1. Introduction
Diagnosis, monitoring, and therapeutic decisions in ischemic heart disease (IHD) rely on the depiction of the coronary anatomy and function. Although the vast majority of the intramyocardial blood volume resides in the microcirculation, high-resolution clinical imaging systems cannot depict the microvascular network due to spatiotemporal limitations. To overcome the limited resolution in clinical imaging systems and provide a more comprehensive depiction, patient-specific myocardial perfusion data can be integrated with computational fluid dynamic analysis to enable automated myocardial arterial network generation and simulations of hemodynamic behavior.
Non-invasive cardiac perfusion stress testing methods, including nuclear or first-pass gadolinium perfusion magnetic resonance imaging (MRI), adopt a framework that is designed for the detection of epicardial coronary stenosis [
1] rather than microvascular disease. Although dynamic stress CT myocardial perfusion imaging (CT-MPI) and coronary CT angiography (CTA) provide whole-heart myocardial perfusion along with anatomic coronary images, CT-MPI is timed to capture the transit of iodinated contrasts during the systolic cardiac phase, whereas coronary CTA is timed to capture anatomic coronary images during mid-diastole [
1]. This discordance in the cardiac phase alignment during the image acquisition makes it difficult to spatially and physiologically correlate anatomic coronary narrowing with myocardial perfusion. Moreover, both iodinated and gadolinium-based contrast agents extravasate almost immediately after injection, requiring a correction for leakage in quantitative perfusion models.
Recently, researchers have proposed the use of ferumoxytol-enhanced (FE) MRI in combination with a two-compartment water exchange model to map the fractional myocardial blood volume (fMBV) as a marker for hypoperfusion [
2,
3,
4]. Ferumoxytol (Feraheme, Azurity Pharmaceuticals, Woburn, MA, USA) is an iron supplement that can be used as a pure intravascular MRI contrast agent to sensitize the intravascular space to changes in blood volume, enabling the accurate estimation of the fMBV. With increased oxygen demand, the body’s autoregulatory system will increase capillary recruitment, leading to increased fMBV, and in areas of microvascular dysfunction, there is decreased fMBV thought to be related to decreased capillary density or dysfunctional microvessels [
5]. Unlike CT-MPI and coronary CTAs, both FE-MRI fMBV maps and whole-heart coronary MRAs are acquired during the steady-state distribution of ferumoxytol and during mid-diastole.
In this study, we propose to develop a synthetic patient-specific framework for the automatic generation of a one-dimensional arterial network in the myocardium based on perfusion data derived from FE-MRI fMBV maps and FE coronary MRA. Consistent with principles of hemodynamic efficiency, whereby the blood volume distribution throughout a solid organ is determined by the local metabolic demand, we hypothesize that FE-MRI fMBV maps, reflecting the blood volume distribution, along with FE coronary MRA will enable the accurate and reliable automatic generation of the myocardial arterial network based on a modified adaptive constrained constructive optimization (CCO) approach. Existing methods for automatic myocardial arterial network generation include fractal models [
6], space-filling methods that encompass optimization algorithms [
7], and continuous models with porous media flow simulations [
8]. Of the space-filling methods, the adaptive CCO approach can be tailored to reflect the physiological and anatomical variations in the myocardial arterial network [
9]. Although existing adaptive CCO approaches excel at capturing intricate network details and accommodating patient-specific physiological variations, they have not been tailored for the coronary circulation. Despite decades of progress in hemodynamic modeling techniques, the generation of a large, physiologically sound, and patient-specific vascular network that accurately supplies blood to the ventricular myocardial segments remains challenging. Our contributions are three-fold: (1) the introduction of a modified framework that constructs adaptable, multiscale, and physiological 1D arterial networks in subject-specific myocardium; (2) the incorporation of FE-MRI fMBV maps to inherently account for the microcirculation’s blood flow autoregulation mechanism; and (3) the computation of reproducible hemodynamic indices at any location across the synthetic arterial network, which is crucial for studying the full spectrum of ischemic heart disease that requires the consideration of the microcirculation.
2. Materials and Methods
This article is a revised and expanded version of a paper entitled “Synthesis of Arterial Networks from Myocardial Blood Volume Maps”, which was presented at the 2024 ASME SB3C Summer Bioengineering Conference in Lake Geneva, WI, USA, on 11–14 June 2024. All animals were treated in accordance with the Guidelines for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the National Institutes of Health. The UCLA Animal Research Committee (ARC-2017-015) approved the animal studies. The human subject research was conducted in accordance with the Declaration of Helsinki. All patients provided written or electronic informed consent prior to participation. The Institutional Review Board at UCLA (IRB-19-2097) and the Veterans Health Administration (VA0004; IRBNet# 1615882) approved the human research studies.
2.1. Study Design
This study was designed to develop and validate a patient-specific and non-invasive framework to synthetically construct coronary arterial networks from MRI. The approach integrates FE MRI to obtain both coronary MRA and fMBV maps, which serve as inputs to a modified adaptive constrained constructive optimization algorithm for generating 1D vascular trees. Validation was performed in swine (n = 3) and healthy volunteers (n = 4), where 126 arterial networks (6000 terminal segments each) were generated using six random seedings per coronary tree and evaluated against empirical morphometric data. Computational simulations of pulsatile blood flow were performed for all generated microvascular networks, and reproducibility across seedings was assessed using mixed-effects models and waveform similarity metrics. Finally, in a patient with IHD, we demonstrate the framework’s ability to recapitulate tissue-dependent changes in morphological and functional parameters of microvasculature and its consistency with coronary autoregulation in areas of ischemia, scar, and remote myocardium.
2.2. Subject Characteristics
Three healthy Yorkshire swine (female, 20–33 kg), four healthy female human volunteers (age 35 ± 10.2 years), and one IHD patient with myocardial ischemia and scar were included. For the swine subjects, intramuscular ketamine (10 mg/kg), midazolam (1 mg/kg), and inhaled 1–2% isoflurane vaporized with 100% oxygen were used to achieve sedation and anesthesia. Intravenous rocuronium (2.5 mg/kg/h) was administered to immobilize the diaphragm during anesthesia and MRI. Hemodynamic and electrocardiographic vital signs were monitored throughout all procedures. This HIPAA-compliant study recruited both healthy volunteers and patients with IHD age ≥ 18 years to undergo an FE-MRI.
