Diagnostic Support in Dentistry Through Artificial Intelligence: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Question
2.2. Protocol and Registration
2.3. Search Processing
2.4. Inclusion and Exclusion Criteria
2.5. Data Processing
3. Results
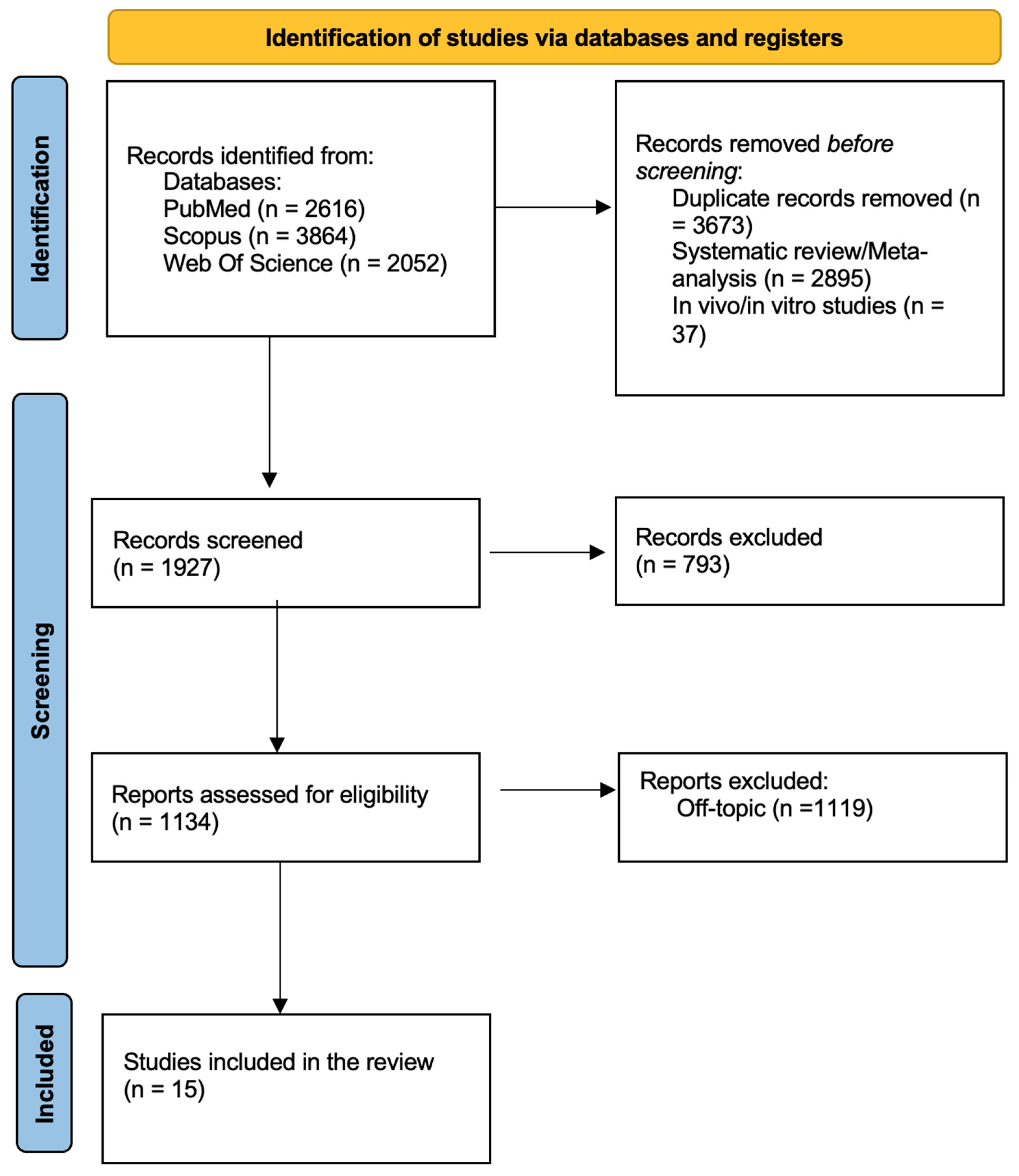
3.1. Study Selection and Characteristics
3.2. Quality Assessment and Risk of Bias of Included Articles
4. Discussion
4.1. Radiographic and Imaging Diagnostics
4.2. Orthodontics and Skeletal Malocclusion Assessment
4.3. Periodontology and Implantology
4.4. Geriatric and Preventive Dentistry
4.5. Orofacial Pain and Temporomandibular Disorders
4.6. Sleep Medicine in Dentistry
4.7. Limitations and Future Directions
4.8. Ethical, Medico-Legal, and Cognitive Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFC | Automated Face Coding |
| AI | Artificial Intelligence |
| CBCT | Cone Beam Computed Tomography |
| CNN | Convolutional Neural Network |
| IAN | Inferior Alveolar Nerve |
| M3M | Mandibular Third Molar |
| MESH | Medical Subject Headings |
| MJA | Mandibular Jaw Movement Analysis |
| ML | Machine Learning |
| OSA | Obstructive Sleep Apnea |
| PCA | Principal Component Analysis |
| RF | Random Forest |
| SVM | Support Vector Machine |
| TMD | Temporomandibular Disorders |
| VAS | Visual Analogue Scale |
References
- Nguyen, T.T.; Larrivée, N.; Lee, A.; Bilaniuk, O.; Durand, R. Use of Artificial Intelligence in Dentistry: Current Clinical Trends and Research Advances. J. Can. Dent. Assoc. 2021, 87, l7. [Google Scholar] [CrossRef]
- Arora, A.; Alderman, J.E.; Palmer, J.; Ganapathi, S.; Laws, E.; McCradden, M.D.; Oakden-Rayner, L.; Pfohl, S.R.; Ghassemi, M.; McKay, F.; et al. The Value of Standards for Health Datasets in Artificial Intelligence-Based Applications. Nat. Med. 2023, 29, 2929–2938. [Google Scholar] [CrossRef]
- Findik, Y.; Yildirim, D.; Baykul, T. Three-Dimensional Anatomic Analysis of the Lingula and Mandibular Foramen: A Cone Beam Computed Tomography Study. J. Craniofac. Surg. 2014, 25, 607–610. [Google Scholar] [CrossRef]
- You, K.-H.; Lee, K.-J.; Lee, S.-H.; Baik, H.-S. Three-Dimensional Computed Tomography Analysis of Mandibular Morphology in Patients with Facial Asymmetry and Mandibular Prognathism. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 540.e1-e8; discussion 540–541. [Google Scholar] [CrossRef] [PubMed]
- Tuzoff, D.V.; Tuzova, L.N.; Bornstein, M.M.; Krasnov, A.S.; Kharchenko, M.A.; Nikolenko, S.I.; Sveshnikov, M.M.; Bednenko, G.B. Tooth Detection and Numbering in Panoramic Radiographs Using Convolutional Neural Networks. Dentomaxillofac. Radiol. 2019, 48, 20180051. [Google Scholar] [CrossRef]
- Zohud, O.; Lone, I.M.; Midlej, K.; Obaida, A.; Masarwa, S.; Schröder, A.; Küchler, E.C.; Nashef, A.; Kassem, F.; Reiser, V.; et al. Towards Genetic Dissection of Skeletal Class III Malocclusion: A Review of Genetic Variations Underlying the Phenotype in Humans and Future Directions. J. Clin. Med. 2023, 12, 3212. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Paduanelli, G.; De Oliveira, L.A.; Inchingolo, A.M.; Georgakopoulos, P.I.; Inchingolo, A.D.; Malcangi, G.; Athanasiou, E.; Fotopoulou, E.; et al. Computer-Based Quantification of an Atraumatic Sinus Augmentation Technique Using CBCT. J. Biol. Regul. Homeost. Agents 2019, 33, 31–39. [Google Scholar] [PubMed]
- Silverman, F.N. Roentgen Standards Fo-Size of the Pituitary Fossa from Infancy through Adolescence. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1957, 78, 451–460. [Google Scholar] [PubMed]
- Aro, K.; Wei, F.; Wong, D.T.; Tu, M. Saliva Liquid Biopsy for Point-of-Care Applications. Front. Public. Health 2017, 5, 77. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Dipalma, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Carpentiere, V.; Garofoli, G.; Azzollini, D.; Campanelli, M.; Paduanelli, G.; Palermo, A.; et al. Artificial Intelligence and Its Clinical Applications in Orthodontics: A Systematic Review. Diagnostics 2023, 13, 3677. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Ceratti, C.; Maspero, C.; Consonni, D.; Caprioglio, A.; Connelly, S.T.; Inchingolo, F.; Tartaglia, G.M. Cone-Beam Computed Tomographic Assessment of the Mandibular Condylar Volume in Different Skeletal Patterns: A Retrospective Study in Adult Patients. Bioengineering 2022, 9, 102. [Google Scholar] [CrossRef]
