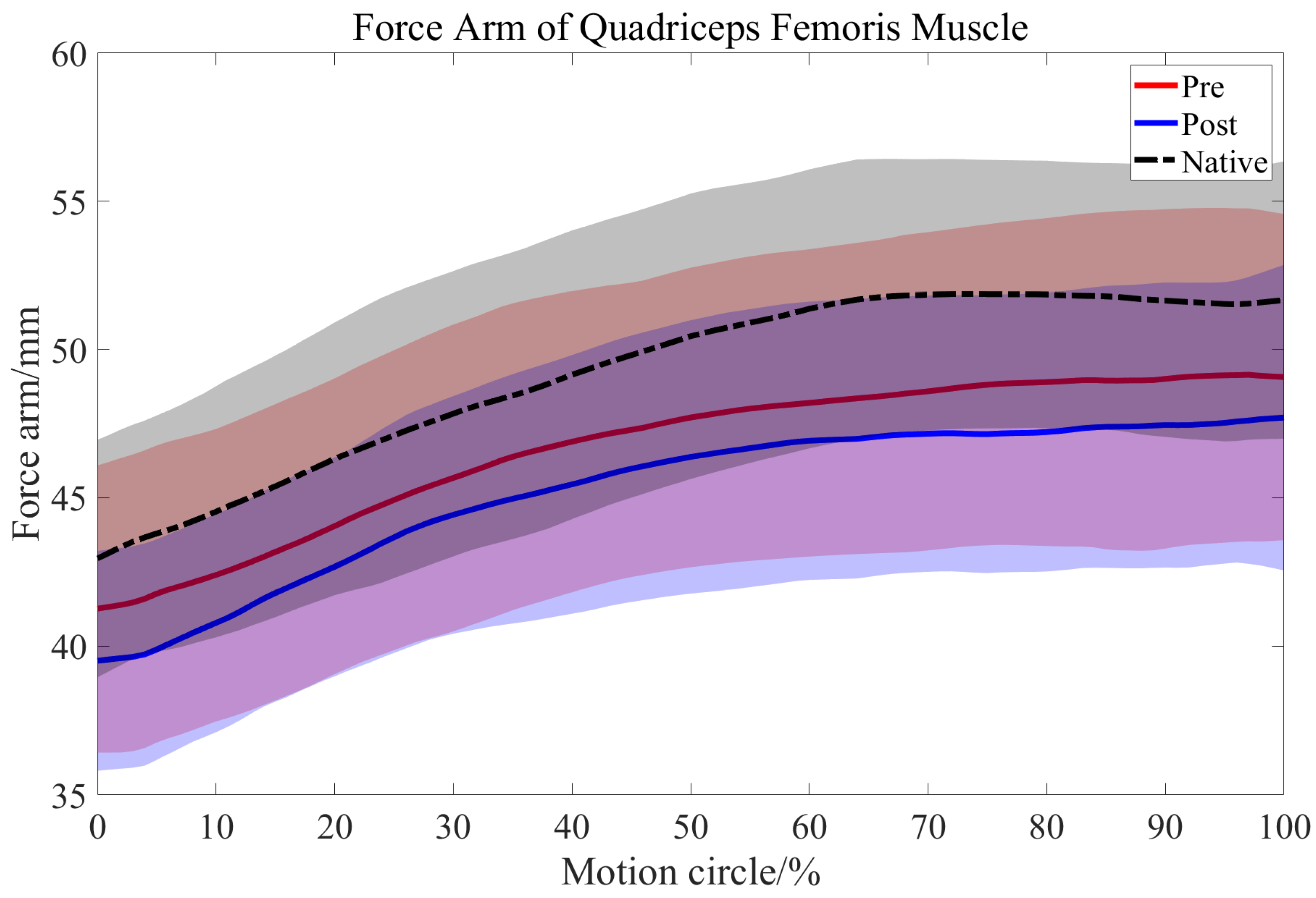
3.2. Length Changes in the QMA
3.3. The Correlation Between PT, MPTL, and the Open-Wedge Angle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ackerman, I.N.; Kemp, J.L.; Crossley, K.M.; Culvenor, A.G.; Hinman, R.S. Hip and Knee Osteoarthritis Affects Younger People, Too. J. Orthop. Sports Phys. Ther. 2017, 47, 67–79. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Gao, Y.; zhang, J.; Jin, Z. High Tibial Osteotomy: Review of Techniques and Biomechanics. J. Healthc. Eng. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, L.; Li, F.; Duan, J. Comparison between Closing-Wedge and Opening-Wedge High Tibial Osteotomy in Patients with Medial Knee Osteoarthritis: A Systematic Review and Meta-analysis. J. Knee Surg. 2017, 30, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.H.; Lee, W.-G.; Song, E.-K.; Jin, C.; Seon, J.-K. Comparison of Long-Term Survival Analysis Between Open-Wedge High Tibial Osteotomy and Unicompartmental Knee Arthroplasty. J. Arthroplast. 2021, 36, 1562–1567.e1. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, N.J.; Hill, N.A.; Fellows, R.A.; Ellis, R.E.; Wilson, D.R. Patellofemoral Joint Kinematics in Individuals with and without Patellofemoral Pain Syndrome. JBJS 2006, 88, 2596–2605. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Kobayashi, H.; Nejima, S.; Schröter, S. Can double-level osteotomy prevent patellofemoral osteoarthritis progression compared with open wedge high tibial osteotomy? Arch. Orthop. Trauma Surg. 2023, 143, 2073–2085. [Google Scholar] [CrossRef]
- Kataoka, K.; Watanabe, S.; Nagai, K.; Kay, J.; Matsushita, T.; Kuroda, R.; de Sa, D. Patellofemoral Osteoarthritis Progresses after Medial Open-Wedge High Tibial Osteotomy: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 3177–3186. [Google Scholar] [CrossRef]
- Amendola, L.; Fosco, M.; Cenni, E.; Tigani, D. Knee joint arthroplasty after tibial osteotomy. Int. Orthop. 2010, 34, 289–295. [Google Scholar] [CrossRef]
- D’ Entremont, A.G.; McCormack, R.G.; Horlick, S.G.D.; Stone, T.B.; Manzary, M.M.; Wilson, D.R. Effect of opening-wedge high tibial osteotomy on the three-dimensional kinematics of the knee. Bone Jt. J. 2014, 96-B, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Grantham, W.J.; Zachary, S.A.; Alex, W.B.; Samuel, I.R.; Travis Hunter, W.S.; Grant, J.D.; Robert, F.L. Medial Patellotibial Ligament Reconstruction Improves Patella Tracking When Combined with Medial Patellofemoral Reconstruction: An In Vitro Kinematic Study. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 2501–2509. [Google Scholar] [CrossRef]