2.3. Magnetic Resonance Image Acquisition and Processing
We performed multi-dose FE-MRI T1 mapping on healthy swine and human subjects using the Modified Look-Locker Inversion (MOLLI) recovery sequence on a 3.0 T (Prisma and Skyra, Siemens, Erlangen, Germany) clinical magnet as previously described [
4]. Using the 5(3)3(3)3 MOLLI recovery sequence, we acquired 7–10 left ventricular (LV) short axis T1 maps from base to apex. A balanced steady-state free precession (SSFP) readout with the following parameters was utilized: FOV = 300 × 240 mm, matrix size = 192 × 156, TR = 338 ms, TE = 1.01 ms, slice thickness = 8 mm, pixel bandwidth = 1085 Hz, flip angle = 35°, minimum T1 = 100 ms, and TI increment 80 ms. Diluted ferumoxytol (Feraheme, Azurity Pharmaceutical, Woburn, MA, USA) was infused for a cumulative dose of 4.0 mg/kg (ferumoxytol increment range 0.0–4.0 mg/kg). An in-house two-compartment water exchange model with three parameter fitting was used to generate pixel-wise fMBV maps [
2,
3] from multi-slice, 2D short axis T1 maps covering the entire ventricular myocardium from base to apex. Using 3D Slicer v5.2.1 [
10], we segmented each pixel-wise fMBV map based on the AHA 16-segment model [
11].
The 4D MUSIC pulse sequence [
12] was used to image the coronaries of the swine subjects, whereas the whole-heart coronary 3D MRA (WIP 1111, Siemens Healthcare, Erlangen, Germany) was used for human subjects. Both were acquired at steady-state ferumoxytol enhancement (4 mg/kg). Representative technical parameters for 4D MUSIC were as follows: TR/TE of 3.0/1.1 ms, flip angle of 25°, pixel bandwidth of 800 Hz/pixel, and isotropic spatial resolution of 1.1 mm
3. The whole-heart coronary MRA is a free-breathing, gradient echo sequence with Dixon imaging for fat suppression and inversion recovery preparation pulses. Representative parameters for the whole-heart coronary MRA sequence were as follows: TR = 985 ms, TE = 1.39 ms, flip angle = 15–18°, and isotropic spatial resolution of 1.1 mm
3.
2.4. Vascular Network Generation
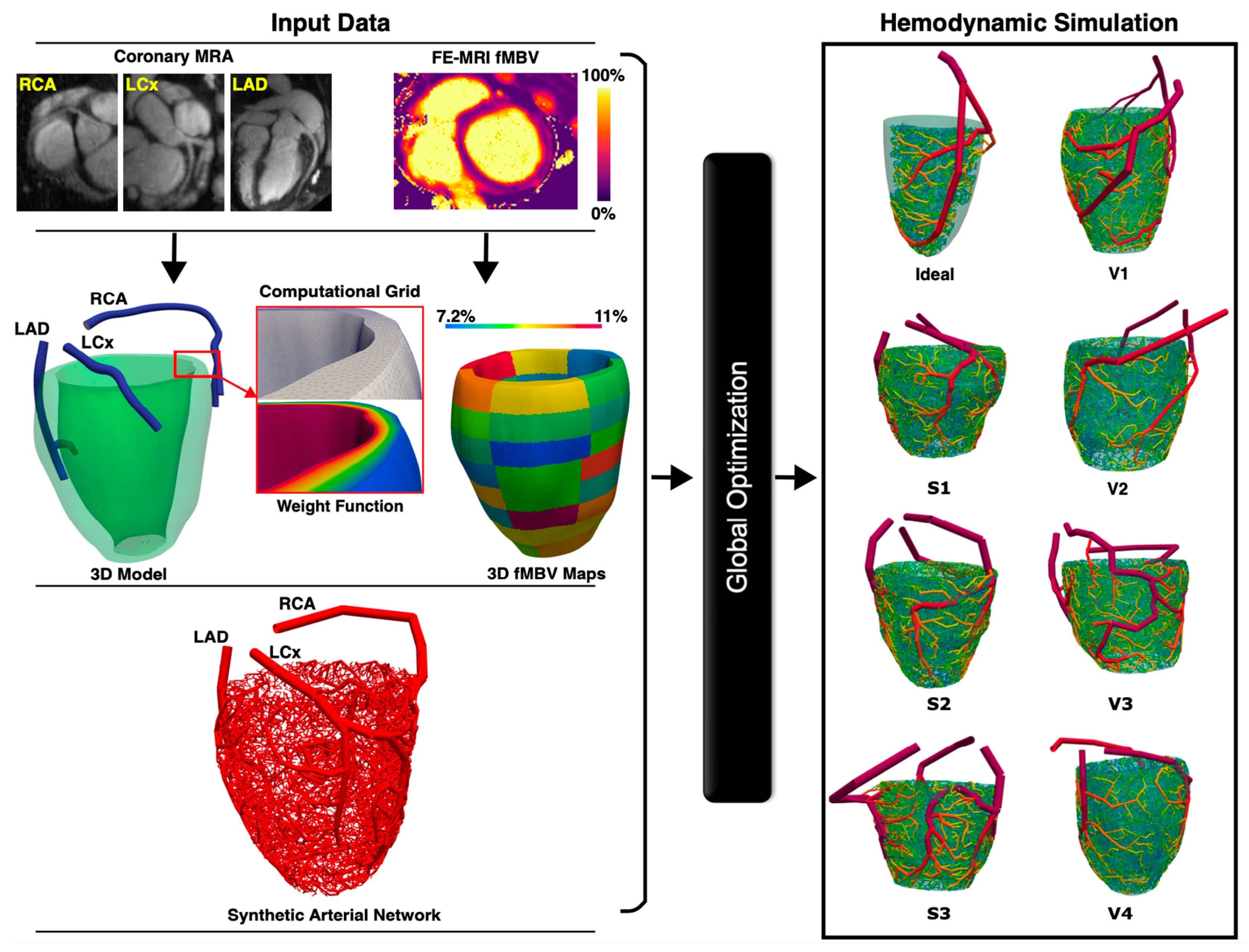
To realistically represent perfusing epicardial coronary arteries and microvasculature, we designed an adaptive, multistage CCO algorithm capable of generating an arterial network initialized from the epicardial coronary arteries and then extending into the myocardium without going beyond the endocardial surface. Inputs into the algorithm included the initial 1D epicardial coronary tree, myocardial perfusion territories represented as spatial myocardial point clouds, corresponding fMBV values, and weight functions. Utilizing the input data, the framework dynamically adjusts bifurcation placements and vessel orientations to reflect the branching gradients observed in physiological conditions. This adaptation allows the synthetic network to maintain a balance between minimizing the vascular volume and supplying blood to various myocardial segments while adhering to the structure of the myocardial geometry.
