Overview of Magnetic Hydrogel Fabrication, Its Basic Characteristics, and Potential Uses in Biomedical Engineering
Abstract
1. Introduction
2. Fabrication and Characteristics of MHs
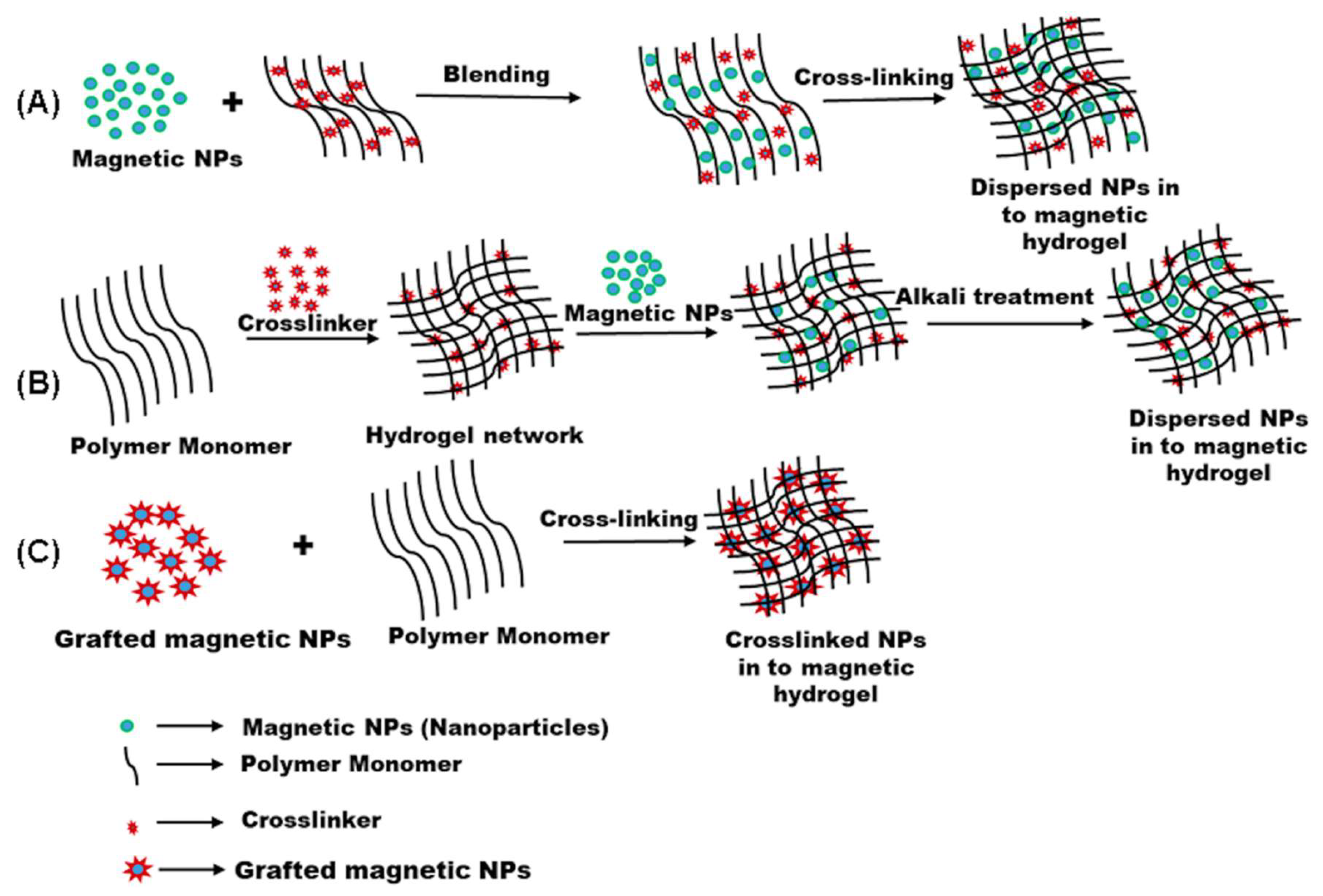
2.1. Strategies for Fabrication of Magnetic Hydrogels with Homogeneous Structure
2.2. Strategies for Fabrication of Magnetic Hydrogels with Ordered Structure
2.3. Fundamental Characteristic of MHs
2.3.1. Surface Properties
2.3.2. Biocompatibility
2.3.3. Diffusive Properties
2.3.4. Biodegradability
2.3.5. Stimuli Sensitivity
2.4. Properties and Functionalities of Magnetic Hydrogels
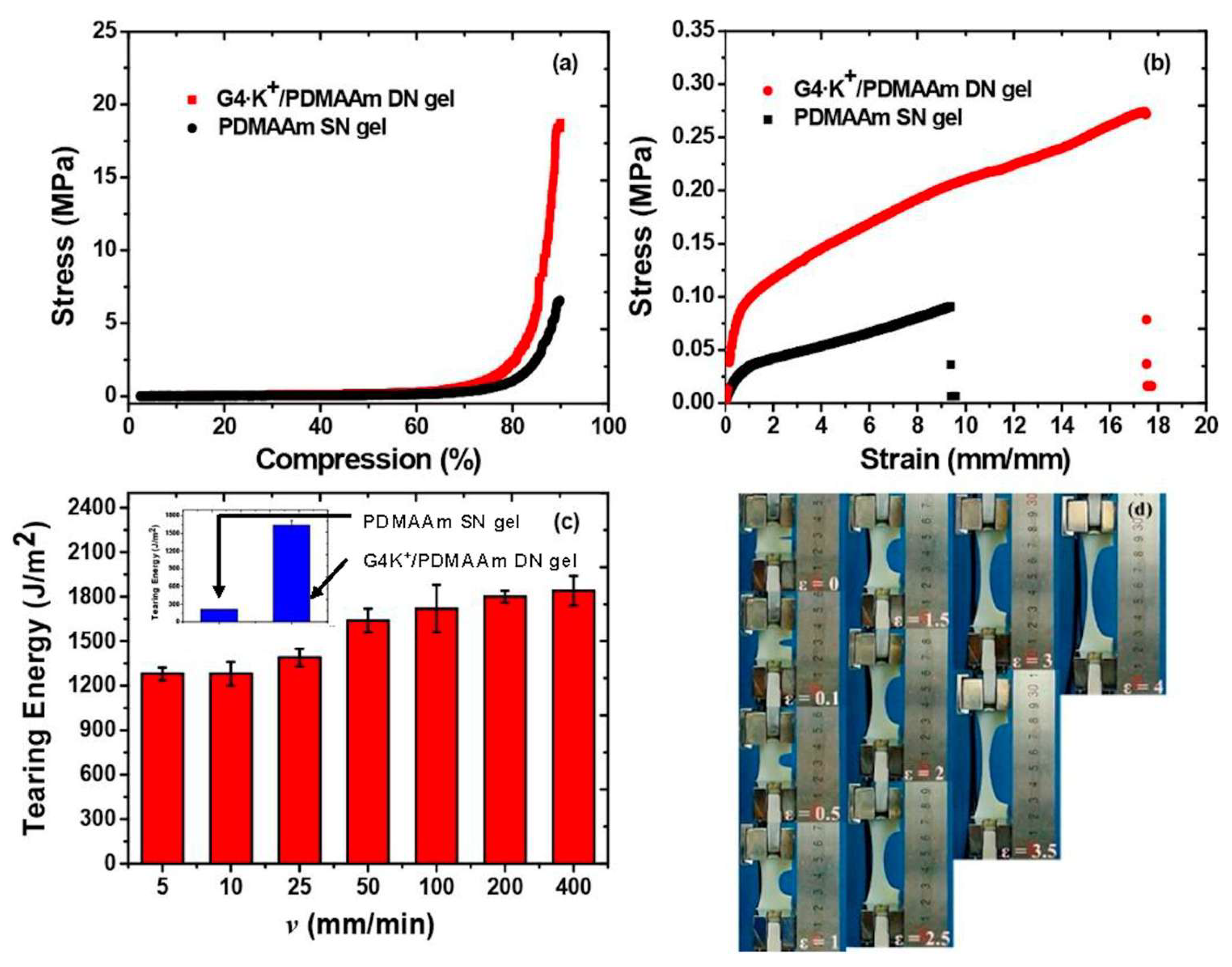
2.4.1. Mechanical Properties
- (1)
- A ‘‘sacrificial bond’’ is used to reduce the increasing energy in the hydrogels by dissipation, which enhances the mechanical characteristics of the hydrogels. Different non-covalent bonds, such as hydrogen bond self-assembly, complexation, supramolecular recognition, and hydrophobic association, have been used in the design of high-strength hydrogels [111].
- (2)
- The “pulley effect” also helps to lower the internal stress in the crosslinking network and significantly improve the mechanical properties of hydrogels. Thus, topological hydrogel such as polyrotaxane is formed by many cyclic molecules threaded on a single polymer chain terminated by bulky end groups. Such hydrogel has high strength due to O ring-shaped crosslinking points that have high mobility along the polymer chain and equalize the tension in the hydrogel [112].
- (3)
- Reversible non-covalent bonds can also give high strength to hydrogels and a self-healing character by reform after breaking [113].