- Desio, S.M.; Burks, R.T.; Bachus, K.N. Soft tissue restraints to lateral patellar translation in the human knee. Am. J. Sports Med. 1998, 26, 59–65. [Google Scholar] [CrossRef]
- Dean, R.S.; Hinckel, B.B.; Arendt, E.A. Combined Medial Patellofemoral Ligament and Medial Patellotibial Ligament Reconstruction. In Anterior Knee Pain and Patellar Instability; Sanchis-Alfonso, V., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 445–459. [Google Scholar]
- Philippot, R.; Boyer, B.; Testa, R.; Farizon, F.; Moyen, B. The role of the medial ligamentous structures on patellar tracking during knee flexion. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 331–336. [Google Scholar] [CrossRef]
- Halloran, J.P.; Esquivel, A.O.; Cracchiolo, A.M.; Chen, C.; Lemos, S.E. The Role of the MPFL and MPTL in Patellar Stability—A Biomechanical Study. Arch. Orthop. 2020, 1, 49–54. [Google Scholar]
- Gokay, N.S.; Erginer, R.; Dervisoglu, S.; Yalcin, M.B.; Gokce, A. Patella infera or patellar tendon adherence after high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1591–1598. [Google Scholar] [CrossRef]
- Davies, G.S.; van Duren, B.; Shorthose, M.; Roberts, P.G.; Morley, J.R.; Monk, A.P.; Murray, D.W.; Pandit, H.G. Changes in patella tendon length over 5 years after different types of knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, H.; Glabgly, P.; Paul, J.; Imhoff, A.B.; Hinterwimmer, S. Patellar Height and Posterior Tibial Slope after Open- and Closed-Wedge High Tibial Osteotomy:A Radiological Study on 100 Patients. Am. J. Sports Med. 2010, 38, 323–329. [Google Scholar] [CrossRef]
- Mason, J.J.; Leszko, F.; Johnson, T.; Komistek, R.D. Patellofemoral joint forces. J. Biomech. 2008, 41, 2337–2348. [Google Scholar] [CrossRef]
- Teitge, R.A. The power of transverse plane limb mal-alignment in the genesis of anterior knee pain—Clinical relevance. Ann. Jt. 2018, 3, 70. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Poole, C.; Chadha, H.; Hermida, J.C.; Mahar, A.; Colwell, C.W.J. Quadriceps Moment Arm and Quadriceps Forces After Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2001, 392, 213–220. [Google Scholar] [CrossRef]
- Fan, J.C.H. Open wedge high tibial osteotomy: Cause of patellar descent. J. Orthop. Surg. Res. 2012, 7, 3. [Google Scholar] [CrossRef]