Arterial segments in our algorithm were modeled as cylindrical tubes, with Poiseuille’s law governing flow as incompressible Newtonian fluid under laminar, steady-state flow conditions. Murray’s law with a power coefficient of 3 determined the relationship between parent and daughter segment radii. fMBV-derived perfusion data were used to ensure that the terminal flow rates accurately reflected the myocardial blood supply, and the density of microvascular network follows the autoregulation mechanism captured by the fMBV maps.
Laplace equation-derived weights were used in the objective function to compute the network’s total weighted volume. The benefit of having a weight function in the computational domain is two-fold: (1) to assist the algorithm in creating a gradient in the number of branching segments from epicardium to endocardium, which more closely matches known physical properties of coronary vessels and (2) to ensure that the vascular network conforms to the patient-specific myocardium geometry. These parameters satisfy the underlying biological assumption that vascular tree growth is geometrically dictated by the organ it perfuses.
The staged growth mechanism involved five phases for each major coronary arterial tree (left anterior descending [LAD], left circumflex [LCX], and right coronary artery [RCA]): (1) Epicardial Completion—five terminal segments per myocardial segment were generated to extend the initial tree. (2) Epicardial Growth—ten terminal segments per myocardial segment were added to fill the gaps on the epicardial surface. (3) Endocardial Access—fifty terminal arteries were generated to enable deeper extension into the mid-myocardium and growth towards the endocardial surface. (4) Epicardial Surface Refinement—one hundred terminal segments were added to refine the epicardial network. (5) Terminal Segment Distribution—remaining terminal segments were designed to distribute fully, covering each myocardial perfusion territory based on fMBV maps.
The arterial segment lengths were calculated based on the number of terminal segments in each perfusion territory, myocardial segment volume, and the stage of arterial network generation. Terminal nodes were randomly placed in each myocardial segment and connected to 10 closest arterial segments, with bifurcation locations optimized to minimize the tree volume. The resulting bifurcation with the smallest arterial network volume was then selected. Bifurcations