- Maspero, C.; Abate, A.; Inchingolo, F.; Dolci, C.; Cagetti, M.G.; Tartaglia, G.M. Incidental Finding in Pre-Orthodontic Treatment Radiographs of an Aural Foreign Body: A Case Report. Children 2022, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Guglielmo, M.; Sardano, R.; Palmieri, G.; Di Pede, C.; de Ruvo, E.; Inchingolo, A.D.; Mancini, A.; Inchingolo, F.; et al. Precision Medicine in Oral Health and Diseases: A Systematic Review. J. Pers. Med. 2023, 13, 725. [Google Scholar] [CrossRef]
- Scarano, A.; Noumbissi, S.; Gupta, S.; Inchingolo, F.; Stilla, P.; Lorusso, F. Scanning Electron Microscopy Analysis and Energy Dispersion X-Ray Microanalysis to Evaluate the Effects of Decontamination Chemicals and Heat Sterilization on Implant Surgical Drills: Zirconia vs. Steel. Appl. Sci. 2019, 9, 2837. [Google Scholar] [CrossRef]
- Segù, M.; Campagnoli, G.; Di Blasio, M.; Santagostini, A.; Pollis, M.; Levrini, L. Pilot Study of a New Mandibular Advancement Device. Dent. J. 2022, 10, 99. [Google Scholar] [CrossRef]
- Maret, D.; Telmon, N.; Treil, J.; Caron, P.; Nabet, C. Pituitary Adenoma as an Incidental Finding in Dental Radiology: A Case Report. Ann. Intern. Med. 2014, 160, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.R.; Spin-Neto, R.; Stavropoulos, A.; Schropp, L.; da Silveira, H.E.D.; Wenzel, A. Planning of Dental Implant Size with Digital Panoramic Radiographs, CBCT-Generated Panoramic Images, and CBCT Cross-Sectional Images. Clin. Oral. Implants Res. 2014, 25, 690–695. [Google Scholar] [CrossRef]
- Dimonte, M.; Inchingolo, F.; Minonne, A.; Arditi, G.; Dipalma, G. Bone SPECT in Management of Mandibular Condyle Hyperplasia. Report of a Case and Review of Literature. Minerva Stomatol. 2004, 53, 281–285. [Google Scholar] [PubMed]
- Inchingolo, F.; Pacifici, A.; Gargari, M.; Acitores Garcia, J.I.; Amantea, M.; Marrelli, M.; Dipalma, G.; Inchingolo, A.M.; Rinaldi, R.; Inchingolo, A.D.; et al. CHARGE Syndrome: An Overview on Dental and Maxillofacial Features. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2089–2093. [Google Scholar]
- Balzanelli, M.; Distratis, P.; Catucci, O.; Amatulli, F.; Cefalo, A.; Lazzaro, R.; Aityan, K.S.; Dalagni, G.; Nico, A.; De Michele, A.; et al. Clinical and Diagnostic Findings in COVID-19 Patients: An Original Research from SG Moscati Hospital in Taranto Italy. J. Biol. Regul. Homeost. Agents 2021, 35, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Talaat, W.M.; Adel, O.I.; Al Bayatti, S. Prevalence of Temporomandibular Disorders Discovered Incidentally during Routine Dental Examination Using the Research Diagnostic Criteria for Temporomandibular Disorders. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 125, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis. Clin. Oral. Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, L.-X.; Meng, H.-Y.; Ren, Y.-H.; Lai, Y.-K.; Kobbelt, L. PRS-Net: Planar Reflective Symmetry Detection Net for 3D Models. IEEE Trans. Vis. Comput. Graph. 2021, 27, 3007–3018. [Google Scholar] [CrossRef]
- Kurusu, A.; Horiuchi, M.; Soma, K. Relationship between Occlusal Force and Mandibular Condyle Morphology. Evaluated by Limited Cone-Beam Computed Tomography. Angle Orthod. 2009, 79, 1063–1069. [Google Scholar] [CrossRef]
- Bashir, N.Z.; Rahman, Z.; Chen, S.L.-S. Systematic Comparison of Machine Learning Algorithms to Develop and Validate Predictive Models for Periodontitis. J. Clin. Periodontol. 2022, 49, 958–969. [Google Scholar] [CrossRef]
- Palmer, J.; Durham, J. Temporomandibular Disorders. BJA Educ. 2021, 21, 44–50. [Google Scholar] [CrossRef]
- Jacobson, A. The “Wits” Appraisal of Jaw Disharmony. Am. J. Orthod. 1975, 67, 125–138. [Google Scholar] [CrossRef]
- Sagl, B.; Schmid-Schwap, M.; Piehslinger, E.; Rausch-Fan, X.; Stavness, I. The Effect of Tooth Cusp Morphology and Grinding Direction on TMJ Loading during Bruxism. Front. Physiol. 2022, 13, 964930. [Google Scholar] [CrossRef]
- La Touche, R.; Fernández-de-las-Peñas, C.; Fernández-Carnero, J.; Escalante, K.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cleland, J.A. The Effects of Manual Therapy and Exercise Directed at the Cervical Spine on Pain and Pressure Pain Sensitivity in Patients with Myofascial Temporomandibular Disorders. J. Oral. Rehabil. 2009, 36, 644–652. [Google Scholar] [CrossRef]