- (4)
- Hydrogels’ mechanical properties also change when NPs are incorporated into them. Several authors have incorporated nanofillers (e.g., MWCNTs, SWCNTs, GO, metal particles, Laponite, polymeric nanoparticles, clay) in hydrogels to achieve better mechanical properties. These nanocomposite hydrogels are made through the process of radical polymerization of a monomer solution incorporating nanoparticles. Specifically, reagents are adsorbed onto the surface of the nanoparticles, then they start the process of polymerization. It was found that the capping of the polymer ends occurred on the nanoparticles, with the formation of clay/brush particles when clay was used as a nanofiller, and the interactions that occurred were adsorption/desorption and were in no way covalent [114].
2.4.2. Adsorption
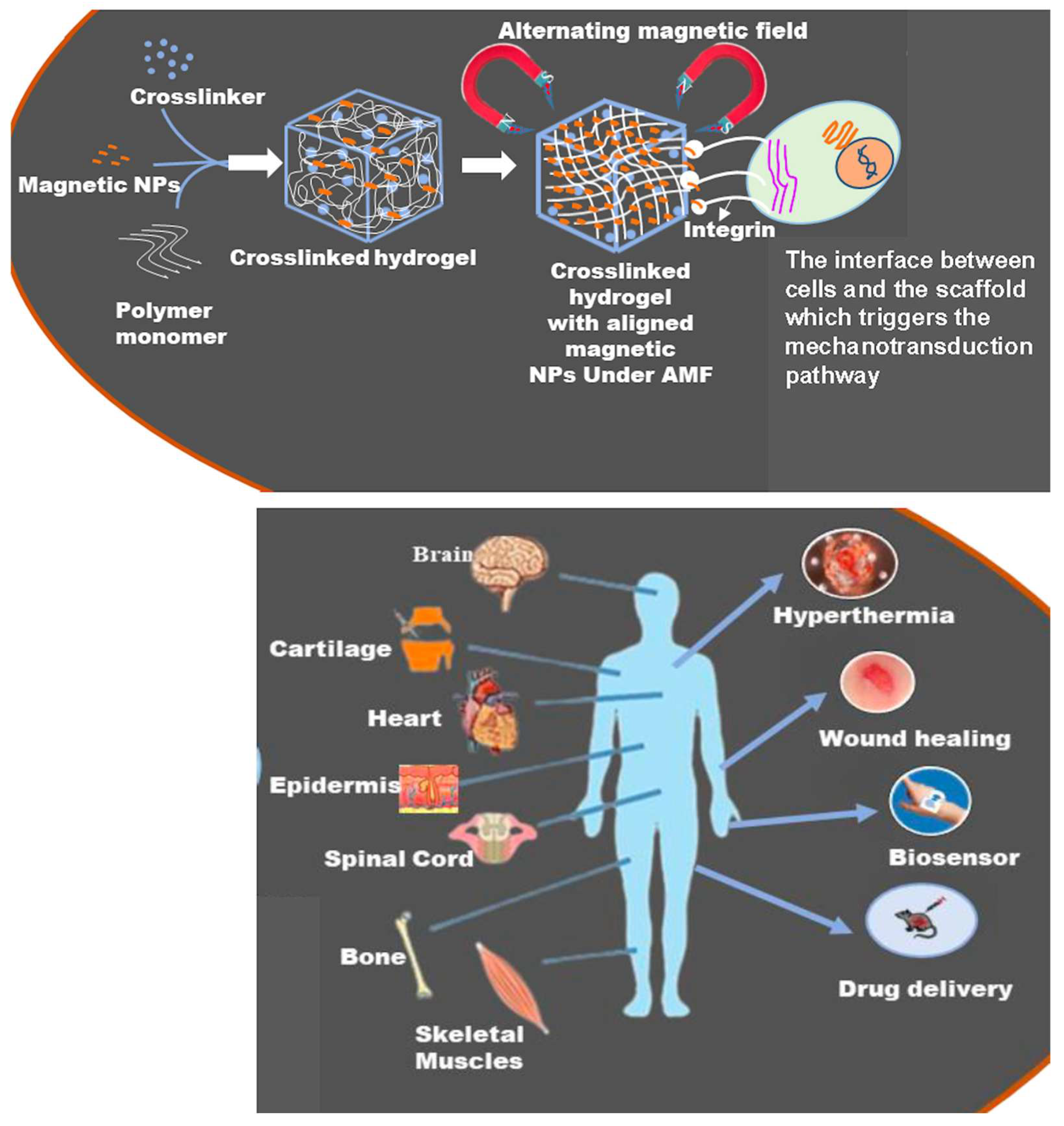
2.4.3. Magnetocaloric Effects
2.4.4. Swelling Behavior
2.4.5. Intelligent Response
3. Biomedical Applications of MHs
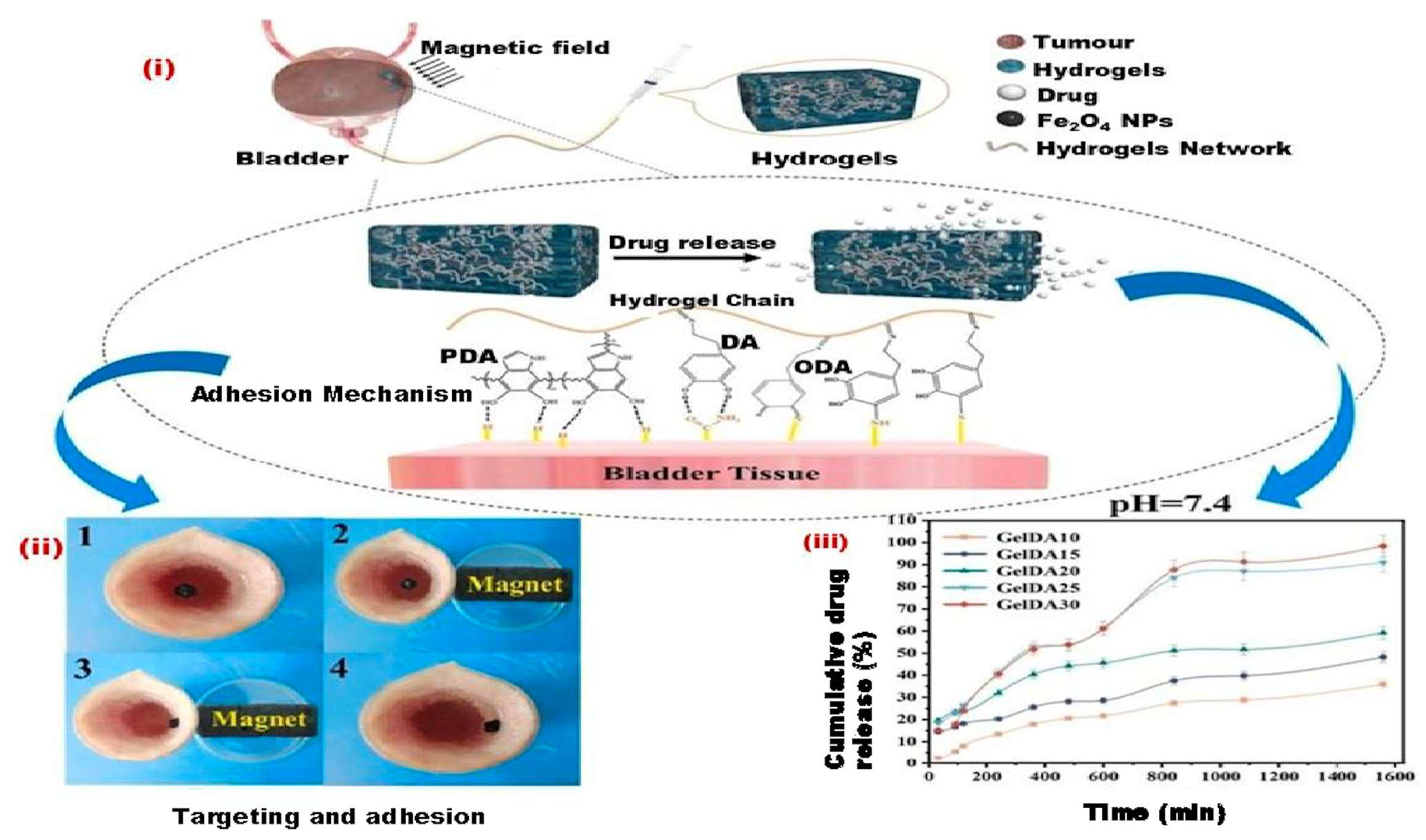
3.1. MHs in Drug Delivery
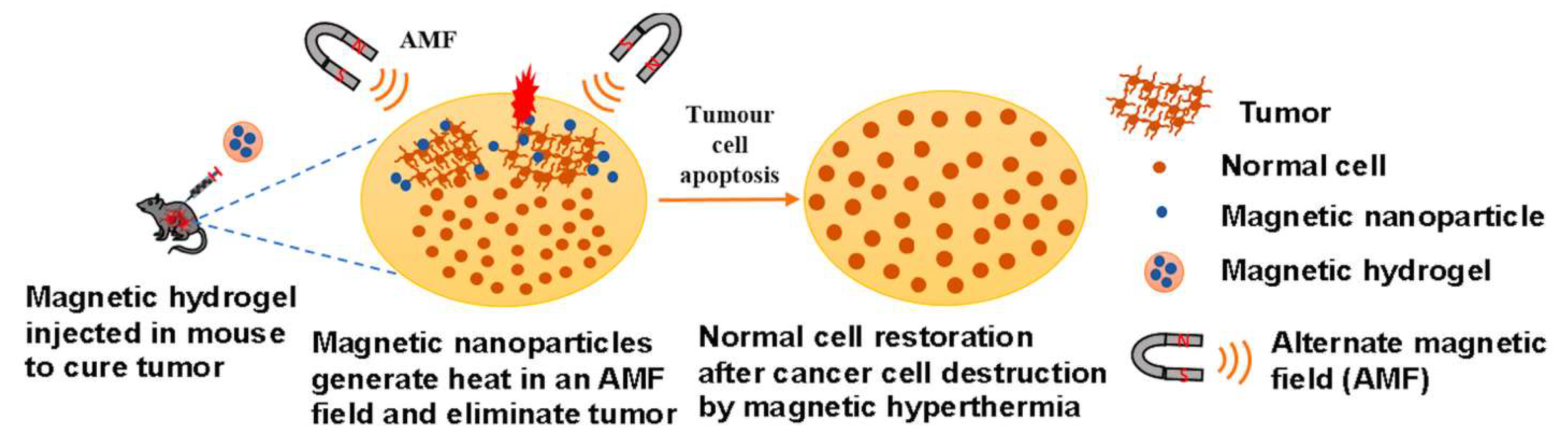
3.2. MHs in Hyperthermia
3.3. MHs in MRI
3.4. MHs in Wound Healing
3.5. MHs in Bio-Sensing
4. Other Applications of MHs in Tissue Engineering
4.1. Applications of MHs in Neural Tissue Engineering
4.2. Applications of MHs in Cartilage Tissue Engineering
4.3. Applications of MHs in Bone Tissue Engineering
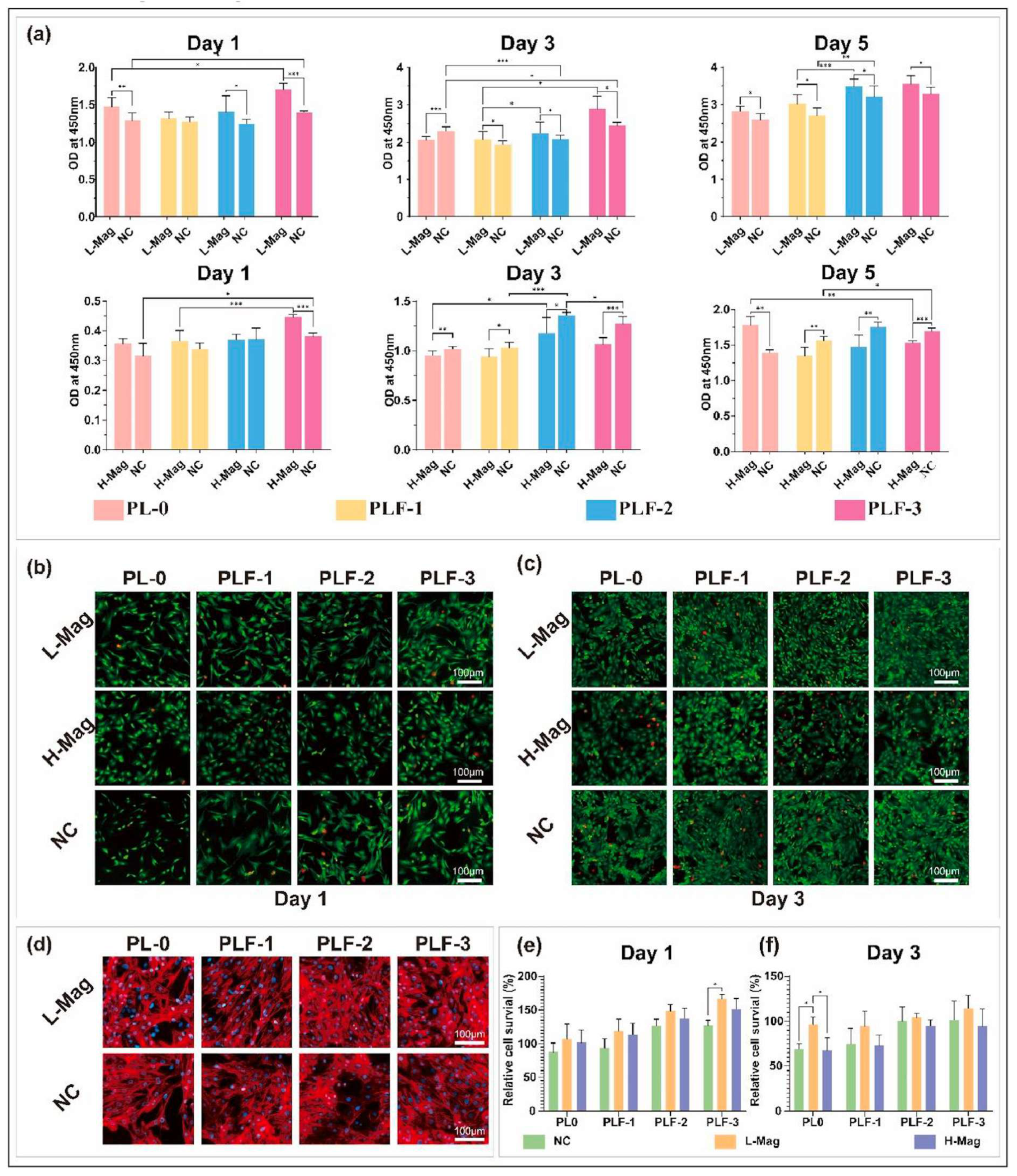
4.4. Application of MHs in Cardiac Tissue Engineering
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AA | Acrylamide |
| AMF | alternating magnetic field |
| CNS | central nervous system |
| CS | Chitosan |
| DOX | Doxorubicin |
| EPC(s) | endothelial progenitor cell(s) |
| ECM | extracellular matrix |
| GelMA | gelatin methacrylate |
| GOx | glucose oxidase |
| LCST | low critical solution temperature |
| MH(s) | magnetic hydrogel(s) |
| MHT | magnetic hyperthermia therapy |
| MNPs | magnetic nanoparticles |
| MRI | magnetic resonance imaging |
| MSC(s) | mesenchymal stem cell(s) |
| MWCNTs | multi-walled carbon nanotubes |
| PCL | polycaprolactone |
| PEG | poly(ethylene glycol) |
| PGA | poly(glycolic acid) |
| PLGA | poly(lactic-co-glycolic) acid |
| PNIPAM | poly(N-isopropylacrylamide) |
| PVA | polyvinyl alcohol |
| SF | silk fibroin |
| SMC(s) | smooth muscle cell(s) |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| SWCNTs | single-walled carbon nanotubes |
| VSA | vinyl sulfonic acid |
References
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta Federico, L.; Chirani, S.; Silvia, F. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 13. [Google Scholar] [CrossRef]
- Yang, D. Recent advances in hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Badeau, B.A.; DeForest, C.A. Programming stimuli-responsive behavior into biomaterials. Annu. Rev. Biomed. Eng. 2019, 21, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front. Chem. 2020, 8, 00124. [Google Scholar] [CrossRef]
- Xue, L.; Sun, J. Magnetic hydrogels with ordered structure for biomedical applications. Front. Chem. 2022, 10, 1040492. [Google Scholar] [CrossRef]
- Frachini, E.C.G.; Petri, D.F.S. Magneto-responsive hydrogels: Preparation, characterization, biotechnological and environmental applications. J. Braz. Chem. Soc. 2019, 30, 2010–2028. [Google Scholar] [CrossRef]
- Gao, F.; Xie, W.; Miao, Y.; Wang, D.; Guo, Z.; Ghosal, A.; Li, Y.; Wei, Y.; Feng, S.-S.; Zhao, L.; et al. Magnetic hydrogel with optimally adaptive functions for breast cancer recurrence prevention. Adv. Healthc. Mater. 2019, 8, 1900203. [Google Scholar] [CrossRef]
- Manjua, A.C.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Magnetic responsive PVA hydrogels for remote modulation of protein sorption. ACS Appl. Mater. Interfaces 2019, 11, 21239–21249. [Google Scholar] [CrossRef]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; Zhao, J.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef]
- Munaweera, I.; Aliev, A.; Balkus, K.J., Jr. Electrospun cellulose acetate-garnet nanocomposite magnetic fibers for bioseparations. ACS Appl. Mater. Interfaces 2014, 6, 244–251. [Google Scholar] [CrossRef]