distribute closer to the epicardial surface to mirror the physical properties of coronary vascular perfusion. The optimization function adhered to several constraints: segment lengths were required to have twice the segment diameter and a dynamic maximum length; bifurcation angles were restricted to a pre-defined range; a dynamic bifurcation diameter symmetry coefficient was enforced; and new or modified segments could not intersect existing arterial segments or the endocardial surface. During the first stage of network generation, arterial segments were constrained to avoid overlapping. These constraints ensured physiologically sound, patient-specific network growth within the myocardium. See
Supplementary Material S2 for parameter specification.
A total of 2000 terminal arterial segments were used for each major coronary arterial tree (
n = 141 synthetic arterial trees from ideal myocardium
n = 6, swine subjects
n = 54, healthy volunteers
n = 72, and IHD patient
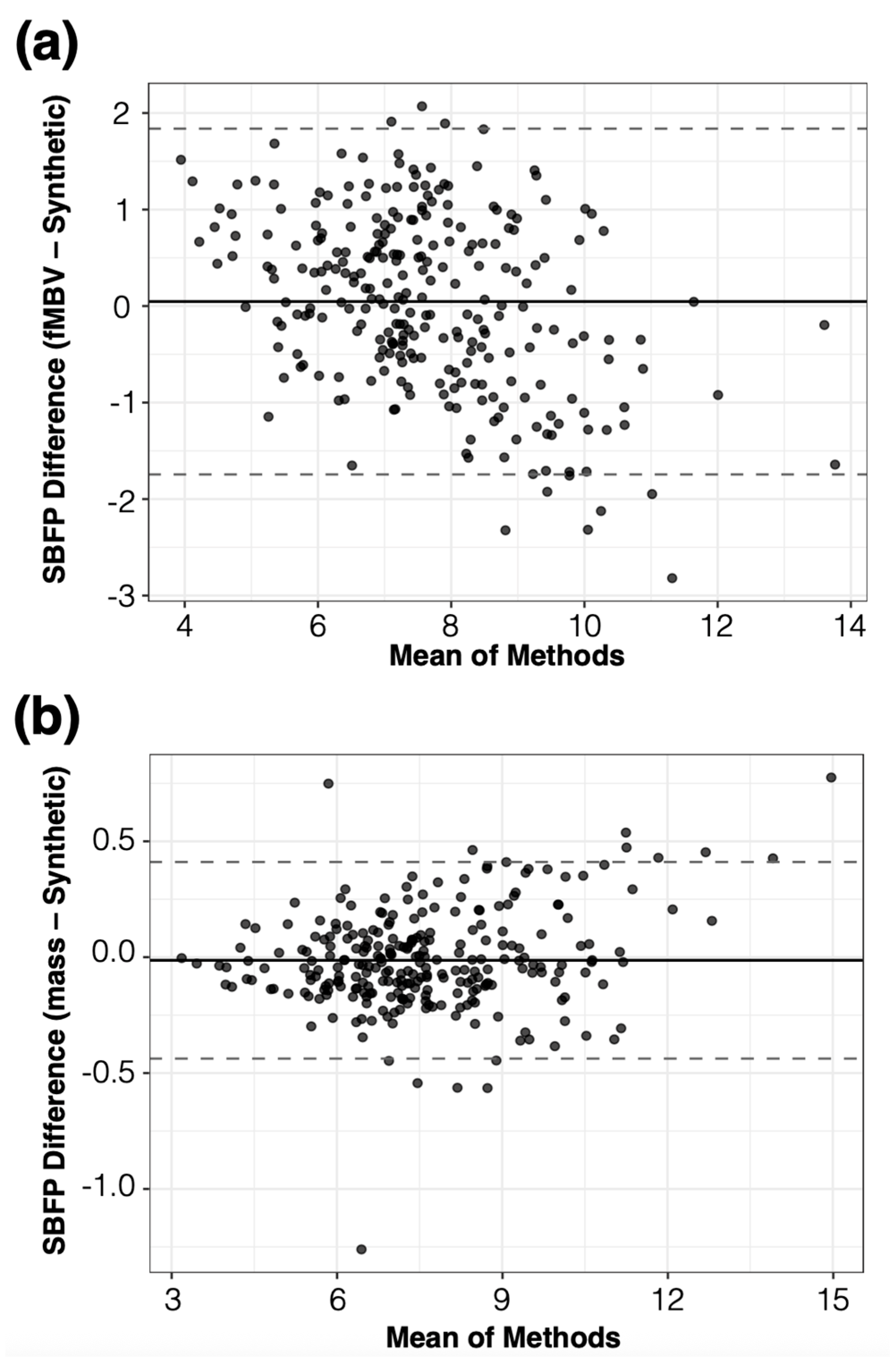
n = 15). The diameter of the most proximal arterial segment (root) of the synthetic arterial network was calculated from the MRA, whereas flow rates and resistances across the network were used to calculate the diameters of each arterial segment in the synthetic network. The FE-MRI fMBV maps were used to define segmental blood flow percentages (SBFPs). SBFP is computed as the average segmental fMBV multiplied by the myocardial volume of each segment, normalized within each epicardial artery. During tree construction, the sum of terminal flows supplying each sub-segment was enforced to equal its allocated share of the artery inflow, based on SBFPs, which served as patient-specific proportional constraints linking myocardial volume and flow distribution. Our automatic arterial network generation was designed to be consistent with principles of hemodynamic efficiency, whereby vascular networks rise to accommodate blood volume distribution governed by local tissue metabolic demands (
Figure 1).
Several factors were critical for the automatic arterial network generation based on FE-MRI fMBV maps. First, our modified adaptive CCO approach [
9] has a multistage component to ensure that larger arterial segments are on the epicardial surface while the microvascular network perfuses the myocardium. Second, the adaptive component enables simultaneous generation of shorter microvascular segments as the network grows, while updating the geometric and hemodynamic parameters based on the segmental fMBV maps (