- Nelson, D.E.; Holtzman, D.; Bolen, J.; Stanwyck, C.A.; Mack, K.A. Reliability and Validity of Measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soz. Praventivmed. 2001, 46 (Suppl. S1), S3–S42. [Google Scholar]
- Martinot, J.-B.; Le-Dong, N.-N.; Malhotra, A.; Pépin, J.-L. Respiratory Effort during Sleep and Prevalent Hypertension in Obstructive Sleep Apnoea. Eur. Respir. J. 2023, 61, 2201486. [Google Scholar] [CrossRef] [PubMed]
- Gaêta-Araujo, H.; Oliveira-Santos, N.; Mancini, A.X.M.; Oliveira, M.L.; Oliveira-Santos, C. Retrospective Assessment of Dental Implant-Related Perforations of Relevant Anatomical Structures and Inadequate Spacing between Implants/Teeth Using Cone-Beam Computed Tomography. Clin. Oral. Investig. 2020, 24, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, S.; Collaert, B.; Cosyn, J.; Deschepper, E.; De Bruyn, H. A Multifactorial Analysis to Identify Predictors of Implant Failure and Peri-Implant Bone Loss. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S1), e298–e307. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A. Peri-Implant Diseases: Diagnosis and Risk Indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio Junior, E.; Romito, G.A.; Shibli, J.A. Peri-Implantitis as a “Burden” Disease. Braz. Oral. Res. 2019, 33, e087. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S68–S77. [Google Scholar] [CrossRef]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal Treatment of Multirooted Teeth. Results after 5 Years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef]
- Chung, J.; Lobbezoo, F.; van Selms, M.K.A.; Chattrattrai, T.; Aarab, G.; Mitrirattanakul, S. Physical, Psychological and Socio-Demographic Predictors Related to Patients’ Self-Belief of Their Temporomandibular Disorders’ Aetiology. J. Oral. Rehabil. 2021, 48, 109–123. [Google Scholar] [CrossRef]
- Stillhart, A.; Häfliger, R.; Takeshita, L.; Stadlinger, B.; Leles, C.R.; Srinivasan, M. Screening for Dental Pain Using an Automated Face Coding (AFC) Software. J. Dent. 2025, 155, 105647. [Google Scholar] [CrossRef]
- Estrella, N.-F.; Alexandra, D.-S.; Yun, C.; Palma-Fernández, J.C.; Alejandro, I.-L. AI-aided volumetric root resorption assessment following personalized forces in orthodontics: Preliminary results of a randomized clinical trial. J. Evid. Based Dent. Pract. 2025, 25, 102095. [Google Scholar] [CrossRef] [PubMed]
- Uribe, S.E.; Issa, J.; Sohrabniya, F.; Denny, A.; Kim, N.N.; Dayo, A.F.; Chaurasia, A.; Sofi-Mahmudi, A.; Büttner, M.; Schwendicke, F. Publicly Available Dental Image Datasets for Artificial Intelligence. J. Dent. Res. 2024, 103, 1365–1374. [Google Scholar] [CrossRef]
- Schwab, J.; Stucki, L.; Fitzek, S.; Tithphit, A.; Hönigl, A.; Stackmann, S.; Horn, I.; Thenner, H.; Dasser, P.; Woitek, R.; et al. Radiological Assessment of Sella Turcica Morphology Correlates with Skeletal Classes in an Austrian Population: An Observational Study. Oral. Radiol. 2025, 41, 169–179. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Christelle, M.; Sun, M.; Wen, Z.; Lin, Y.; Zhang, H.; Xu, J. Automated Localization of Mandibular Landmarks in the Construction of Mandibular Median Sagittal Plane. Eur. J. Med. Res. 2024, 29, 84. [Google Scholar] [CrossRef]
- Al-Sarem, M.; Al-Asali, M.; Alqutaibi, A.Y.; Saeed, F. Enhanced Tooth Region Detection Using Pretrained Deep Learning Models. Int. J. Environ. Res. Public. Health 2022, 19, 15414. [Google Scholar] [CrossRef]
- Deng, K.; Zonta, F.; Yang, H.; Pelekos, G.; Tonetti, M.S. Development of a Machine Learning Multiclass Screening Tool for Periodontal Health Status Based on Non-Clinical Parameters and Salivary Biomarkers. J. Clin. Periodontol. 2024, 51, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Heidrich, V.; Bazzani, D.; Asnicar, F.; Armanini, F.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; Vicentini, D.; et al. Shotgun Metagenomics Identifies in a Cross-Sectional Setting Improved Plaque Microbiome Biomarkers for Peri-Implant Diseases. J. Clin. Periodontol. 2025, 52, 999–1010. [Google Scholar] [CrossRef]