- Wu, H.; Song, L.; Chen, L.; Huang, Y.; Wu, Y.; Zang, F.; An, Y.; Lyu, H.; Ma, M.; Chen, J.; et al. Injectable thermosensitive magnetic nanoemulsion hydrogel for multimodal-imaging-guided accurate thermoablative cancer therapy. Nanoscale 2017, 9, 16175–16182. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Jose, V.K.; Lee, J.-M. Hydrogels for medical and environmental applications. Small Methods 2020, 4, 1900735. [Google Scholar] [CrossRef]
- Hu, P.; Lu, J.; Li, C.; He, Z.; Wang, X.; Pan, Y.; Zhao, L. Injectable magnetic hydrogel filler for synergistic bone tumor hyperthermia chemotherapy. ACS Appl. Bio Mater. 2024, 7, 1569–1578. [Google Scholar] [CrossRef]
- Chen, X.; Tian, C.; Zhang, H.; Xie, H. Biodegradable magnetic hydrogel robot with multimodal locomotion for targeted cargo delivery. ACS Appl. Mater. Interfaces 2023, 15, 28922–28932. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Xue, L.; Dong, G.; Su, W.; Cui, M.J.; Wang, Z.G.; Liu, M.; Zhou, Z.; Zhang, X. Ultrasoft and biocompatible magnetic-hydrogel-based strain sensors for wireless passive biomechanical monitoring. ACS Nano 2022, 16, 21555–21564. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Narita, Y.; Teramura, Y.; Fukazawa, K. Preparation of magnetic hydrogel microparticles with cationic surfaces and their cell-assembling performance. ACS Biomater. Sci. Eng. 2021, 7, 5107–5117. [Google Scholar] [CrossRef]
- Singh, R.; Pal, D.; Chattopadhyay, S. Target-specific superparamagnetic hydrogel with excellent pH sensitivity and reversibility: A promising platform for biomedical applications. ACS Omega 2020, 5, 21768–21780. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yin, Q.; Qiao, Y.; Wang, T. Shape morphing of hydrogels in alternating magnetic field. ACS Appl. Mater. Interfaces 2019, 11, 21194–21200. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Review on magnetic natural polymer constructed hydrogels as vehicles for drug delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: From strategic design to biomedical applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Phalake, S.S.; Somvanshi, S.B.; Tofail, S.A.M.; Thorat, N.D.; Khot, V.M. Functionalized manganese iron oxide nanoparticles: A dual potential magneto-chemotherapeutic cargo in a 3D breast cancer model. Nanoscale 2023, 15, 15686–15699. [Google Scholar] [CrossRef]
- Esmaeili, J.; Barati, A.; Ai, J.; Nooshabadi, V.T.; Mirzaei, Z. Employing hydrogels in tissue engineering approaches to boost conventional cancer-based research and therapies. RSC Adv. 2021, 11, 10646–10669. [Google Scholar] [CrossRef]
- Goranov, V. Biomaterials functionalized with magnetic nanoparticles for tissue engineering: Between advantages and challenges. Biomater. Biosys. 2024, 15, 100100. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Song, S.-C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Fuentes-Caparrós, A.M.; Sharkey, J.; Daniels, L.M.; Mandal, P.; Park, B.K.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. SPIONs for cell labelling and tracking using MRI: Magnetite or maghemite? Biomater. Sci. 2018, 6, 101–106. [Google Scholar] [CrossRef]
- Haas, W.; Zrinyi, M.; Kilian, H.G.; Heise, B. Structural analysis of anisometric colloidal iron(III)-hydroxide particles and particle-aggregates incorporated in poly(vinyl-acetate) networks. Colloid Polym. Sci. 1993, 271, 1024–1034. [Google Scholar] [CrossRef]
- Fiejdasz, S.; Gilarska, A.; Horak, W.; Radziszewska, A.; Strączek, T.; Szuwarzyński, M.; Nowakowska, M.; Kapusta, C. Structurally stable hybrid magnetic materials based on natural polymers – preparation and characterization. J. Mater. Res. Technol. 2021, 15, 3149–3160. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhou, Y.; Jia, D. In situ mineralization of magnetite nanoparticles in chitosan hydrogel. Nanoscale Res. Lett. 2009, 4, 1041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting functionality to the hydrogel by magnetic-field-induced nano-assembly and macro-response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; et al. Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Adv. Mater. 2013, 25, 4028–4034. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Sun, J.; Chen, B.; Li, Y.; Hu, K.; Wang, P.; Ma, M.; Gu, N. Rotating magnetic field-controlled fabrication of magnetic hydrogel with spatially disk-like microstructures. Sci. China Mater. 2018, 61, 1112–1122. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Shefi, O. Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Shen, Z.; Zhang, M.; Jin, D.; Zheng, K.; Liu, X.; Chai, M.; Wang, Z.; Chi, A.; et al. Magnetic soft microrobots for erectile dysfunction therapy. Proc. Natl. Acad. Sci. USA 2024, 121, e2407809121. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chan, T.-Y.; Wang, K.-S.; Tsou, H.-M. Influence of magnetic nanoparticle arrangement in ferrogels for tunable biomolecule diffusion. RSC Adv. 2015, 5, 90098–90102. [Google Scholar] [CrossRef]
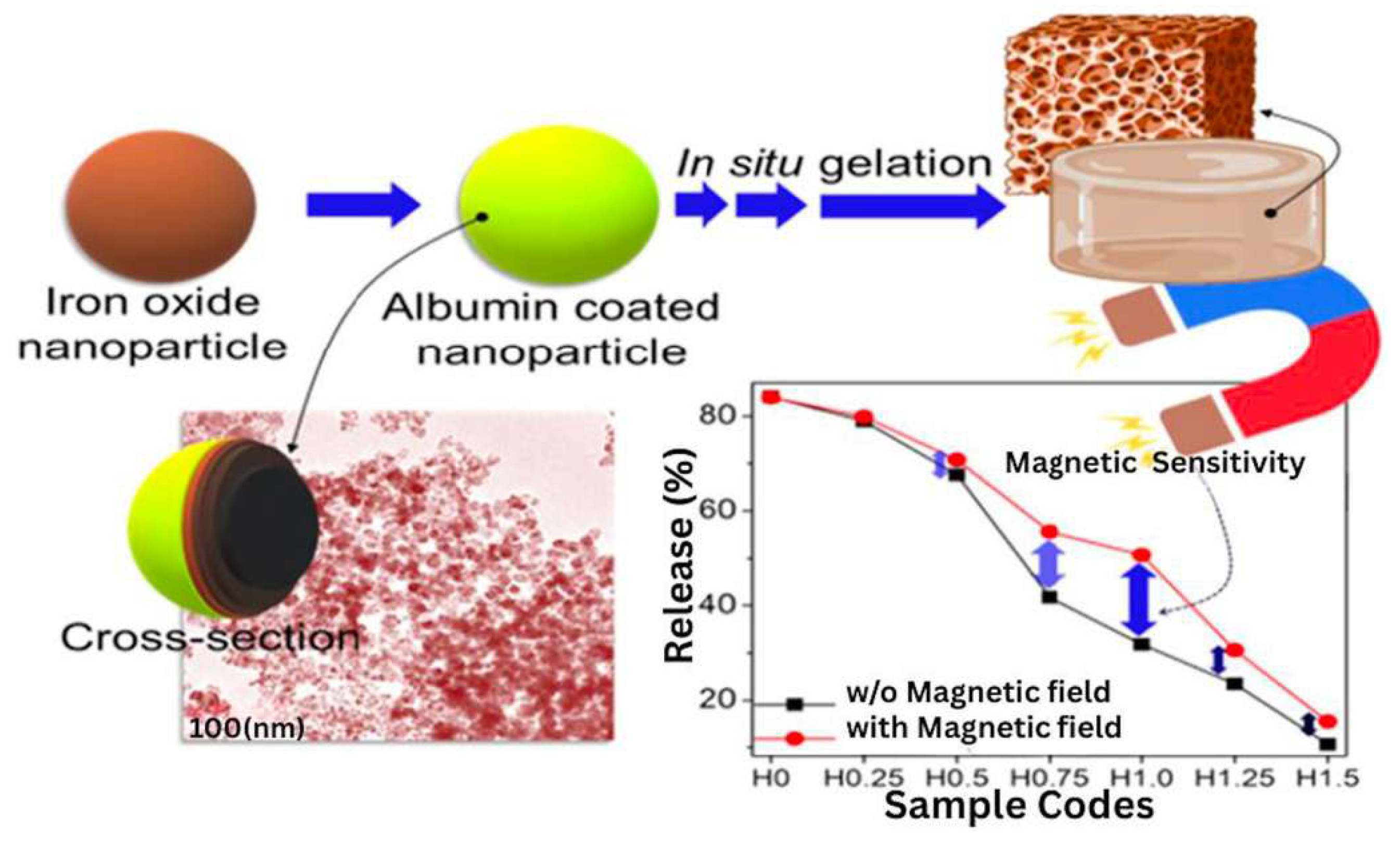
- Ganguly, S.; Das, P.; Srinivasan, S.; Rajabzadeh, A.R.; Tang, X.S.; Margel, S. Superparamagnetic Amine-Functionalized Maghemite Nanoparticles as a Thixotropy Promoter for Hydrogels and Magnetic Field-Driven Diffusion-Controlled Drug Release. ACS Appl. Nano Mater. 2024, 7, 5272–5286. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Tumarkin, E.; Kumacheva, E. Microfluidic generation of microgels from synthetic and natural polymers. Chem. Soc. Rev. 2009, 38, 2161–2168. [Google Scholar] [CrossRef]
- Thomas, R.G.; Unnithan, A.R.; Moon, M.J.; Surendran, S.P.; Batgerel, T.; Park, C.H.; Kim, C.S.; Jeong, Y.Y. Electromagnetic manipulation enabled calcium alginate Janus microsphere for targeted delivery of mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 110, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yu, Y.; Zou, M.; Liu, Y.; Bian, F.; Zhao, Y. Peanut-inspired anisotropic microparticles from microfluidics. Compos. Commun. 2018, 10, 129–135. [Google Scholar] [CrossRef]
- Cai, L.; Bian, F.; Chen, H.; Guo, J.; Wang, Y.; Zhao, Y. Anisotropic microparticles from microfluidics. Chem 2021, 7, 93–136. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, J.; Wang, Y.; Shao, C.; Wang, Y.; Zhao, Y. Bioinspired helical micromotors as dynamic cell microcarriers. ACS Appl. Mater. Interfaces 2020, 12, 16097–16103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Valenzuela, C.; Xue, P.; Zhang, X.; Zhang, X.; Chen, Y.; Yang, Y.; Wang, L.; Xu, X. Magnetic structural color hydrogels for patterned photonic crystals and dynamic camouflage. ACS Appl. Polym. Mater. 2022, 4, 3618–3626. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol 2023, 11, 991821. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Murugan, R.; Hojae, B.; Gorka, O.; Toshinori, F.; Xuetao, S.; Kaji, H. Latest developments in engineered skeletal muscle tissues for drug discovery and development. Expert Opin. Drug Discov. 2023, 18, 47–63. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in skeletal muscle tissue engineering. Small 2019, 15, 1805530. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Jiang, L.; Tang, D.; Xu, H.; Zhao, P.; Fu, J.; Zhou, Q.; Chen, Y. 3D Printing of functional magnetic materials: From design to applications. Adv. Funct. Mater. 2021, 31, 2102777. [Google Scholar] [CrossRef]
- Simińska-Stanny, J.; Nizioł, M.; Szymczyk-Ziółkowska, P.; Brożyna, M.; Junka, A.; Shavandi, A.; Podstawczyk, D. 4D printing of patterned multimaterial magnetic hydrogel actuators. Addit. Manuf. 2022, 49, 102506. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-drying methods to produce a range of collagen–glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2009, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Rouhollahi, A.; Ilegbusi, O.; Florczyk, S.; Xu, K.; Foroosh, H. Effect of mold geometry on pore size in freeze-cast chitosan-alginate scaffolds for tissue engineering. Ann. Biomed. Eng. 2020, 48, 1090–1102. [Google Scholar] [CrossRef]
- Rossi, A.; Furlani, F.; Bassi, G.; Cunha, C.; Lunghi, A.; Molinari, F.; Teran, F.J.; Lista, F.; Bianci, M.; Piperno, A.; et al. Contactless magnetically responsive injectable hydrogel for aligned tissue regeneration. Mater. Today Bio 2024, 27, 101110. [Google Scholar] [CrossRef]