- Li, G.; Kozanek, M.; Hosseini, A.; Liu, F.; Van de Velde, S.K.; Rubash, H.E. New fluoroscopic imaging technique for investigation of 6DOF knee kinematics during treadmill gait. J. Orthop. Surg. Res. 2009, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Hosseini, A.; Tsai, T.-Y.; Li, J.-S.; Rubash, H.E.; Li, G. In vivo kinematics of the knee during weight bearing high flexion. J. Biomech. 2013, 46, 1576–1582. [Google Scholar] [CrossRef]
- Liu, F.; Gadikota, H.R.; Kozánek, M.; Hosseini, A.; Yue, B.; Gill, T.J.; Rubash, H.E.; Li, G. In vivo length patterns of the medial collateral ligament during the stance phase of gait. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Feller, J.A.; Bartlett, R.J.; Lang, D.M. Patellar Resurfacing versus Retention in Total Knee Arthroplasty. J. Bone Jt. Surg. Br. Vol. 1996, 78-B, 226–228. [Google Scholar] [CrossRef]
- Annika, T.; Donald, C.B.; Andrew, C.S.; Susan, W.K.; Jeganath, K. The Use of Scoring Systems in Knee Arthroplasty: A Systematic Review of the Literature. J. Arthroplast. 2016, 31, 2364–2370.e8. [Google Scholar]
- Matthew, P.S.; Kade, S.M.; Adam, M.F.; Lisa, A.G.; Kevin, J.S.; Mark, S.K. Current Trends in Patient-Reported Outcome Measures in Total Joint Arthroplasty: A Study of 4 Major Orthopaedic Journals. J. Arthroplast. 2018, 33, 3416–3421. [Google Scholar]
- Bae, D.K.; Song, S.J.; Park, C.H.; Liang, H.; Bae, J.K. Comparison of mid-term results between conversion total knee arthroplasties following closed wedge high tibial osteotomy and primary total knee arthroplasties: A matched pair study including patellar symptom and position. J. Orthop. Sci. 2017, 22, 495–500. [Google Scholar] [CrossRef]
- Schwiesau, J.; Schilling, C.; Kaddick, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blömer, W.; Grupp, T. Definition and evaluation of testing scenarios for knee wear simulation under conditions of highly demanding daily activities. Med. Eng. Phys. 2013, 35, 591–600. [Google Scholar] [CrossRef]
- Sanchis-Alfonso, V.; Marín-Roca, S.; Montesinos-Berry, E.; Baydal-Bertomeu, J.M.; Moya, M.F.P.-D. Kinetic and Kinematic Analysis in Evaluating Patients with Anterior Knee Pain. In Anterior Knee Pain and Patellar Instability; Sanchis-Alfonso, V., Ed.; Springer: London, UK, 2011; pp. 317–327. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Yokoyama, M.; Nakamura, Y.; Onishi, T.; Hirano, K.; Doi, M. Healing Period after Open High Tibial Osteotomy Relat. Factors: Can We Really Say That It Is Long? SpringerPlus 2016, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Pauls, J.L. Safety standards, requirements, and litigation in relation to building use and safety, especially safety from falls involving stairs. Saf. Sci. 1991, 14, 125–154. [Google Scholar] [CrossRef]