Video S1).
2.5. Hemodynamic Simulation
To evaluate the functionality of the synthetic networks, 1D pulsatile blood flow was simulated for all subjects and seeds. Global optimization was applied prior to each simulation to tune deformable arterial segment diameters, ensuring the mean standard error between synthetic and FE-MRI fMBV-derived flow distributions remained below
. A general physiological flow rate waveform was prescribed at the root segment of each coronary arterial tree [
13,
14], and the Olufsen material model was considered for the arterial walls [
15]. Distal outlets were prescribed with a fixed perfusion pressure of 80 mmHg, consistent with CCO-based coronary morphometry studies [
9]. Intramyocardial pressure (IMP) was not explicitly modeled. At resting end-diastolic cardiac phase, the IMP ranges from approximately 5 mmHg in the epicardium to 20 mmHg in the endocardium [
16], which represents a relatively constant offset and does not alter the relative segmental flow distribution. A 20% perturbation in distal pressure was imposed to evaluate the sensitivity of SBFP (see
Section 2.10). Hemodynamic simulations used a time step of 0.001 s over 10 cardiac cycles to reach periodic steady state, with a convergence tolerance of 10
−6 for flow and pressure residuals. See
Supplementary Material S2 and S3 for details of numerical solver configuration and pipeline runtime.
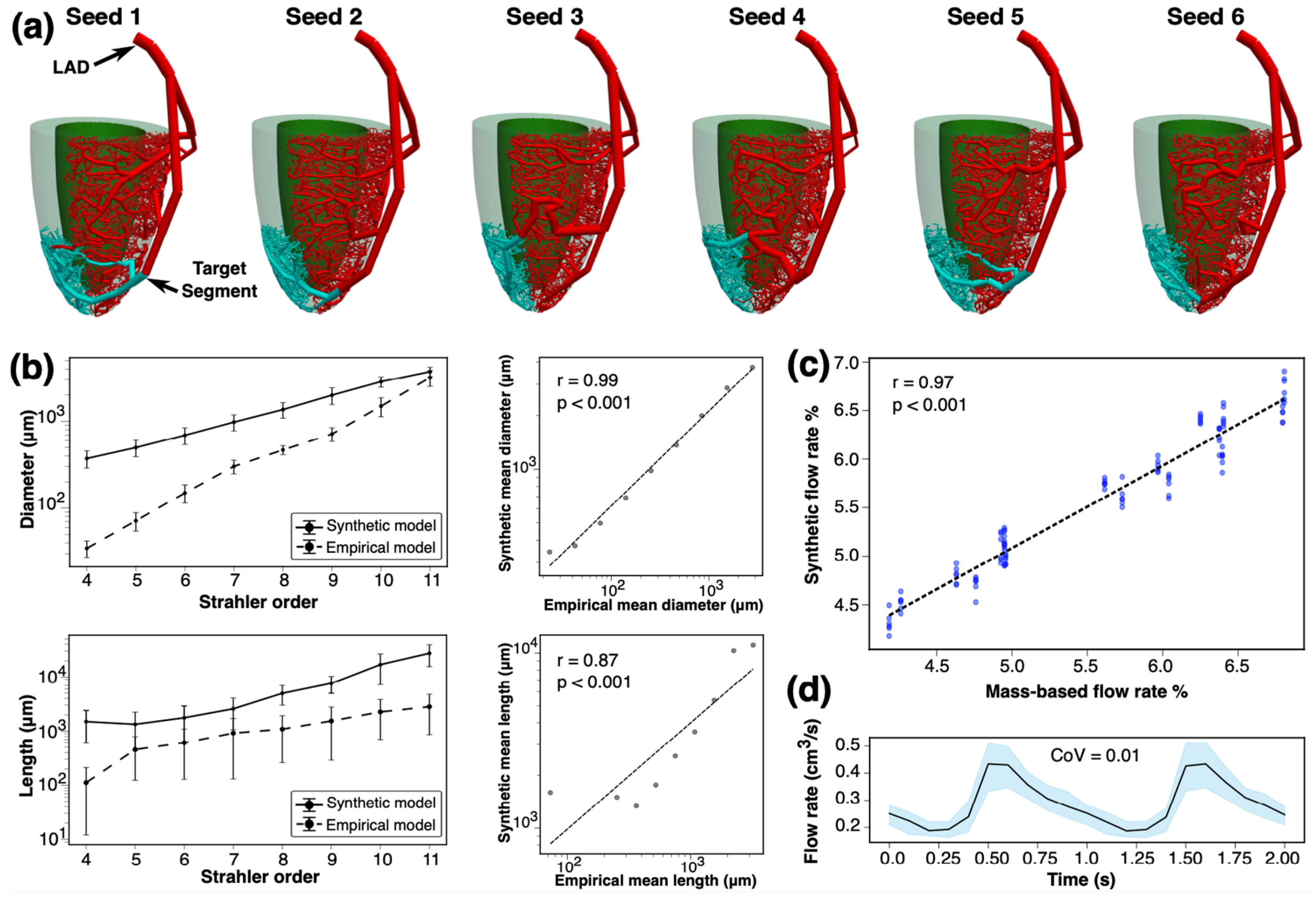
2.6. Modeling in Idealized Myocardium
For idealized myocardium, we generated six arterial networks using different initial seedings. The LAD artery territory was considered, and a patient-specific 3D model of the LAD was generated from coronary CTA images. The LAD perfusion territory was segmented using the AHA’s 16-myocardial segment model, and the SBFPs were calculated using an empirical model. The morphology of the constructed synthetic trees was compared against Kassab’s empirical model [
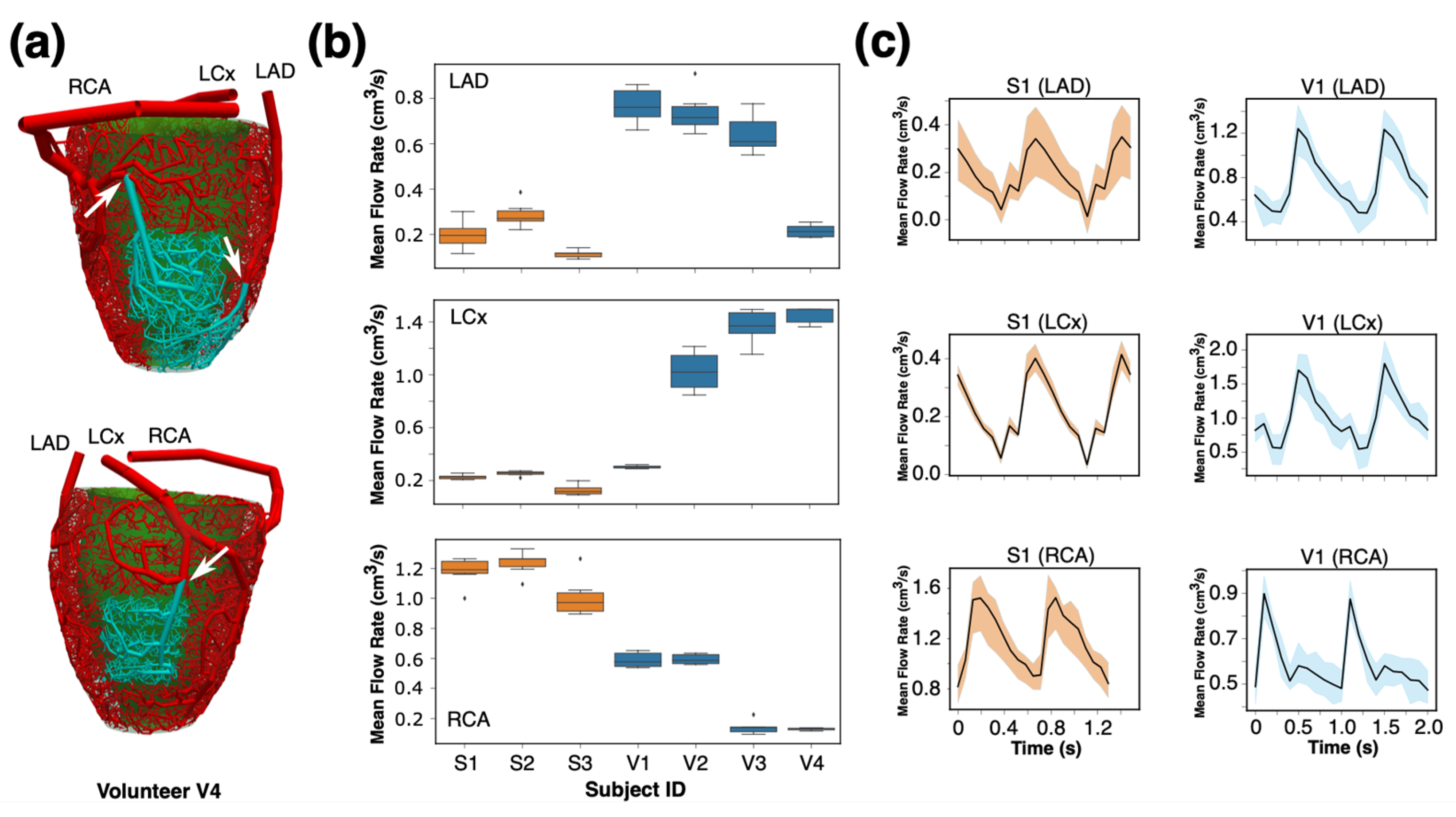
17], and the geometrical features of the networks were studied. We also solved 1D pulsatile blood flow simulations for each synthetic arterial network. The blood flow rate waveforms and flow resistances at three distal epicardial arterial segments were compared across different seedings. The resulting synthetic SBFPs were compared against those derived from the empirical model.