- Shi, M.; Bai, L.; Xu, M.; Dong, R.; Yin, Z.; Zhao, W.; Guo, B.; Hu, J. Magnetically induced anisotropic conductive in situ hydrogel for skeletal muscle regeneration by promoting cell alignment and myogenic differentiation. Chem. Eng. J. 2024, 484, 149019. [Google Scholar] [CrossRef]
- Jain, E.; Zhang, K.; Tiwari, R.M. Properties and Characterization of cryogels: Structural, mechanical, and functional insights. ACS Omega 2025, 10, 36771–36787. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Park, I.K. Mechanically robust magnetic Fe3O4 nanoparticles/polyvinylidene fluoride composite nanofiber and its application in a triboelectric nanogenerator. ACS Appl. Mater. Interfaces 2018, 10, 25660–25665. [Google Scholar] [CrossRef]
- Roskov, K.E.; Atkinson, J.E.; Bronstein, L.M.; Spontak, R.J. Magnetic field-induced alignment of nanoparticles in electrospun microfibers. RSC Adv. 2012, 2, 4603–4607. [Google Scholar] [CrossRef]
- Almeida, D.; Sanjuan-Alberte, P.; Silva, J.C.; Ferreira, F.C. 3D bioprinting of magnetic hydrogels: Formulation and applications in tissue engineering. Int. J. Bioprint. 2023, 10, 965. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, L.; Liu, Z. Fabrication of patterned magnetic hydrogels by ion transfer printing. Soft Matter 2021, 17, 8059. [Google Scholar] [CrossRef]
- Hinojosa-Ventura, G.; Acosta-Cuevas, J.M.; Velazquez-Carriles, C.A.; Navarro-Lopez, D.E.; Lopez-Alvarez, M.A.; Ortega de la Rosa, N.; Silva-Jara, J.M. From basic to breakthroughs: The journey of microfluidic devices in hydrogel droplet generation. Gels 2025, 11, 309. [Google Scholar] [CrossRef]
- Moharramzadeh, F.; Ebrahimi, S.A.S.; Zarghami, V.; Lalegani, Z.; Hamawandi, B. Synthesis and characterization of hydrogel droplets containing magnetic nanoparticles in a microfluidic flow-focusing chip. Gels 2023, 9, 501. [Google Scholar] [CrossRef]
- Wu, J.; Gong, X.; Fan, Y.; Xia, H. Physically crosslinked poly(vinyl alcohol) hydrogels with magnetic field controlled modulus. Soft Matter 2011, 7, 6205–6212. [Google Scholar] [CrossRef]
- Chen, L.; Deng, X.; Tian, L.; Xie, J.; Xiang, Y.; Liang, X.; Jiang, L.; Jiang, L. Preparation and properties of chitosan/dialdehyde sodium alginate/dopamine magnetic drug-delivery hydrogels. Colloids Surf. A Phys. Eng. Asp. 2024, 680, 132739. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ebrahimi, M.; Bae, H.; Nguyen, H.K.; Salehi, S.; Kim, S.B.; Kumatani, A.; Matsue, T.; Shi, X.; Nakajima, K.; et al. Gelatin–polyaniline composite nanofibers enhanced excitation–contraction coupling system maturation in myotubes. ACS Appl. Mater. Interfaces 2017, 9, 42444–42458. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Shi, X.; Zhang, L.; Liang, X.; Kim, S.B.; Fujie, T.; Ramalingam, M.; Chen, M.; Nakajima, K.; Al-Hazmi, F.; et al. Myotube formation on gelatin nanofibers – Multi-walled carbon nanotubes hybrid scaffolds. Biomaterials 2014, 35, 6268–6277. [Google Scholar] [CrossRef]
- Eom, S.; Park, S.M.; Hong, H.; Kwon, J.; Oh, S.-R.; Kim, J.; Kim, D.S. Hydrogel-assisted electrospinning for fabrication of a 3D complex tailored nanofiber macrostructure. ACS Appl. Mater. Interfaces 2020, 12, 51212–51224. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023, 162, 157–178. [Google Scholar] [CrossRef]
- Sousa, J.P.M.; Monteiro, C.F.; Deus, I.A.; Completo, A.; Stratakis, E.; Mano, J.F.; Marques, P.A.A.P. Magnetoresponsive anisotropic fiber-integrating hydrogels for neural tissue regeneration. Small Struct. 2024, 5, 2400213. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, Q.; Zhou, G.; Wei, T.; Zhong, S.; Lu, L.; Yan, C.; Wang, Y.; Fang, M.; Yang, M.; et al. Electrospinning aligned SF/magnetic nanoparticles-blend nanofiber scaffolds for inducing skeletal myoblast alignment and differentiation. ACS Appl. Bio Mater. 2024, 7, 7710–7718. [Google Scholar] [CrossRef]
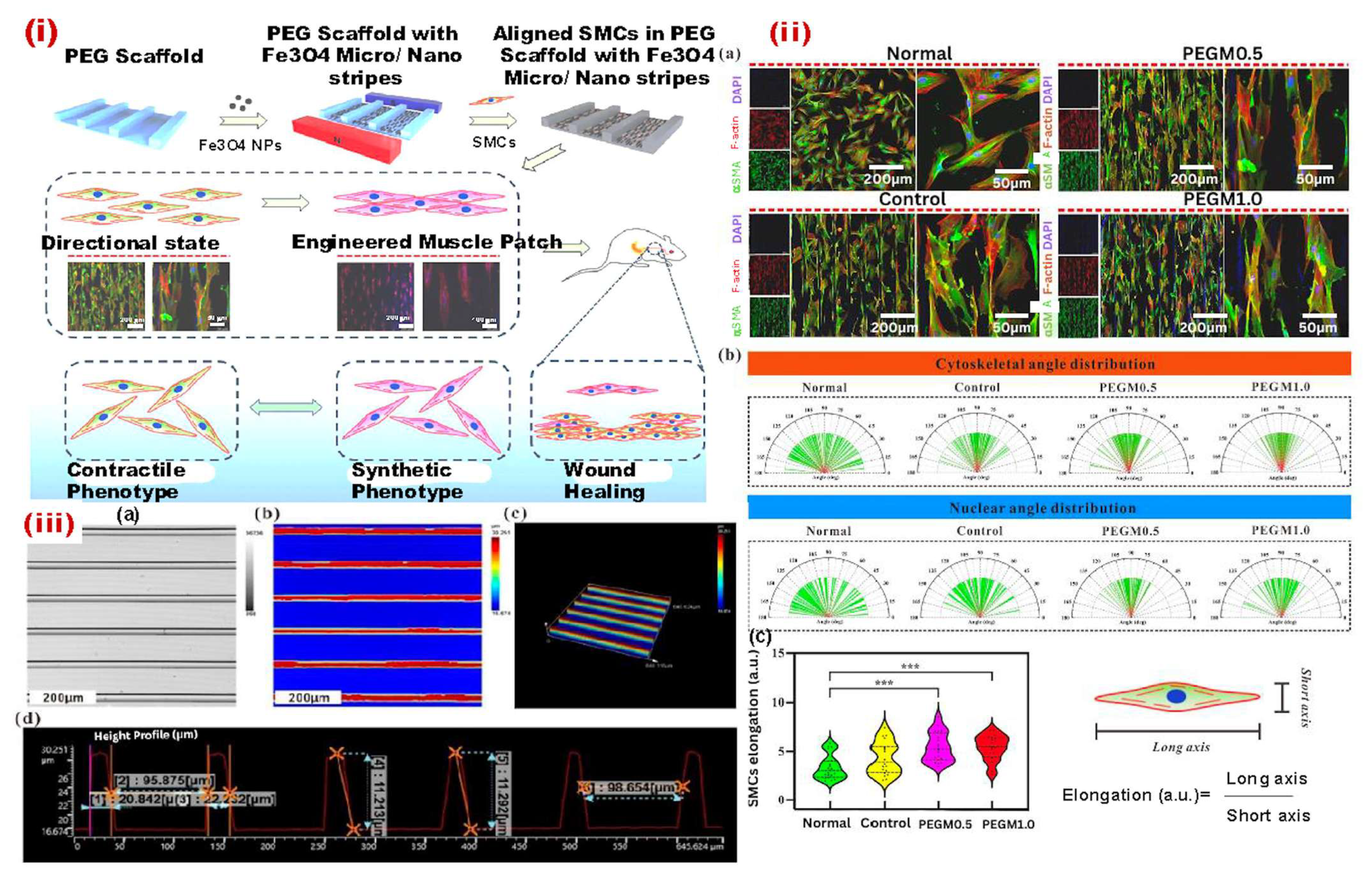
- Luo, Y.; Chen, Y.; Gu, Z.; Ni, R.; Feng, P.; Hu, Z.; Song, L.; Shen, X.; Gu, C.; Li, J.; et al. Engineered muscle from micro-channeled PEG scaffold with magnetic Fe3O4 fixation towards accelerating esophageal muscle repair. Mater. Today Bio 2023, 23, 100853. [Google Scholar] [CrossRef]