- Bingham, J.; Li, G. An Optimized Image Matching Method for Determining In-Vivo TKA Kinematics with a Dual-Orthogonal Fluoroscopic Imaging System. J. Biomech. Eng. 2006, 128, 588–595. [Google Scholar] [CrossRef]
- Li, G.; Papannagari, R.; Nha, K.W.; Defrate, L.E.; Rubash, H.E. The Coupled Motion of the Femur and Patella During In Vivo Weightbearing Knee Flexion. J. Biomech. Eng. 2007, 129, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hosseini, A.; Li, J.S.; Gill, T.J.T.; Li, G. In vivo patellar tracking and patellofemoral cartilage contacts during dynamic stair ascending. J. Biomech. 2012, 45, 2432–2437. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 4th ed.; LWW: Baltimore, MD, USA, 2014; Volume 123. [Google Scholar]
- Tsai, T.-Y.; Dimitriou, D.; Li, G.; Kwon, Y.-M. Does total hip arthroplasty restore native hip anatomy? Three-dimensional reconstruction analysis. Int. Orthop. 2014, 38, 1577–1583. [Google Scholar] [CrossRef]
- Wilson, N.A.; Press, J.M.; Koh, J.L.; Hendrix, R.W.; Zhang, L.-Q. In Vivo Noninvasive Evaluation of Abnormal Patellar Tracking during Squatting in Patients with Patellofemoral Pain. JBJS 2009, 91, 558–566. [Google Scholar] [CrossRef]
- Alexander, E.; Dyrby, C.; Andriacchi, T. Kinematic patterns and knee cartilage thickness. Trans. Orthop. Res. Soc. 2003, 28, 1266. [Google Scholar]
- Kim, J.I.; Jang, J.; Lee, K.W.; Han, H.S.; Lee, S.; Lee, M.C. Anterior tibial curved cortex is a reliable landmark for tibial rotational alignment in total knee arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 252. [Google Scholar] [CrossRef]
- Defrate, L.E.; Nha, K.W.; Papannagari, R.; Moses, J.M.; Gill, T.J.; Li, G. The biomechanical function of the patellar tendon during in-vivo weight-bearing flexion. J. Biomech. 2007, 40, 1716–1722. [Google Scholar] [CrossRef]
- Peterson, C.Y.; Krzyzaniak, M.; Coimbra, R.; Chang, D.C. Vagus Nerve and Postinjury Inflammatory Response. Arch. Surg. 2012, 147, 76–80. [Google Scholar] [CrossRef]
- Sarasua, S.M.; Floyd, S.; Bridges, W.C.; Pill, S.G. The epidemiology and etiology of adhesive capsulitis in the U.S. Medicare population. BMC Musculoskelet. Disord. 2021, 22, 828. [Google Scholar] [CrossRef]
- O’Brien, T.D.; Reeves, N.D.; Baltzopoulos, V.; Jones, D.A.; Maganaris, C.N. Mechanical properties of the patellar tendon in adults and children. J. Biomech. 2010, 43, 1190–1195. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.-M.; Liu, J.-Y. Patella infera following patellar tendon contracture after closed trauma. Chin. Med. J. 2013, 126, 3990–3991. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, X.; Li, Y.; Liu, X.; Wang, S.; Lu, M.; Yan, X.; Deng, L.; Liu, S.; Wang, F.; et al. Self-Healing Hydrogel Embodied with Macrophage-Regulation and Responsive-Gene-Silencing Properties for Synergistic Prevention of Peritendinous Adhesion. Adv. Mater. 2022, 34, 2106564. [Google Scholar] [CrossRef]