- Muramatsu, M.; Muramatsu, M.; Takahashi, N.; Hagiwara, A.; Hagiwara, J.; Takamatsu, Y.; Morooka, R.; Ochi, M.; Kaitani, T. Image Diagnosis Models for the Oral Assessment of Older People Using Convolutional Neural Networks: A Retrospective Observational Study. J. Clin. Nurs. 2022, 31, 3550–3559. [Google Scholar] [CrossRef]
- Yıldız, N.T.; Kocaman, H.; Yıldırım, H.; Canlı, M. An Investigation of Machine Learning Algorithms for Prediction of Temporomandibular Disorders by Using Clinical Parameters. Medicine 2024, 103, e39912. [Google Scholar] [CrossRef] [PubMed]
- Pul, U.; Tichy, A.; Pitchika, V.; Schwendicke, F. Impact of Artificial Intelligence Assistance on Diagnosing Periapical Radiolucencies: A Randomized Controlled Trial. J. Dent. 2025, 160, 105868. [Google Scholar] [CrossRef] [PubMed]
- Picoli, F.F.; Fontenele, R.C.; Van der Cruyssen, F.; Ahmadzai, I.; Trigeminal Nerve Injuries Research Group; Politis, C.; Silva, M.A.G.; Jacobs, R. Risk Assessment of Inferior Alveolar Nerve Injury after Wisdom Tooth Removal Using 3D AI-Driven Models: A within-Patient Study. J. Dent. 2023, 139, 104765. [Google Scholar] [CrossRef]
- Midlej, K.; Watted, N.; Awadi, O.; Masarwa, S.; Lone, I.M.; Zohud, O.; Paddenberg, E.; Krohn, S.; Kuchler, E.; Proff, P.; et al. Lateral Cephalometric Parameters among Arab Skeletal Classes II and III Patients and Applying Machine Learning Models. Clin. Oral. Investig. 2024, 28, 511. [Google Scholar] [CrossRef]
- Pépin, J.-L.; Cistulli, P.A.; Crespeigne, E.; Tamisier, R.; Bailly, S.; Bruwier, A.; Le-Dong, N.-N.; Lavigne, G.; Malhotra, A.; Martinot, J.-B. Mandibular Jaw Movement Automated Analysis for Oral Appliance Monitoring in Obstructive Sleep Apnea: A Prospective Cohort Study. Ann. Am. Thorac. Soc. 2024, 21, 814–822. [Google Scholar] [CrossRef]
- Sui, H.; Xiao, M.; Jiang, X.; Li, J.; Qiao, F.; Yin, B.; Wang, Y.; Wu, L. Development and Validation of a Predictive Nomogram for Bilateral Posterior Condylar Displacement Using Cone-Beam Computed Tomography and Machine-Learning Algorithms: A Retrospective Observational Study. BMC Oral. Health 2025, 25, 916. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Huang, D.; Wang, Z.; Hu, M.; Liu, H.; Jiang, H. A Comparative Study of Condyle Position in Temporomandibular Disorder Patients with Chewing Side Preference Using Cone-Beam Computed Tomography. J. Oral. Rehabil. 2022, 49, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ozsari, S.; Güzel, M.S.; Yılmaz, D.; Kamburoğlu, K. A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases. Diagnostics 2023, 13, 2700. [Google Scholar] [CrossRef] [PubMed]
- Pittayapat, P.; Jacobs, R.; Bornstein, M.M.; Odri, G.A.; Kwon, M.S.; Lambrichts, I.; Willems, G.; Politis, C.; Olszewski, R. A New Mandible-Specific Landmark Reference System for Three-Dimensional Cephalometry Using Cone-Beam Computed Tomography. Eur. J. Orthod. 2016, 38, 563–568. [Google Scholar] [CrossRef]
- de Melo, F.; Milanesi, F.C.; Angst, P.D.M.; Oppermann, R.V. A Systematic Review of the Microbiota Composition in Various Peri-Implant Conditions: Data from 16S rRNA Gene Sequencing. Arch. Oral. Biol. 2020, 117, 104776. [Google Scholar] [CrossRef]
- Martín-Salvador, A.; Torres-Sánchez, I.; Sáez-Roca, G.; López-Torres, I.; Rodríguez-Alzueta, E.; Valenza, M.C. Age Group Analysis of Psychological, Physical and Functional Deterioration in Patients Hospitalized for Pneumonia. Arch. Bronconeumol. 2015, 51, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of Matrix Metalloproteinases, Especially MMP-8, in Gingival Creviclular Fluid, Mouthrinse and Saliva for Monitoring Periodontal Diseases. Periodontology 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Ikeda, K.; Kawamura, A.; Ikeda, R. Assessment of Optimal Condylar Position in the Coronal and Axial Planes with Limited Cone-Beam Computed Tomography. J. Prosthodont. 2011, 20, 432–438. [Google Scholar] [CrossRef]
- Gada, S.K.; Nagda, S.J. Assessment of Position and Bilateral Symmetry of Occurrence of Mental Foramen in Dentate Asian Population. J. Clin. Diagn. Res. 2014, 8, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, M.; Shahidi, S. Association between Mandibular Condylar Position and Clinical Dysfunction Index. J. Craniomaxillofac. Surg. 2015, 43, 432–436. [Google Scholar] [CrossRef]
- Gerhardt, M.d.N.; Fontenele, R.C.; Leite, A.F.; Lahoud, P.; Van Gerven, A.; Willems, H.; Smolders, A.; Beznik, T.; Jacobs, R. Automated Detection and Labelling of Teeth and Small Edentulous Regions on Cone-Beam Computed Tomography Using Convolutional Neural Networks. J. Dent. 2022, 122, 104139. [Google Scholar] [CrossRef]