- Noh, M.; Choi, Y.H.; An, Y.-H.; Tahk, D.; Cho, S.; Yoon, J.W.; Jeon, N.L.; Park, T.H.; Kim, J.; Hwang, N.S. Magnetic nanoparticle-embedded hydrogel sheet with a groove pattern for wound healing application. ACS Biomater. Sci. Eng. 2019, 5, 3909–3921. [Google Scholar] [CrossRef]
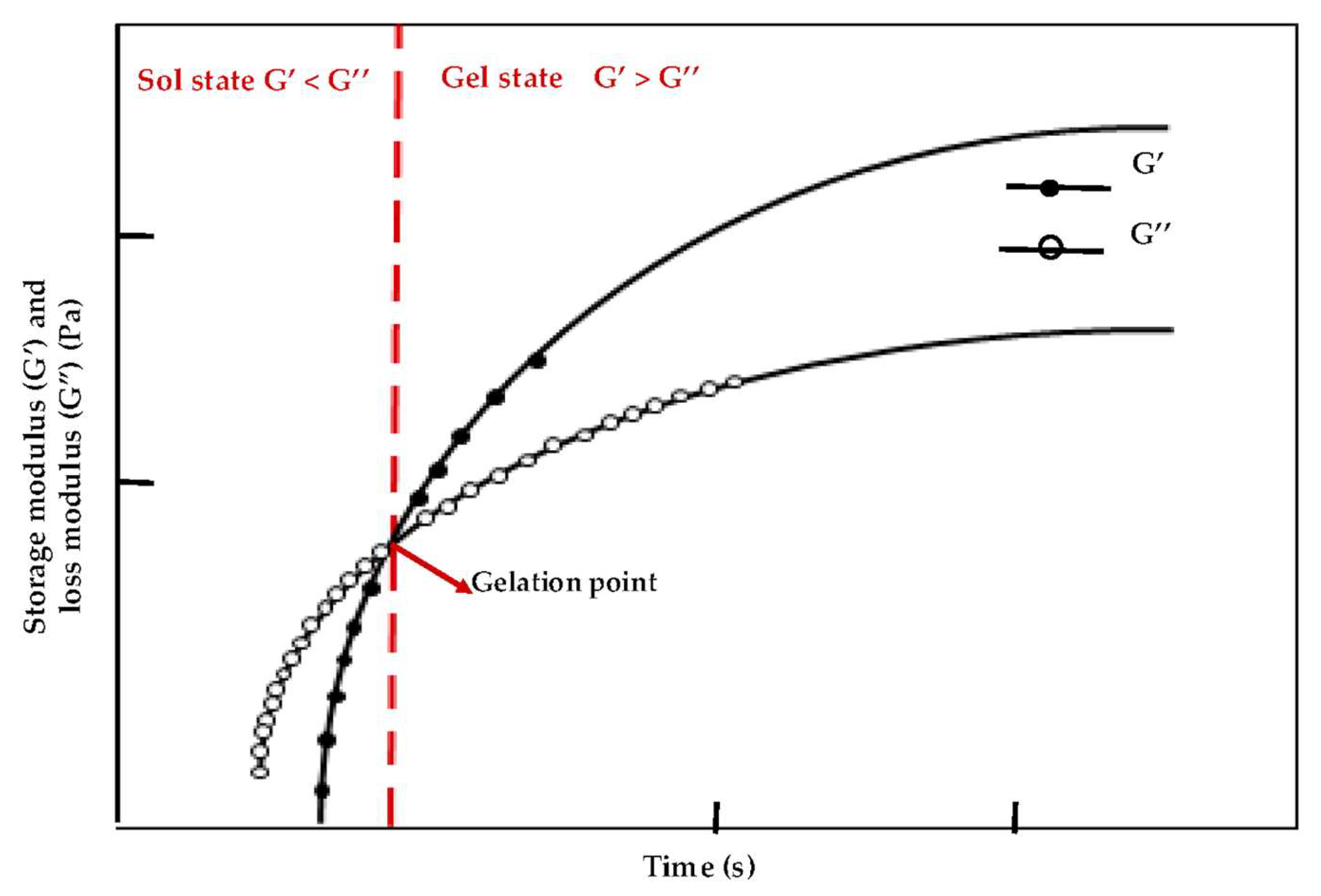
- Sahiner, N.; Singh, M.; De Kee, D.; John, V.T.; McPherson, G.L. Rheological characterization of a charged cationic hydrogel network across the gelation boundary. Polymer 2006, 47, 1124–1131. [Google Scholar] [CrossRef]
- Shi, X.; Ostrovidov, S.; Shu, Y.; Liang, X.; Nakajima, K.; Wu, H.; Khademhosseini, A. Microfluidic generation of polydopamine gradients on hydrophobic surfaces. Langmuir 2014, 30, 832–838. [Google Scholar] [CrossRef]
- He, J.; Burgess, D.J. Chapter 5—Impact of biomaterials’ physical properties on cellular and molecular responses. In Handbook of Biomaterials Biocompatibility; Mozafari, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 69–84. [Google Scholar]
- Kim, D.-N.; Park, J.; Koh, W.-G. Control of cell adhesion on poly(ethylene glycol) hydrogel surfaces using photochemical modification and micropatterning techniques. J. Ind. Eng. Chem. 2009, 15, 124–128. [Google Scholar] [CrossRef]
- Cui, L.; Yao, Y.; Yim, E.K.F. The effects of surface topography modification on hydrogel properties. APL Bioeng. 2021, 5, 031509. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Crippa, F.; Moore, T.L.; Mortato, M.; Geers, C.; Haeni, L.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Dynamic and biocompatible thermo-responsive magnetic hydrogels that respond to an alternating magnetic field. J. Magn. Magn. Mater. 2017, 427, 212–219. [Google Scholar] [CrossRef]
- Lan, W.; Xu, M.; Qin, M.; Cheng, Y.; Zhao, Y.; Huang, D.; Wei, X.; Guo, Y.; Chen, W. Physicochemical properties and biocompatibility of the bi-layer polyvinyl alcohol-based hydrogel for osteochondral tissue engineering. Mater. Des. 2021, 204, 109652. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Shi, Z.; Lan, G.; Hu, E.; Lu, F.; Qian, P.; Liu, J.; Dai, F.; Xie, R. Targeted delivery of hemostats to complex bleeding wounds with magnetic guidance for instant hemostasis. Chem. Eng. J. 2022, 427, 130916. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
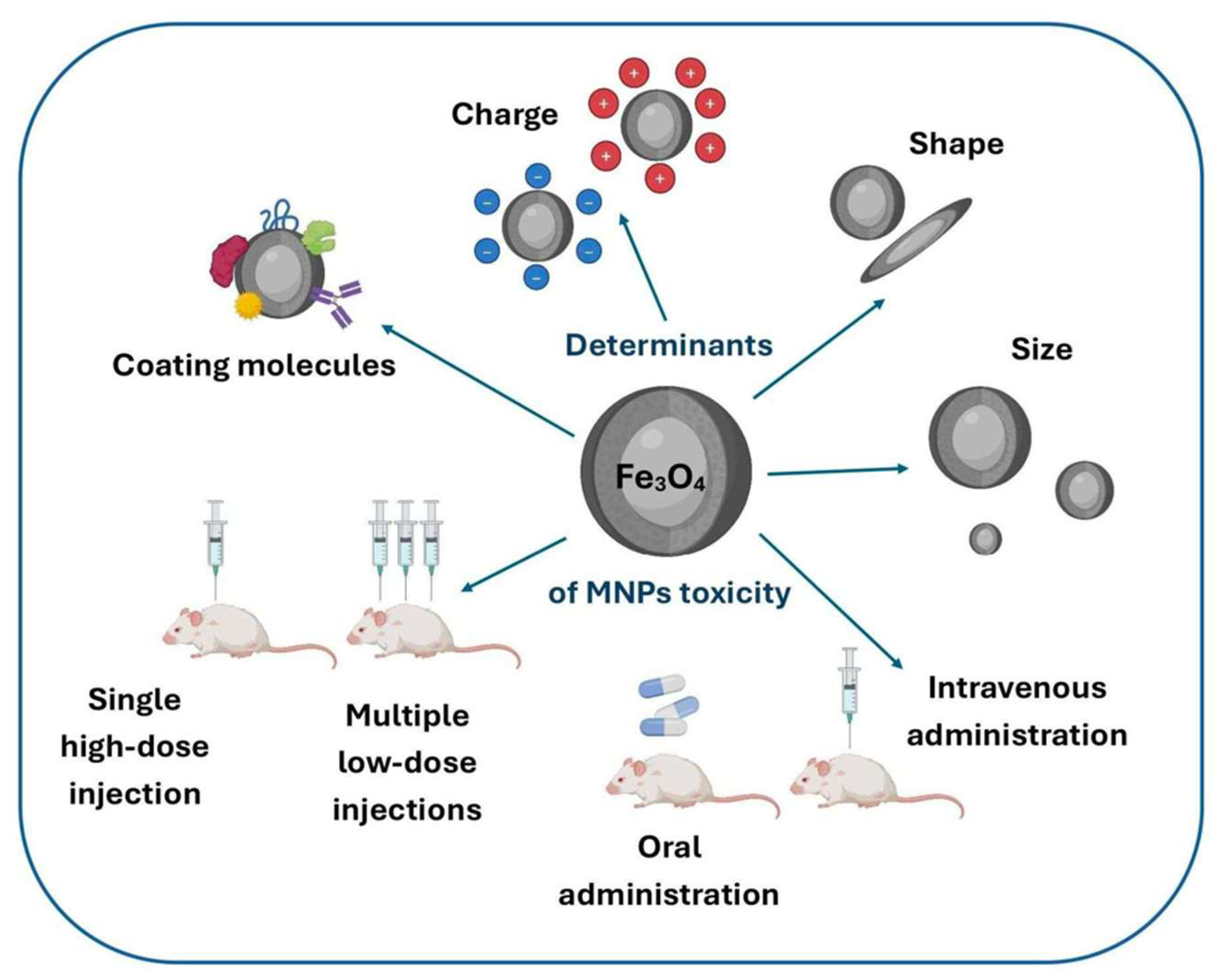
- Nowak-Jary, J.; Machnicka, B. Toxicity of magnetic nanoparticles in medicine: Contributing factors and modern assessment methods. Int. J. Mol. Sci. 2025, 26, 8586. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Comprehensive analysis of the potential toxicity of magnetic iron oxide nanoparticles for medical applications: Cellular mechanisms and systemic effects. Int. J. Mol. Sci. 2024, 25, 12013. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Mai, T.; Hilt, J.Z. Magnetic nanoparticles: Reactive oxygen species generation and potential therapeutic applications. J. Nanopart. Res. 2017, 19, 253. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Parveen, N.; Amhad, M.F.; Wani, A.L.; Afrin, S.; Rahman, Y.; Jameel, S.; Khan, Y.A.; Siddique, H.R.; Tabish, M.; et al. Evaluation of DNA interaction, genotoxicity and oxidative stress, induced by iron oxide nanoparticles both in vitro and in vivo: Attenuation by thymoquinone. Sci. Rep. 2019, 9, 6912. [Google Scholar] [CrossRef]
- Yang, W.J.; Lee, J.H.; Hong, S.C.; Lee, J.; Lee, J.; Han, D.W. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqi, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef]
- Ying, H.; Ruan, Y.; Zeng, Z.; Bai, Y.; Xu, J.; Chen, S. Iron oxide nanoparticle size-dependently activate mouse primary macrophages via oxidative stress and endoplasmic reticulum stress. Int. Immunopharmacol. 2022, 105, 108533. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Chan, D.; Offeddu, G.S.; Chang, Y.; Merida, D.; Hernandez, H.L.; Appel, E.A. A multiscale model for solute diffusion in hydrogels. Macromolecules 2019, 52, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Nicolella, P.; Koziol, M.F.; Löser, L.; Saalwächter, K.; Ahmadi, M.; Seiffert, S. Defect-controlled softness, diffusive permeability, and mesh-topology of metallo-supramolecular hydrogels. Soft Matter 2022, 18, 1071–1081. [Google Scholar] [CrossRef]