- Forbes, K.E.; Cavallaro, D.; Power, D. A Systematic Review of Anti-Adhesion Agents in Hand Trauma. Hand 2024, 5, 15589447241238374. [Google Scholar] [CrossRef]
- Yin, W.; Liu, X.; Wang, K.; Shen, L.; Li, Y.; Cai, Q.; Chen, S.; Chen, J.; Liu, S. Ultrasound-guided Hydrogel Injection Provides Better Therapeutic Effects After Hand Tendon Surgery Than Intraoperative Injection: A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2022, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Rajasekaran, S. Ultrasound-Guided Scraping for Chronic Patellar Tendinopathy: A Case Presentation. PM&R 2016, 8, 593–596. [Google Scholar]
- Malinowski, K.; Szalbot, K.; Pękala, P.A.; LaPrade, R.F.; Mostowy, M. Patella Baja Revisited: Interposition of a Pedunculated Flap of the Hoffa Fat Pad to Treat Adhesions Between the Tibia and Patellar Tendon and Restore the Functional Length of the Patellar Tendon. Arthrosc. Tech. 2024, 13, 103108. [Google Scholar] [CrossRef] [PubMed]
- Westrich, G.H.; Peters, L.E.; Haas, S.B.; Buly, R.L.; Windsor, R.E. Patella Height After High Tibial Osteotomy with Internal Fixation and Early Motion. Clin. Orthop. Relat. Res. 1998, 354, 169–174. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Hu, B.; Sun, Q.; Wan, M.; Carr, A.; Liu, S.; Cao, X. Neutralization of excessive levels of active TGF-β1 reduces MSC recruitment and differentiation to mitigate peritendinous adhesion. Bone Res. 2023, 11, 24. [Google Scholar] [CrossRef]
- Sim, J.A.; Na, Y.G.; Lee, B.K.; Lee, B.H. Alignment changes after open-wedge high tibial osteotomy result in offloading in the patellofemoral joint: A SPECT/CT analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 437–446. [Google Scholar] [CrossRef]
- Ellis, M.; Seedhom, B.; Wright, V.; Dowson, D. An evaluation of the ratio between the tensions along the quadriceps tendon and the patellar ligament. Eng. Med. 1980, 9, 189–194. [Google Scholar] [CrossRef]
- van Eijden, T.M.G.J.; Weijs, W.A.; Kouwenhoven, E.K.; Verburg, J. Forces Acting on the Patella during Maximal Voluntary Contraction of the Quadriceps femoris Muscle at Different Knee Flexion/Extension Angles. Acta Anat. 2008, 129, 310–314. [Google Scholar] [CrossRef]