2.7. Subject-Specific Modeling
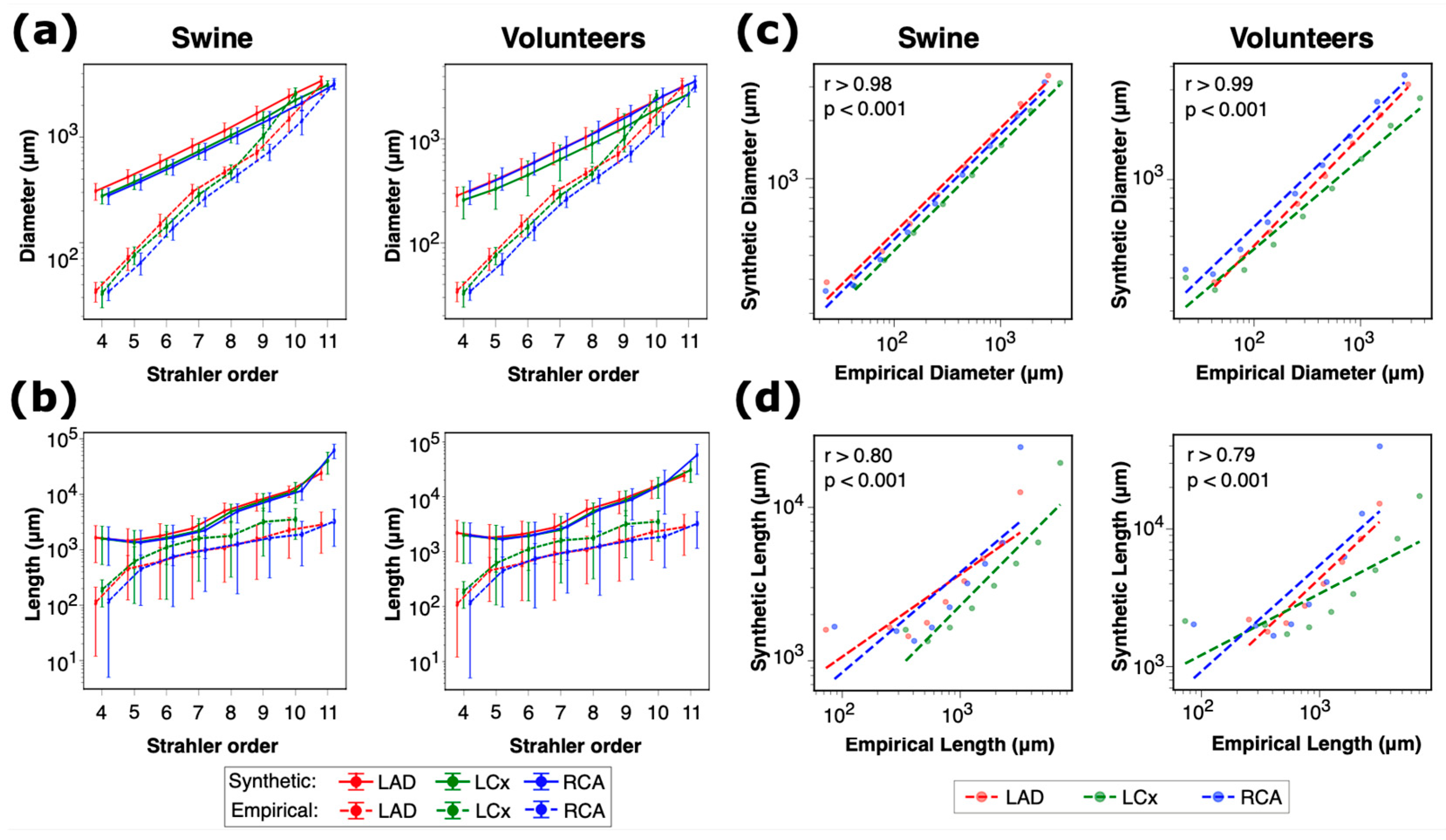
The 3D models of myocardium were extracted from whole-heart coronary MRA for swine and from multi-slice cine images for human subjects. Segmental fMBV values from MRI were mapped onto the 3D model of the left ventricle, and the volume of blood supplying each left ventricular segment was calculated based on the ratio of average fMBV values in different myocardial segments. Three-dimensional models of the LAD, LCx, and RCA were constructed in Simvascular v2021.06 [
18] and then converted to one-dimensional models using the software’s reduced order modeling feature. The morphology of the constructed healthy synthetic trees was compared against Kassab’s empirical model [
17], and the geometric features of the networks were studied. One-dimensional pulsatile blood flow simulations were performed for each synthetic arterial network. The blood flow rate waveforms and flow resistances at three distal epicardial arterial segments were compared across different seedings.
2.8. Arterial Network Generation and Subject-Specific Modeling in a Patient with Ischemic Heart Disease
To investigate the performance of our proposed framework in several myocardial tissue types, we selected a 74-year-old patient with prior myocardial infarction status post coronary stenting of the LAD vessel with progressive complaints of chest pain on exertion. The patient had undergone stress cardiac positron emission tomography (PET) with Rubidium-82 tracer, late gadolinium enhancement (LGE) MRI, and FE-MRI. Five microvascular networks with 2000 terminal segments using 5 random seedings were constructed for each main epicardial coronary artery based on the patient-specific FE-MRI fMBV maps. The average diameter of the arterial network at Strahler order numbers 1, 2, and 3 as well as the vascular tissue flow delivery (= % terminal flow/tissue volume) from the synthetic microvascular networks were extracted and compared between different myocardial tissue types (ischemia, scar, and remote). Ischemic tissue was defined as areas with reversible myocardial perfusion defect on PET. Scar tissue was defined as areas with LGE on gadolinium-based MRI. Remote myocardial tissue was defined as regions with no perfusion defect on PET and without LGE on MRI.