- Vinchhi, P.; Rawal, S.U.; Patel, M.M. Chapter 19—Biodegradable hydrogels. In Drug Delivery Devices and Therapeutic Systems; Chappel, E., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 395–419. [Google Scholar]
- Zong, H.; Wang, B.; Li, G.; Yan, S.; Zhang, K.; Shou, Y.; Yin, J. Biodegradable high-strength hydrogels with injectable performance based on poly(l-glutamic acid) and gellan gum. ACS Biomater. Sci. Eng. 2020, 6, 4702–4713. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. VIEW 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Gang, F.; Jiang, L.; Xiao, Y.; Zhang, J.; Sun, X. Multi-functional magnetic hydrogel: Design strategies and applications. Nano Sel. 2021, 2, 2291–2307. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Q.; Zhu, L.; Tang, Z.; Li, Q.; Qin, G.; Yang, J.; Zhang, Y.; Ren, B.; Zheng, J. General strategy to fabricate strong and tough low-molecular-weight gelator-based supramolecular hydrogels with double network structure. Chem. Mater. 2018, 30, 1743–1754. [Google Scholar] [CrossRef]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, F.; Qian, D.; He, Y.-D.; Nie, W.-C.; Wang, X.-L.; Wang, Y.-Z. Strong and tough fully physically crosslinked double network hydrogels with tunable mechanics and high self-healing performance. Chem. Eng. J. 2018, 349, 588–594. [Google Scholar] [CrossRef]
- Haraguchi, K. Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym. J. 2011, 43, 223–241. [Google Scholar] [CrossRef]
- Ispas, G.-M.; Porav, S.; Gligor, D.; Turcu, R.; Crăciunescu, I. Magnetic hydrogel composites based on cross-linked poly (acrylic acid) used as a recyclable adsorbent system for nitrates. Water Environ. J. 2020, 34, 916–928. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Soleymani, M.; Etemadi, H.; Sabzi, M.; Atlasi, Z. Model protein BSA adsorption onto novel magnetic chitosan/PVA/laponite RD hydrogel nanocomposite beads. Int. J. Biol. Macromol. 2018, 107, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, W.; Shen, H.; Zhou, Z.; Li, M.; Su, P.; Yang, Y. Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability. Chem. Commun. 2019, 55, 2449–2452. [Google Scholar] [CrossRef]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magnetothermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef]
- Ram, N.R.; Prakash, M.; Naresh, U.; Kumar, N.S.; Sarmash, T.S.; Subbarao, T.; Kumar, R.J.; Kumar, G.R.; Naidu, K.C.B. Review on magnetocaloric effect and materials. J. Supercond. Nov. Magn. 2018, 31, 1971–1979. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of nanoparticles and nanomaterials in thermal ablation therapy of cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Z.; Liang, Y.; Huang, Z.; Rong, Z.; Xiong, J.; Wang, D. Pulse electromagnetic fields enhance the repair of rabbit articular cartilage defects with magnetic nano-hydrogel. RSC Adv. 2020, 10, 541–550. [Google Scholar] [CrossRef]
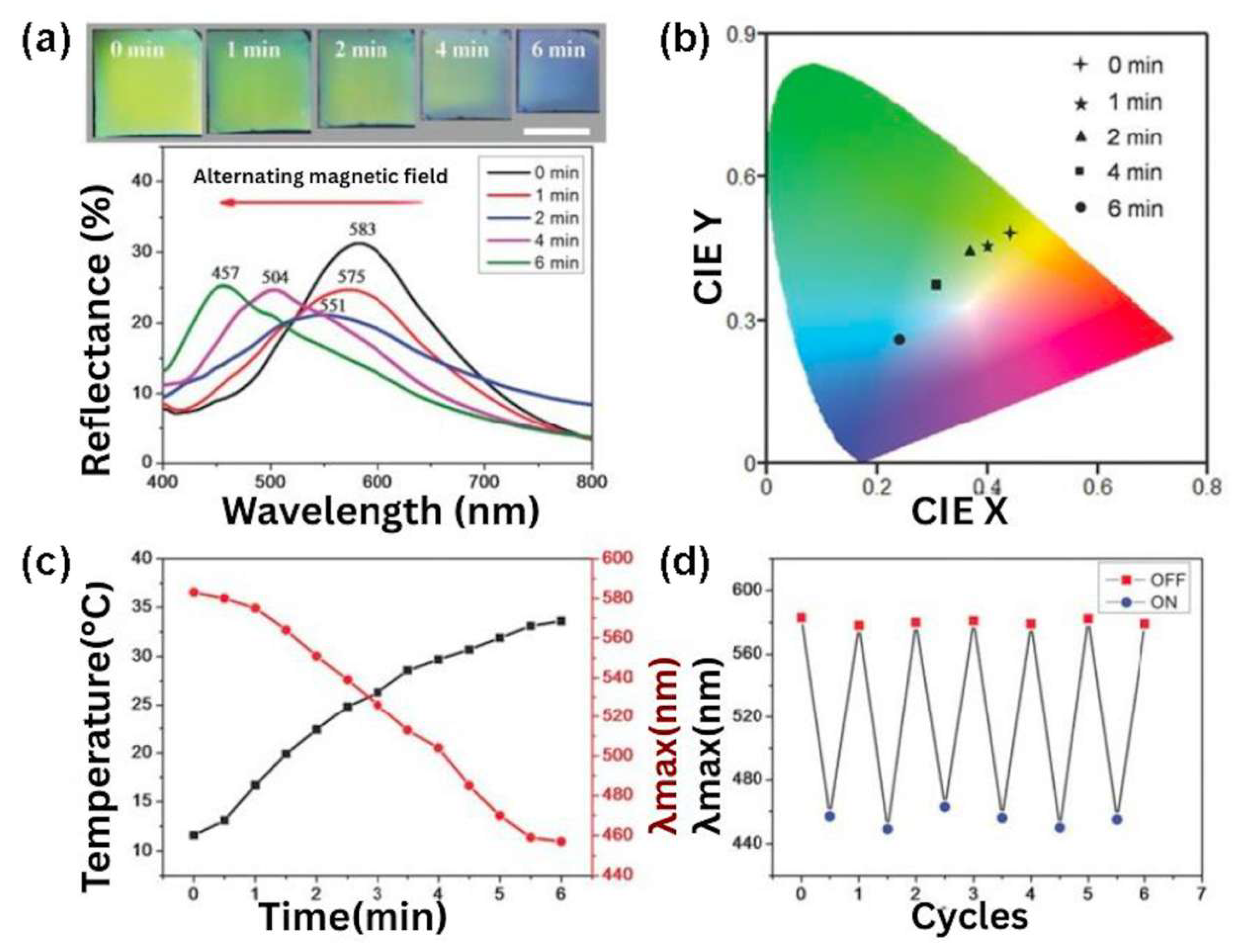
- Wang, W.; Fan, X.; Li, F.; Qiu, J.; Umair, M.M.; Ren, W.; Ju, B.; Zhang, S.; Tang, B. Magnetochromic photonic hydrogel for an alternating magnetic field-responsive color display. Adv. Opt. Mater. 2018, 6, 1701093. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the swelling-shrinkable behavior of hydrogels for biomedical applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Kamata, H.; Kushiro, K.; Takai, M.; Chung, U.-i.; Sakai, T. Non-osmotic hydrogels: A rational strategy for safely degradable hydrogels. Angew. Chem. Int. Ed. 2016, 55, 9282–9286. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci 2022, 23, 3665. [Google Scholar] [CrossRef]