- Ryo, G.; Takehiko, M.; Yuya, U.; Shibata, Y.; Miura, D.; Ono, K.; Kida, A.; Nishida, K.; Nagai, K.; Kanzaki, N.; et al. Quadriceps strength can improve twelve months after opening wedge high tibial osteotomy and opening wedge distal tibial tubercle osteotomy, particularly after opening wedge high tibial osteotomy. Knee 2024, 51, 258–267. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kruckeberg, B.M.; Chahla, J.; Moatshe, G.; Cinque, M.E.; Muckenhirn, K.J.; Godin, J.A.; Ridley, T.J.; Brady, A.W.; Arendt, E.A.; LaPrade, R.F. Quantitative and Qualitative Analysis of the Medial Patellar Ligaments: An Anatomic and Radiographic Study. Am. J. Sports Med. 2018, 46, 153–162. [Google Scholar] [CrossRef] [PubMed]



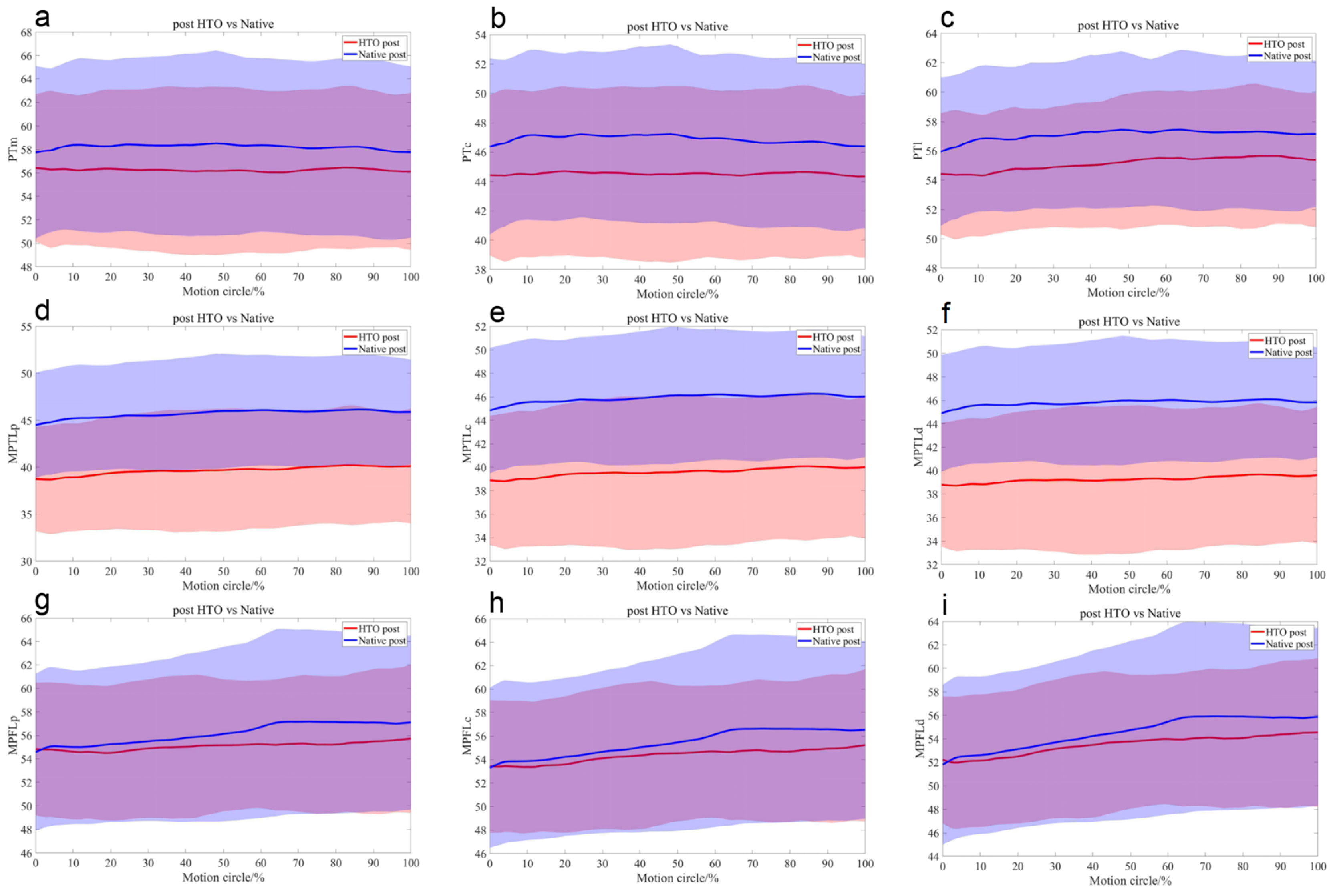
| Ligament | Bundles | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | M (mm) | −0.55 ± 3.43 | −1.3 ± 3.24 | −1.06 ± 3.32 | −0.81 ± 3.35 | −0.76 ± 3.48 | −0.62 ± 3.57 | −0.83 ± 3.13 | −0.81 ± 3.15 | −0.82 ± 3.02 | −0.86 ± 2.96 | −0.41 ± 2.94 |
| C (mm) | −1.31 ± 2.76 | −1.63 ± 2.52 * | −1.11 ± 2.5 | −0.76 ± 2.63 | −0.69 ± 2.73 | −0.73 ± 2.9 | −1.18 ± 2.28 | −1.18 ± 2.23 | −1.11 ± 2.13 | −1.25 ± 2.25 | −0.7 ± 2.11 | |
| L (mm) | −1.85 ± 2.04 * | −2.11 ± 2.63 * | −1.5 ± 3.05 | −0.98 ± 3.26 | −0.82 ± 3.31 | −1.17 ± 3.35 | −1.84 ± 2.61 * | −1.85 ± 2.32 * | −1.77 ± 2.25 * | −2.07 ± 2.55 * | −1.58 ± 2.7 * | |
| MPTL | P (mm) | −0.77 ± 3.5 | −1.47 ± 3.12 | −1 ± 3.07 | −0.77 ± 3.21 | −0.9 ± 3.36 | −0.79 ± 3.39 | −0.93 ± 2.81 | −0.92 ± 2.82 | −0.96 ± 2.53 | −0.98 ± 2.55 | −0.59 ± 2.47 |
| C (mm) | −0.92 ± 3.35 | −1.6 ± 2.96 * | −1.1 ± 2.88 | −0.83 ± 3 | −0.94 ± 3.13 | −0.84 ± 3.19 | −0.99 ± 2.63 | −0.96 ± 2.65 | −1 ± 2.4 | −1.04 ± 2.45 | −0.62 ± 2.37 | |
| D (mm) | −1.06 ± 3.17 | −1.7 ± 2.79 * | −1.19 ± 2.69 | −0.9 ± 2.77 | −0.99 ± 2.86 | −0.9 ± 2.94 | −1.07 ± 2.38 | −1.02 ± 2.42 | −1.05 ± 2.22 | −1.11 ± 2.28 | −0.67 ± 2.2 | |
| MPFL | P (mm) | 0.12 ± 4.03 | −0.22 ± 3.79 | 0.32 ± 3.6 | 0.66 ± 3.75 | 0.64 ± 3.47 | 0.49 ± 3.46 | 0.28 ± 4.09 | −0.13 ± 4.14 | −0.55 ± 4.13 | −0.71 ± 4.34 | −0.43 ± 3.58 |
| C (mm) | −0.11 ± 4.11 | −0.16 ± 3.7 | 0.2 ± 3.56 | 0.7 ± 3.62 | 0.55 ± 3.32 | 0.29 ± 3.21 | 0.18 ± 3.93 | −0.16 ± 4.13 | −0.53 ± 4.1 | −0.62 ± 4.29 | −0.53 ± 3.7 | |
| D (mm) | −0.1 ± 3.97 | −0.29 ± 3.66 | 0.28 ± 3.37 | 0.66 ± 3.44 | 0.51 ± 3.19 | 0.22 ± 3.11 | 0.07 ± 3.83 | −0.23 ± 3.99 | −0.58 ± 4 | −0.67 ± 4.33 | −0.45 ± 3.67 |
| Ligament | Bundles | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | M (mm) | 0.14 ± 2.5 | 0.2 ± 3.01 | 0.18 ± 3.11 | −0.11 ± 3.26 | −0.23 ± 3.22 | −0.45 ± 3.17 | −0.26 ± 2.85 | −0.12 ± 3.08 | 0.17 ± 3.19 | 0.04 ± 3.1 | −0.47 ± 3.17 |