2.9. Statistical Analysis
The interquartile range (IQR) for the density of vessels per vessel diameter size was calculated as
. A wider range of four times the IQR was used to show the consistency of resultant terminal diameter density across various seeds. Coefficient of variation (CoV) was used to assess the relative variability of structural and geometrical parameters in the synthetic arterial networks. This metric was of importance in quantifying the consistency of our synthetic arterial trees across various seeds and subjects. Pearson and Spearman correlation coefficients were used to compare the arterial segment diameters and lengths in our synthetic networks with Kassab’s empirical model [
17]. Mixed-effects models were employed to analyze the variability in arterial diameters, flow rates, and flow resistance across various seeds, main coronary arteries, and subjects. These models account for both fixed effects (seeds) and random effects (coronary arteries, myocardial segments, and subjects) and provide an analysis of the factors affecting the observed variability. Dynamic Time Warping (DTW) [
19] was employed to measure the dissimilarity between flow rate waveforms from different seeds for each subject and artery. This technique aligns flow rate waveforms to minimize the distance between them, allowing for an accurate comparison of their shapes. Coupled with mixed-effect model, DTW was used to quantify the effect of subject, artery, and initial seeds on the variability in flow rate waveforms. This analysis was important for determining the repeatability of the hemodynamic results of our synthetic framework. See
Supplementary Material S5 for complete results of statistical analyses.
2.10. Uncertainty Quantification
To test the robustness of the SBFP-based flow allocation across myocardial segments, we performed steady-state 1D simulations in the LAD network of subject V3 (Seed 1) while varying four physiological parameters within ±20% of their baseline values: (i) blood viscosity, (ii) wall stiffness in the Olufsen model, (iii) distal pressure offset, and (iv) inlet flow rate. The resulting segmental SBFP distributions were compared with baseline using the root-mean-square deviation and coefficient of variation. See
Supplementary Material S2 for physiological values.
4. Discussion
This proof-of-concept study explores the feasibility of constructing subject-specific synthetic coronary arterial networks from ferumoxytol-enhanced coronary MRA and MRI fMBV maps. By coupling an adaptive, multistage CCO algorithm with fMBV maps, the framework aims to represent the microvascular anatomy and autoregulatory behavior using a physiologically constrained, image-based pipeline. Our findings in healthy swine, human volunteers, and a patient with ischemic heart disease support the potential of the proposed framework to non-invasively compute key coronary hemodynamic measures.
Despite an exponential increase in the cost burden [
24], the management of chronic IHD focuses on obstructive epicardial coronary arteries as the primary cause of angina [
25]. However, three out of four patients with angina undergoing invasive angiography do not show signs of epicardial coronary obstruction [
26]. Instead, coronary microvascular dysfunction (CMD) is the main contributor. In women, CMD results in a five-fold increase in major adverse cardiovascular events [
25,
27]. Current strategies to definitively diagnose symptomatic CMD rely on an invasive coronary evaluation, which includes (1) the exclusion of significant anatomic obstructive epicardial coronary disease, (2) provocative vasoreactivity testing, and (3) quantification of the index of microcirculatory resistance (IMR) [
25].
The findings from this work support the use of patient-specific MRI data to non-invasively evaluate microvascular health through the synthetic arterial network generation and non-invasive computation of coronary hemodynamics. The distinguishing feature of our approach relies on the incorporation of FE-MRI fMBV maps into microvascular network construction. When used in combination with a two-compartment water exchange model, the fMBV maps account for the underlying autoregulatory mechanisms and provide accurate representations of the ferumoxytol distribution as a surrogate for the intravascular blood volume—unlike gadolinium-based contrast agents in MRI or iodine-based CT contrast agents that immediately leak into the extravascular space. At a concentration of 4 mg/kg or less, the relationship between the ferumoxytol concentration and MRI signal is also linear, which differs from many nuclear tracers that have nonlinear relationships. These properties provide a physiological surrogate for the intravascular blood volume and are used to encode aspects of autoregulation in the microvascular network construction.
Recent computational efforts have used a Darcy model to approximate the microcirculation while integrating CT-MPI with truncated synthetic coronary tree generation models to reproduce the myocardial blood flow estimates [
8,
28]. Although porous media models are effective in specific applications, they oversimplify the anatomic branching and flow directionality. On the other hand, CT-MPI has limitations related to the leakage of the contrast and the misalignment between acquisitions used to capture the coronary tree vs. perfusion information. Our synthetic framework directly generates the microvascular network and extends the epicardial coronaries based on the distribution of FE-MRI fMBV maps. Unlike the majority of CCO-based methods used to generate arterial trees [
7,
29,
30], the arterial networks generated by our proposed algorithm are not binary, and the synthetically generated arterial segments represented the extension of previous terminal segments. The terminal segment flow rate across the AHA’s 16 myocardial segments is also different and represents fMBV map values. Additionally, this framework is capable of scaling the network resolution to 6000 terminal segments in the myocardium, embedding the autoregulatory aspects into the structure of the distal vasculature. Hence, it provides a more reliable surrogate for the complete arterial network to synthetically estimate the hemodynamic environment of a patient-specific LV using flow waveforms and resistances. Compared to empirical swine models, our preliminary results showed high correlation coefficients while maintaining minimal variability across various random seedings in healthy subjects. In a subject with mixed myocardial perfusion defects, our framework reconstructed heterogeneous microvascular networks with an increased number of terminal vessels in the myocardial segment with reversible perfusion defects while distributing a less dense microvascular tree in segments with fixed perfusion defects. These results indicate that the proposed adaptive CCO approach may address the oversimplification of Darcy-based models of the microcirculation while observing anatomic accuracy and blood flow distributions, which are needed for modeling the key coronary hemodynamic measures such as the fractional flow reserve (FFR), IMR, and coronary flow reserve (CFR).