- Dot, G.; Schouman, T.; Chang, S.; Rafflenbeul, F.; Kerbrat, A.; Rouch, P.; Gajny, L. Automatic 3-Dimensional Cephalometric Landmarking via Deep Learning. J. Dent. Res. 2022, 101, 1380–1387. [Google Scholar] [CrossRef]
- Verhelst, P.-J.; Matthews, H.; Verstraete, L.; Van der Cruyssen, F.; Mulier, D.; Croonenborghs, T.M.; Da Costa, O.; Smeets, M.; Fieuws, S.; Shaheen, E.; et al. Automatic 3D Dense Phenotyping Provides Reliable and Accurate Shape Quantification of the Human Mandible. Sci. Rep. 2021, 11, 8532. [Google Scholar] [CrossRef] [PubMed]
- Kao, Z.-K.; Chiu, N.-T.; Wu, H.-T.H.; Chang, W.-C.; Wang, D.-H.; Kung, Y.-Y.; Tu, P.-C.; Lo, W.-L.; Wu, Y.-T. Classifying Temporomandibular Disorder with Artificial Intelligent Architecture Using Magnetic Resonance Imaging. Ann. Biomed. Eng. 2023, 51, 517–526. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, Y.; Yang, H.; Sun, Y.; Wang, Y. Comparison Between Interactive Closest Point and Procrustes Analysis for Determining the Median Sagittal Plane of Three-Dimensional Facial Data. J. Craniofac Surg. 2016, 27, 441–444. [Google Scholar] [CrossRef]
- Ileșan, R.R.; Beyer, M.; Kunz, C.; Thieringer, F.M. Comparison of Artificial Intelligence-Based Applications for Mandible Segmentation: From Established Platforms to In-House-Developed Software. Bioengineering 2023, 10, 604. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Yeom, H.-G.; Shin, W.; Yun, J.P.; Lee, J.H.; Jeong, S.H.; Lim, H.J.; Lee, J.; Kim, B.C. Deep Learning Based Prediction of Extraction Difficulty for Mandibular Third Molars. Sci. Rep. 2021, 11, 1954. [Google Scholar] [CrossRef]
- Vinayahalingam, S.; Berends, B.; Baan, F.; Moin, D.A.; van Luijn, R.; Bergé, S.; Xi, T. Deep Learning for Automated Segmentation of the Temporomandibular Joint. J. Dent. 2023, 132, 104475. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Crowley, K.E.; Rajaratnam, S.M.W.; Shea, S.A.; Epstein, L.J.; Czeisler, C.A.; Lockley, S.W. Harvard Work Hours, Health and Safety Group Evaluation of a Single-Channel Nasal Pressure Device to Assess Obstructive Sleep Apnea Risk in Laboratory and Home Environments. J. Clin. Sleep. Med. 2013, 9, 109–116. [Google Scholar] [CrossRef]
- Duyan Yüksel, H.; Orhan, K.; Evlice, B.; Kaya, Ö. Evaluation of Temporomandibular Joint Disc Displacement with MRI-Based Radiomics Analysis. Dentomaxillofac. Radiol. 2025, 54, 19–27. [Google Scholar] [CrossRef]
- Chaitanya, B.; Pai, K.M.; Chhaparwal, Y. Evaluation of the Effect of Age, Gender, and Skeletal Class on the Dimensions of Sella Turcica Using Lateral Cephalogram. Contemp. Clin. Dent. 2018, 9, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Míguez, A.; Beghini, F.; Cumbo, F.; McIver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and Improving Metagenomic Taxonomic Profiling with Uncharacterized Species Using MetaPhlAn 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Trzepizur, W.; Cistulli, P.A.; Glos, M.; Vielle, B.; Sutherland, K.; Wijkstra, P.J.; Hoekema, A.; Gagnadoux, F. Health Outcomes of Continuous Positive Airway Pressure versus Mandibular Advancement Device for the Treatment of Severe Obstructive Sleep Apnea: An Individual Participant Data Meta-Analysis. Sleep 2021, 44, zsab015. [Google Scholar] [CrossRef] [PubMed]
- Katakami, K.; Shimoda, S.; Kobayashi, K.; Kawasaki, K. Histological Investigation of Osseous Changes of Mandibular Condyles with Backscattered Electron Images. Dentomaxillofac. Radiol. 2008, 37, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Hallberg, I.R.; Renvert, S. Inter-Rater Reliability of an Oral Assessment Guide for Elderly Patients Residing in a Rehabilitation Ward. Spec. Care Dent. 2002, 22, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kudo, M.; Endo, M.; Nakayama, Y.; Amano, A.; Naito, M.; Ota, Y. Inter-Rater Reliability of the Oral Assessment Guide for Oral Cancer Patients between Nurses and Dental Hygienists: The Difficulties in Objectively Assessing Oral Health. Support. Care Cancer 2019, 27, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Dobai, A.; Markella, Z.; Vízkelety, T.; Fouquet, C.; Rosta, A.; Barabás, J. Landmark-Based Midsagittal Plane Analysis in Patients with Facial Symmetry and Asymmetry Based on CBCT Analysis Tomography. J. Orofac. Orthop. 2018, 79, 371–379. [Google Scholar] [CrossRef]