| C (mm) | −0.42 ± 2.49 | −0.49 ± 2.86 | −0.56 ± 2.94 | −0.88 ± 3.11 | −1 ± 3.21 | −1.04 ± 3.08 | −0.52 ± 2.66 | −0.36 ± 2.81 | −0.12 ± 2.96 | −0.07 ± 2.99 | −0.61 ± 3.03 | |
| L (mm) | −0.31 ± 3.03 | −0.63 ± 3.23 | −0.55 ± 3.44 | −0.9 ± 3.4 | −0.97 ± 3.43 | −0.72 ± 3.22 | −0.05 ± 2.99 | 0.02 ± 3.03 | 0.18 ± 3.12 | 0.45 ± 3.24 | −0.1 ± 3.32 | |
| MPTL | P (mm) | −4.11 ± 1.65 * | −3.99 ± 1.77 * | −4 ± 1.81 * | −4.1 ± 2.06 * | −4.2 ± 2.26 * | −4.44 ± 2.32 * | −4.17 ± 2.14 * | −4.08 ± 2.28 * | −3.94 ± 2.11 * | −4.11 ± 1.78 * | −4.44 ± 1.97 * |
| C (mm) | −4.18 ± 1.62 * | −4.15 ± 1.79 * | −4.2 ± 1.8 * | −4.37 ± 1.96 * | −4.45 ± 2.21 * | −4.67 ± 2.23 * | −4.34 ± 2.05 * | −4.25 ± 2.25 * | −4.13 ± 2.12 * | −4.26 ± 1.79 * | −4.63 ± 2.03 * | |
| D (mm) | −4.26 ± 1.55 * | −4.32 ± 1.81 * | −4.4 ± 1.86 * | −4.62 ± 2 * | −4.7 ± 2.3 * | −4.9 ± 2.28 * | −4.54 ± 2.06 * | −4.44 ± 2.32 * | −4.33 ± 2.21 * | −4.43 ± 1.85 * | −4.83 ± 2.13 * | |
| MPFL | P (mm) | 0.65 ± 2.35 | 0.35 ± 2.57 | −0.16 ± 2.21 | −0.27 ± 2.05 | −0.26 ± 2.27 | −0.29 ± 2.18 | −0.34 ± 2.19 | −0.41 ± 2.26 | −0.6 ± 2.34 | −0.37 ± 2.6 | −0.48 ± 2.4 |
| C (mm) | 0.65 ± 2.48 | 0.22 ± 2.43 | −0.09 ± 2.16 | −0.27 ± 2.08 | −0.21 ± 2.22 | −0.22 ± 2.17 | −0.29 ± 2.23 | −0.33 ± 2.37 | −0.57 ± 2.44 | −0.47 ± 2.67 | −0.5 ± 2.41 | |
| D (mm) | 0.94 ± 2.69 | 0.31 ± 2.45 | −0.11 ± 2.12 | −0.26 ± 2.08 | −0.21 ± 2.26 | −0.19 ± 2.18 | −0.2 ± 2.32 | −0.32 ± 2.38 | −0.55 ± 2.46 | −0.36 ± 2.65 | −0.58 ± 2.41 |
| Ligament | Bundles | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | M (mm) | −1.33 ± 3.61 | −2.13 ± 3.91 * | −1.91 ± 4.17 | −2.09 ± 4.53 | −2.22 ± 4.6 | −2.32 ± 4.46 * | −2.27 ± 4.51 * | −1.99 ± 4.52 | −1.77 ± 4.37 | −1.74 ± 4.44 | −1.64 ± 4.35 |
| C (mm) | −1.95 ± 3.41 * | −2.66 ± 3.55 * | −2.34 ± 3.7 * | −2.49 ± 4 * | −2.69 ± 3.98 * | −2.68 ± 3.81 * | −2.45 ± 3.91 * | −2.22 ± 4.01 * | −2.07 ± 3.88 * | −2.06 ± 3.84 * | −2.06 ± 3.76 | |
| L (mm) | −1.51 ± 3.62 | −2.48 ± 3.8 * | −2.01 ± 3.94 * | −2.12 ± 4.03 * | −2.28 ± 3.92 * | −2.13 ± 3.78 * | −1.88 ± 3.77 | −1.83 ± 3.92 | −1.72 ± 3.94 | −1.58 ± 3.92 | −1.77 ± 3.81 | |
| MPTL | P (mm) | −5.76 ± 2.25 * | −6.29 ± 2.64 * | −5.97 ± 2.98 * | −5.91 ± 3.51 * | −6.11 ± 3.78 * | −6.3 ± 3.68 * | −6.32 ± 3.94 * | −6 ± 3.85 * | −5.92 ± 3.39 * | −5.97 ± 3.15 * | −5.76 ± 3.16 * |
| C (mm) | −5.95 ± 2.26 * | −6.54 ± 2.61 * | −6.23 ± 2.93 * | −6.2 ± 3.4 * | −6.4 ± 3.64 * | −6.54 ± 3.5 * | −6.54 ± 3.78 * | −6.23 ± 3.71 * | −6.18 ± 3.33 * | −6.21 ± 3.06 * | −6.01 ± 3.11 * | |