Recent machine learning surrogates have demonstrated a remarkable capability to estimate coronary hemodynamics directly from anatomical data, including geometry-based predictions of the FFR and wall shear stress distributions in near real time [
31,
32,
33]. These models achieve a rapid inference suitable for high-throughput triages but are typically limited to the epicardial coronary level and do not explicitly encode tissue-specific microvascular density, autoregulatory mechanisms, or transmural loading conditions that govern the myocardial perfusion reserve. In contrast, the proposed FE-MRI fMBV-based, physiology-constrained framework integrates quantitative microvascular information derived from ferumoxytol-enhanced MRI into a mechanistic arterial network model, enabling the simulation of flow distributions and resistances. While machine learning surrogates emphasize speed and data scalability, the present framework prioritizes physiological interpretability and the ability to compute functional indices (e.g., CFR, IMR, and FFR) within a patient-specific arterial network. Together, these approaches are complementary, such that machine learning algorithms can accelerate initial geometric screening and provide boundary conditions, whereas the proposed framework captures the multiscale hemodynamic environment underlying microvascular dysfunction. The combination of these methods, leveraging data-driven surrogates for efficiency and physiology-based modeling for interpretability, represents a promising direction for translational coronary hemodynamics. Future hybrid approaches that combine physiology-based vascular generation with learned surrogates for flow or boundary condition estimation may enable physiologically accurate, real-time predictions of complete coronary arterial networks, bridging the current gap between data-driven and mechanistic methodologies.
Our findings in the patient with IHD demonstrate the potential of the proposed framework to reflect the autoregulation mechanism in the microvascular bed captured through the use of fMBV maps. In the IHD subject with mixed ischemia and scar tissues, the microvascular networks generated across five random seedings showed a tissue-dependent geometry and vascular-tissue flow delivery (
, consistent with coronary autoregulation [
34,
35]. In tissues with ischemia, the average terminal arterial segments were larger than the terminal tree in the remote tissue. This behavior is expected under reduced perfusion pressure, where the microvascular dilation lowers the distal resistance and compensates for the segmental flow at rest, increasing the ability of the microvasculature to deliver blood to the tissue [
35]. On the other hand, smaller terminal diameters were observed in the tissue with scar (fixed perfusion defect and late gadolinium enhancement) and hence were related to the lower terminal flow delivery capability. This behavior is consistent with the microvascular rarefaction and increased extravascular resistance in fibrotic tissue [
36,
37].
4.1. Limitations
Our study has several limitations. This study was conducted in a small cohort, and the findings should be interpreted as preliminary. However, we show the potential of the framework in idealized myocardium, healthy swine, human subjects, and one patient with mixed myocardial perfusion defects. Arterial segments were assumed to be cylindrical without explicit tapering to reduce the computational cost. Although tapering is implicitly represented via Murray’s law at bifurcations, continuous axial tapering was not imposed. Incorporating smooth taper functions will further improve the morphological realism. The synthetic arterial network morphometry was validated against empirical swine models, which may not fully capture the complexity of the swine coronary circulation, specifically in diseased myocardium. Population-averaged coronary waveforms and the constant outlet pressure were used as inflow and outflow boundary conditions, respectively. While this approach is common in coronary modeling, it neglects subject-specific variations in the heart rate and distal impedance. A detailed microvascular rheology, including the Fåhræus–Lindqvist viscosity model and red cell phase separation at bifurcations, was not modeled. These effects primarily influence vessels < 100 μm [
38] and are secondary to the macro-scale flow constraints considered in this study. Sensitivity analyses with varying viscosities and wall stiffnesses across physiological ranges produced negligible changes in the SBFP, suggesting a limited impact on the hemodynamic environment. The present analysis focuses on steady-state perfusion at rest and does not model time-dependent myocardial deformation or cyclic intramyocardial pressure. This simplification is justified because the coronary flow occurs predominantly during diastole, when the myocardial contraction is minimal. The ferumoxytol-enhanced MRI data used as input were acquired under steady-state, end-diastolic conditions and therefore already reflect the net effects of the wall stress on the microvascular filling. While the framework showed low variability across various seeds and subjects, the influence of epicardial coronary artery disease as well as microcirculatory diseases (such as microvascular spasm) on our synthetic arterial networks was not investigated. The computational cost of the microvascular network construction and hemodynamic simulation in large networks needs to be reduced. The end-to-end pipeline requires approximately two days per subject on a single CPU core (see
Supplementary Material S3), with arterial network generation as the principal bottleneck. The parallelization and optimization of the framework performance and improving its efficiency will be necessary for clinical applications. The fMBV values were quantified from six to eight left ventricular short axis images with a slice thickness of 8 mm and an interpolated spatial resolution of 0.93 × 0.93 mm
2, which may limit the volumetric precision.
Despite the aforementioned limitations, the proposed framework generated a consistent network morphometry and hemodynamic environment across healthy and IHD subjects and initial seedings, while conforming to patient-specific left ventricular anatomy and flow boundary conditions. These findings demonstrate potential for using the fMBV to generate synthetic microvascular networks, which could provide a powerful non-invasive tool for estimating key hemodynamic indices.
4.2. Future Work
Future studies could extend the current validation to patients with ischemic and microvascular disease. In these cohorts, PET- or CT-derived perfusion indices and invasive measurements such as the FFR and IMR will provide quantitative benchmarks. This will test how disease-related alterations in vessel density and epicardial coronary artery disease affect the morphology and flow distribution. While the present study uses steady-state fMBV maps to derive SBFPs, future work could integrate conventional flow-based indices from dynamic perfusion imaging modalities to directly compare perfusion-derived and fMBV-derived metrics within the same framework, thereby bridging intravascular volume and flow-based physiological assessments. Additionally, incorporating non-Newtonian viscosity models will enable a more realistic simulation of the microvascular rheology. These additions will improve accuracy for vessels under 100 µm in diameter. The long-term goal is to achieve a fully automated, physiology-aware framework capable of the same-day computation of perfusion and functional indices from standard perfusion imaging modalities. Integration with machine learning surrogates may accelerate the initial network generation, while the mechanistic model will retain physiological interpretability and support personalized risk assessments.