- Sajda, P. Machine Learning for Detection and Diagnosis of Disease. Annu. Rev. Biomed. Eng. 2006, 8, 537–565. [Google Scholar] [CrossRef]
- Asnicar, F.; Thomas, A.M.; Passerini, A.; Waldron, L.; Segata, N. Machine Learning for Microbiologists. Nat. Rev. Microbiol. 2024, 22, 191–205. [Google Scholar] [CrossRef]
- Le-Dong, N.-N.; Martinot, J.-B.; Coumans, N.; Cuthbert, V.; Tamisier, R.; Bailly, S.; Pépin, J.-L. Machine Learning-Based Sleep Staging in Patients with Sleep Apnea Using a Single Mandibular Movement Signal. Am. J. Respir. Crit. Care Med. 2021, 204, 1227–1231. [Google Scholar] [CrossRef]
- Sousa, R.V.d.; Pinto-Monteiro, A.K.d.A.; Martins, C.C.; Granville-Garcia, A.F.; Paiva, S.M. Malocclusion and Socioeconomic Indicators in Primary Dentition. Braz. Oral. Res. 2014, 28, 54–60. [Google Scholar] [CrossRef]
- Iskra, T.; Stachera, B.; Możdżeń, K.; Murawska, A.; Ostrowski, P.; Bonczar, M.; Gregorczyk-Maga, I.; Walocha, J.; Koziej, M.; Wysiadecki, G.; et al. Morphology of the Sella Turcica: A Meta-Analysis Based on the Results of 18,364 Patients. Brain Sci. 2023, 13, 1208. [Google Scholar] [CrossRef]
- Muhammed, F.K.; Abdullah, A.O.; Liu, Y. Morphology, Incidence of Bridging, Dimensions of Sella Turcica, and Cephalometric Standards in Three Different Racial Groups. J. Craniofac Surg. 2019, 30, 2076–2081. [Google Scholar] [CrossRef]
- Heimer, M.V.; Tornisiello Katz, C.R.; Rosenblatt, A. Non-Nutritive Sucking Habits, Dental Malocclusions, and Facial Morphology in Brazilian Children: A Longitudinal Study. Eur. J. Orthod. 2008, 30, 580–585. [Google Scholar] [CrossRef]
- Katz, C.R.T.; Rosenblatt, A.; Gondim, P.P.C. Nonnutritive Sucking Habits in Brazilian Children: Effects on Deciduous Dentition and Relationship with Facial Morphology. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 53–57. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.-L. Obstructive Sleep Apnoea Syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Mongan, J.; Halabi, S.S. On the Centrality of Data: Data Resources in Radiologic Artificial Intelligence. Radiol. Artif. Intell. 2023, 5, e230231. [Google Scholar] [CrossRef]
- Lindqvist, L.; Seleskog, B.; Wårdh, I.; von Bültzingslöwen, I. Oral Care Perspectives of Professionals in Nursing Homes for the Elderly. Int. J. Dent. Hyg. 2013, 11, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, S.; Olai, L.; Ståhlnacke, K.; Fält, A.; Ehrenberg, A. Oral Health-Related Quality of Life and Associated Factors among Older People in Short-Term Care. Int. J. Dent. Hyg. 2020, 18, 163–172. [Google Scholar] [CrossRef]
- Yoneyama, T.; Hashimoto, K.; Fukuda, H.; Ishida, M.; Arai, H.; Sekizawa, K.; Yamaya, M.; Sasaki, H. Oral Hygiene Reduces Respiratory Infections in Elderly Bed-Bound Nursing Home Patients. Arch. Gerontol. Geriatr. 1996, 22, 11–19. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Liu, H.; Yu, P.; Jiang, X.; Liu, R. Co-Mask R-CNN: Collaborative Learning-Based Method for Tooth Instance Segmentation. J. Clin. Pediatr. Dent. 2024, 48, 161–172. [Google Scholar] [CrossRef]
- Pascadopoli, M.; Zampetti, P.; Nardi, M.G.; Pellegrini, M.; Scribante, A. Smartphone Applications in Dentistry: A Scoping Review. Dent. J. 2023, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-H.; Beam, A.L.; Kohane, I.S. Artificial Intelligence in Healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Carra, M.C.; Gueguen, A.; Thomas, F.; Pannier, B.; Caligiuri, G.; Steg, P.G.; Zins, M.; Bouchard, P. Self-Report Assessment of Severe Periodontitis: Periodontal Screening Score Development. J. Clin. Periodontol. 2018, 45, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Pike, F.; Alvarez, K.; Angus, D.; Newman, A.B.; Lopez, O.; Tate, J.; Kapur, V.; Wilsdon, A.; Krishnan, J.A.; et al. Bidirectional Relationship between Cognitive Function and Pneumonia. Am. J. Respir. Crit. Care Med. 2013, 188, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Rollman, G.B.; Gillespie, J.M. The Role of Psychosocial Factors in Temporomandibular Disorders. Curr. Rev. Pain. 2000, 4, 71–81. [Google Scholar] [CrossRef] [PubMed]