| D (mm) | −6.09 ± 2.3 * | −6.76 ± 2.65 * | −6.45 ± 2.97 * | −6.46 ± 3.41 * | −6.65 ± 3.62 * | −6.76 ± 3.45 * | −6.74 ± 3.73 * | −6.44 ± 3.68 * | −6.4 ± 3.35 * | −6.42 ± 3.07 * | −6.22 ± 3.14 * | |
| MPFL | P (mm) | 0.26 ± 3.35 | −0.4 ± 3.16 | −0.75 ± 2.83 | −0.61 ± 2.95 | −0.77 ± 3.32 | −0.97 ± 3.95 | −1.46 ± 3.85 | −1.86 ± 3.82 | −1.92 ± 3.62 | −1.61 ± 3.47 | −1.39 ± 3.66 |
| C (mm) | 0.11 ± 3.45 | −0.5 ± 3.1 | −0.64 ± 2.73 | −0.55 ± 2.78 | −0.7 ± 3.14 | −0.93 ± 3.86 | −1.46 ± 3.73 | −1.83 ± 3.76 | −1.94 ± 3.58 | −1.65 ± 3.46 | −1.3 ± 3.63 | |
| D (mm) | 0.42 ± 3.63 | −0.47 ± 3.3 | −0.63 ± 2.83 | −0.55 ± 2.88 | −0.76 ± 3.27 | −0.98 ± 3.95 | −1.48 ± 3.82 | −1.82 ± 3.85 | −1.84 ± 3.68 | −1.45 ± 3.51 | −1.33 ± 3.82 |
| Comparison | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre vs. Post (mm) | −1.74 ± 2.47 * | −1.62 ± 2.68 * | −1.47 ± 2.95 | −1.39 ± 3.77 | −1.4 ± 3.59 | −1.2 ± 3.85 | −1.23 ± 3.87 | −1.36 ± 3.64 | −1.6 ± 3.33 | −1.48 ± 3.18 | −1.45 ± 3.47 |
| Post vs. Native (mm) | −3.44 ± 2.73 * | −3.75 ± 3.23 * | −3.64 ± 3.86 * | −3.41 ± 4.24 * | −3.7 ± 3.94 * | −4.07 ± 3.96 * | −4.45 ± 3.83 * | −4.69 ± 3.51 * | −4.64 ± 3.61 * | −4.2 ± 3.88 * | −3.96 ± 4.41 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zheng, N.; He, A.; Zhang, G.; Lin, W.; Qu, Y.; Tsai, T.-Y.; Liu, W.; Mao, Y. Digging into the Cause of Abnormal Patellar Kinematics After Open-Wedge High Tibial Osteotomy via a Quantitative Study on In Vivo Soft Tissue Functional Changes. Bioengineering 2025, 12, 123. https://doi.org/10.3390/bioengineering12020123
Jiang Z, Zheng N, He A, Zhang G, Lin W, Qu Y, Tsai T-Y, Liu W, Mao Y. Digging into the Cause of Abnormal Patellar Kinematics After Open-Wedge High Tibial Osteotomy via a Quantitative Study on In Vivo Soft Tissue Functional Changes. Bioengineering. 2025; 12(2):123. https://doi.org/10.3390/bioengineering12020123
Chicago/Turabian StyleJiang, Zheng, Nan Zheng, Axiang He, Guoqiang Zhang, Weiming Lin, Yang Qu, Tsung-Yuan Tsai, Wanjun Liu, and Yanjie Mao. 2025. "Digging into the Cause of Abnormal Patellar Kinematics After Open-Wedge High Tibial Osteotomy via a Quantitative Study on In Vivo Soft Tissue Functional Changes" Bioengineering 12, no. 2: 123. https://doi.org/10.3390/bioengineering12020123
APA StyleJiang, Z., Zheng, N., He, A., Zhang, G., Lin, W., Qu, Y., Tsai, T.-Y., Liu, W., & Mao, Y. (2025). Digging into the Cause of Abnormal Patellar Kinematics After Open-Wedge High Tibial Osteotomy via a Quantitative Study on In Vivo Soft Tissue Functional Changes. Bioengineering, 12(2), 123. https://doi.org/10.3390/bioengineering12020123