| KEYWORDS | A: Artificial Intelligence; Machine learning; Deep learning; Neural networks B: Dental; Dentistry; Oral health; Odontology; C: Diagnosis; Diagnostic; Detection; Screening |
| BOOLEAN INDICATORS | “A” AND “B” AND “C” |
| TIMESPAN | From 1 January 2015, to 30 June 2025 |
| ELECTRONIC DATABASES | PubMed, Scopus and Web of Science |
| Authors | Study Type | Study Sample | Aim of Study | Materials and Methods | Conclusions |
|---|---|---|---|---|---|
| Stillhart et al. (2024) [41] | Observational study | 57 patients | Evaluate Automated Face Coding software’s effectiveness in detecting facial expressions related to dental pain | Facial expressions recorded and analyzed at baseline and post-treatment using AFC software. Pain assessed with VAS. | AFC software showed limited sensitivity to changes in pain-related expressions; more research needed for integration in diagnostics. |
| Navarro-Fraile et al. (2024) [42] | Randomized clinical trial | 43 patients | Assess root resorption using AI-aided segmentation with different orthodontic forces | CBCT images segmented manually and with AI; resorption compared in control and experimental groups | AI showed comparable accuracy to manual in length detection but less sensitivity in volume loss; promising for clinical use. |
| Uribe et al. (2024) [43] | Observational study | 131,028 records screened; 16 datasets analyzed | Evaluate publicly available dental image datasets for AI development. | Systematic search across databases; analysis of dataset characteristics and FAIR metrics. | Limited publicly available datasets and inconsistent metadata; better quality and access needed for robust AI in dentistry. |
| Schwab et al. (2024) [44] | Observational study | 208 cephalometric radiographs | Assess Sella Turcica morphology and correlation with skeletal class | Cephalometric analysis with demographic correlation using statistical methods | Identified normative ST values for Austrian population; useful for orthodontic diagnostics and future AI integration. |
| Wang et al. (2025) [45] | Experimental study | 400 CBCT scans (360 training, 40 validations, 50 test) | Automate mandibular landmark detection using AI for midsagittal plane construction | Deep learning models (PointRend, PoseNet) used to segment mandible and identify landmarks | Accurate automatic landmark detection and segmentation support use in mandibular asymmetry analysis. |
| Al-Sarem et al. (2024) [46] | Experimental study | 500 CBCT images | Enhance tooth region detection using pretrained deep learning models | Six pretrained CNNs applied to segmented CBCT data; models tested with and without segmentation | DenseNet169 achieved best performance; supports automated implant planning systems. |
| Deng et al. (2023) [47] | Cross-sectional diagnostic study | 408 participants | Develop machine learning tool to screen periodontal health using non-clinical parameters | Random forest models using questionnaire, biomarkers, and demographic data | High accuracy in classifying periodontal stages; promising for population screening applications. |
| Ghensi et al. (2025) [48] | Observational study | 102 individuals, 158 samples | Evaluate plaque microbiome as a biomarker for peri-implant diseases using shotgun metagenomics | Shotgun sequencing of submucosal plaque; machine learning for taxonomic/functional profile analysis | Identified disease-specific microbial signatures; supports future diagnostic and personalized treatment strategies. |
| Muramatsu et al. (2021) [49] | Retrospective observational study | 3201 images from 114 older patients | Construct CNN models to assess oral status of elderly using image data | CNNs trained to classify oral health features into assessment scores | Models demonstrated high diagnostic accuracy for multiple oral conditions in elderly; enhances remote assessment capability. |
| Yıldız et al. (2023) [50] | Cross-sectional observational study | 228 participants (125 TMD, 103 non-TMD) | Predict TMD using machine learning based on clinical features | 20+ ML models trained on physical and psychological metrics; best model identified by validation | Bagging MARS model achieved best performance; useful for preliminary diagnosis in clinics lacking imaging. |
| Pul et al. (2024) [51] | Randomized controlled trial | 30 dentists, 50 panoramic radiographs | To evaluate the impact of AI on diagnostic accuracy and confidence for periapical radiolucency | Cross-over design with AI-aided and unaided assessments; CBCT as reference standard | AI reduced false positives and improved diagnostic accuracy and confidence, especially for junior dentists |
| Picoli et al. (2023) [52] | Within-patient controlled trial | 25 patients with bilateral M3M removal | To assess risk of inferior alveolar nerve injury using 3D AI-driven models compared to CBCT and PANO | 3D models created from CBCT using AI platform; examiners scored IAN risk from different modalities | 3D AI had similar sensitivity to CBCT; promising tool for pre-surgical planning of M3M removal |
| Midlej et al. (2024) [53] | Observational study | 502 patients (Class II and III malocclusion) | To establish ML models for classifying skeletal malocclusions in Arab orthodontic patients | Cephalometric data analyzed with PCA and ML models including LDA, SVM, KNN, RF, CART | High accuracy (up to 0.99) achieved in classifying skeletal classes using ML models with cephalometric inputs |
| Pépin et al. (2024) [54] | Observational study | Obstructive sleep apnea patients (exact N not specified) | Automate mandibular jaw movement analysis to monitor oral appliance treatment in OSA patients | Machine learning applied to mandibular jaw movement signals to classify sleep/OSA events | Automated MJA analysis provided reliable classification of respiratory events and sleep stages |
| Zhou et al. (2025) [55] | Experimental study | 50 panoramic radiographs, evaluated by 30 dentists | Evaluate AI’s impact on dentist performance in identifying periapical radiolucency | Dentists interpreted images with/without AI assistance; diagnostic metrics compared | AI improved diagnostic accuracy and reduced inter-observer variability, particularly for less experienced dentists |
| Authors | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Stillhart et al. (2024) [41] |  |  |  |  |  |  |  |  |
| Navarro-Fraile et al. (2024) [42] |  |  |  |  |  |  |  |  |
| Uribe et al. (2024) [43] |  |  |  |  |  |  |  |  |
| Schwab et al. (2024) [44] |  |  |  |  |  |  |  |  |
| Wang et al. (2025) [45] |  |  |  |  |  |  |  |  |
| Al-Sarem et al. (2024) [46] |  |  |  |  |  |  |  |  |
| Deng et al. (2023) [47] |  |  |  |  |  |  |  |  |
| Ghensi et al. (2025) [48] |  |  |  |  |  |  |  |  |
| Muramatsu et al. (2021) [49] |  |  |  |  |  |  |  |  |
| Yıldız et al. (2023) [50] |  |  |  |  |  |  |  |  |
| Pul et al. (2024) [51] |  |  |  |  |  |  |  |  |
| Picoli et al. (2023) [52] |  |  |  |  |  |  |  |  |
| Midlej et al. (2024) [53] |  |  |  |  |  |  |  |  |
| Pépin et al. (2024) [54] |  |  |  |  |  |  |  |  |
| Zhou et al. (2025) [55] |  |  |  |  |  |  |  |  |
 Very High;
Very High;  High;
High;  Some Concerns;
Some Concerns;  Low;
Low;  No information.
No information.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Marinelli, G.; Fiore, A.; Balestriere, L.; Carone, C.; Inchingolo, F.; Corsalini, M.; Di Venere, D.; Palermo, A.; Inchingolo, A.M.; et al. Diagnostic Support in Dentistry Through Artificial Intelligence: A Systematic Review. Bioengineering 2025, 12, 1244. https://doi.org/10.3390/bioengineering12111244
Inchingolo AD, Marinelli G, Fiore A, Balestriere L, Carone C, Inchingolo F, Corsalini M, Di Venere D, Palermo A, Inchingolo AM, et al. Diagnostic Support in Dentistry Through Artificial Intelligence: A Systematic Review. Bioengineering. 2025; 12(11):1244. https://doi.org/10.3390/bioengineering12111244
Chicago/Turabian StyleInchingolo, Alessio Danilo, Grazia Marinelli, Arianna Fiore, Liviana Balestriere, Claudio Carone, Francesco Inchingolo, Massimo Corsalini, Daniela Di Venere, Andrea Palermo, Angelo Michele Inchingolo, and et al. 2025. "Diagnostic Support in Dentistry Through Artificial Intelligence: A Systematic Review" Bioengineering 12, no. 11: 1244. https://doi.org/10.3390/bioengineering12111244
APA StyleInchingolo, A. D., Marinelli, G., Fiore, A., Balestriere, L., Carone, C., Inchingolo, F., Corsalini, M., Di Venere, D., Palermo, A., Inchingolo, A. M., & Dipalma, G. (2025). Diagnostic Support in Dentistry Through Artificial Intelligence: A Systematic Review. Bioengineering, 12(11), 1244. https://doi.org/10.3390/bioengineering12111244