- Rashidzadeh, B.; Shokri, E.; Mahdavinia, G.R.; Moradi, R.; Mohamadi-Aghdam, S.; Abdi, S. Preparation and characterization of antibacterial magnetic-/pH-sensitive alginate/Ag/Fe3O4 hydrogel beads for controlled drug release. Int. J. Biol. Macromol. 2020, 154, 134–141. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.-O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Kim, D.-i.; Lee, H.; Kwon, S.-h.; Choi, H.; Park, S. Magnetic nano-particles retrievable biodegradable hydrogel microrobot. Sens. Actuat. B Chem. 2019, 289, 65–77. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Smirnova, M.E.; Kessel, D.E.; Bedin, S.A.; Razumovskaya, I.V.; Philippova, O.E. Remotely self-healable, shapeable and pH-sensitive dual cross-linked polysaccharide hydrogels with fast response to magnetic field. Nanomaterials 2021, 11, 1271. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.-L.; Ma, H.; Xie, W.; Huang, S.; Wen, H.; Jing, G.; Zhao, L.; Liang, X.-J.; Fan, H.M. AMF responsive DOX-loaded magnetic microspheres: Transmembrane drug release mechanism and multimodality postsurgical treatment of breast cancer. J. Mater. Chem. B 2018, 6, 2289–2303. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Annabi, N.; Seidi, A.; Ramalingam, M.; Dehghani, F.; Kaji, H.; Khademhosseini, A. controlled release of drugs from gradient hydrogels for high-throughput analysis of cell–drug interactions. Anal. Chem. 2012, 84, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, J.; Liu, L.; Gong, C. Injectable hydrogel-based drug delivery systems for enhancing the efficacy of radiation therapy: A review of recent advances. Chin. Chem. Lett. 2024, 35, 109225. [Google Scholar] [CrossRef]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Jalili, N.A.; Muscarello, M.; Gaharwar, A.K. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng. Transl. Med. 2016, 1, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release. Cellulose 2019, 26, 6861–6877. [Google Scholar] [CrossRef]
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 nanoparticle composite hydrogels for non-invasive magnetic resonance imaging and magnetically assisted drug delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729. [Google Scholar] [CrossRef]
- Chen, Z.; Jian, W.; Huixiang, J.; Fang, Z. Magnetic nano-Fe3O4 particles targeted gathering and bio-effects on nude mice loading human hepatoma Bel-7402 cell lines model under external magnetic field exposure in vivo. Electromag. Biol. Med. 2015, 34, 309–316. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Soleimani, K.; Derakhshankhah, H.; Haghshenas, B.; Rezaei, A.; Massoumi, B.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. Multi-stimuli-responsive magnetic hydrogel based on Tragacanth gum as a de novo nanosystem for targeted chemo/hyperthermia treatment of cancer. J. Mater. Res. 2021, 36, 858–869. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, M.R.; de Almeida, E.A.M.S.; Pereira, A.G.B.; Muniz, E.C.; Tambourgi, E.B. One-pot synthesis of a chitosan-based hydrogel as a potential device for magnetic biomaterial. J. Magn. Magn. Mater. 2009, 321, 2636–2642. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achievements Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Moros, M.; Idiago-López, J.; Asín, L.; Moreno-Antolín, E.; Beola, L.; Grazú, V.; Fratila, R.M.; Gutiérrez, L.; de la Fuente, J.M. Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Adv. Drug Deliv. Rev. 2019, 138, 326–343. [Google Scholar] [CrossRef]
- Sumitha, N.S.; Krishna, N.G.; Sailaja, G.S. Chitosan-TEMPO-oxidized nanocellulose magnetic responsive patches with hyperthermia potential for smart melanoma therapy. ACS Appl. Polym. Mater. 2023, 5, 9170–9179. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, Z.; Wang, X.; Wang, H.; Zhai, H.; Ding, J.; Yu, L. Autophagy inhibition mediated via an injectable and NO-releasing hydrogel for amplifying the antitumor efficacy of mild magnetic hyperthermia. Bioact. Mater. 2024, 39, 336–353. [Google Scholar] [CrossRef]
- Barra, A.; Wychowaniec, J.K.; Winning, D.; Cruz, M.M.; Ferreira, L.P.; Rodriguez, B.J.; Oliveira, H.; Ruiz-Hitzky, E.; Nunes, C.; Brougham, D.F.; et al. Magnetic chitosan bionanocomposite films as a versatile platform for biomedical hyperthermia. Adv. Healthc. Mater. 2024, 13, 2303861. [Google Scholar] [CrossRef]
- Cheng, T.; Mishkovsky, M.; Junk, M.J.N.; Münnemann, K.; Comment, A. Producing radical-free hyperpolarized perfusion agents for in vivo magnetic resonance using spin-labeled thermoresponsive hydrogel. Macromol. Rapid Commun. 2016, 37, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
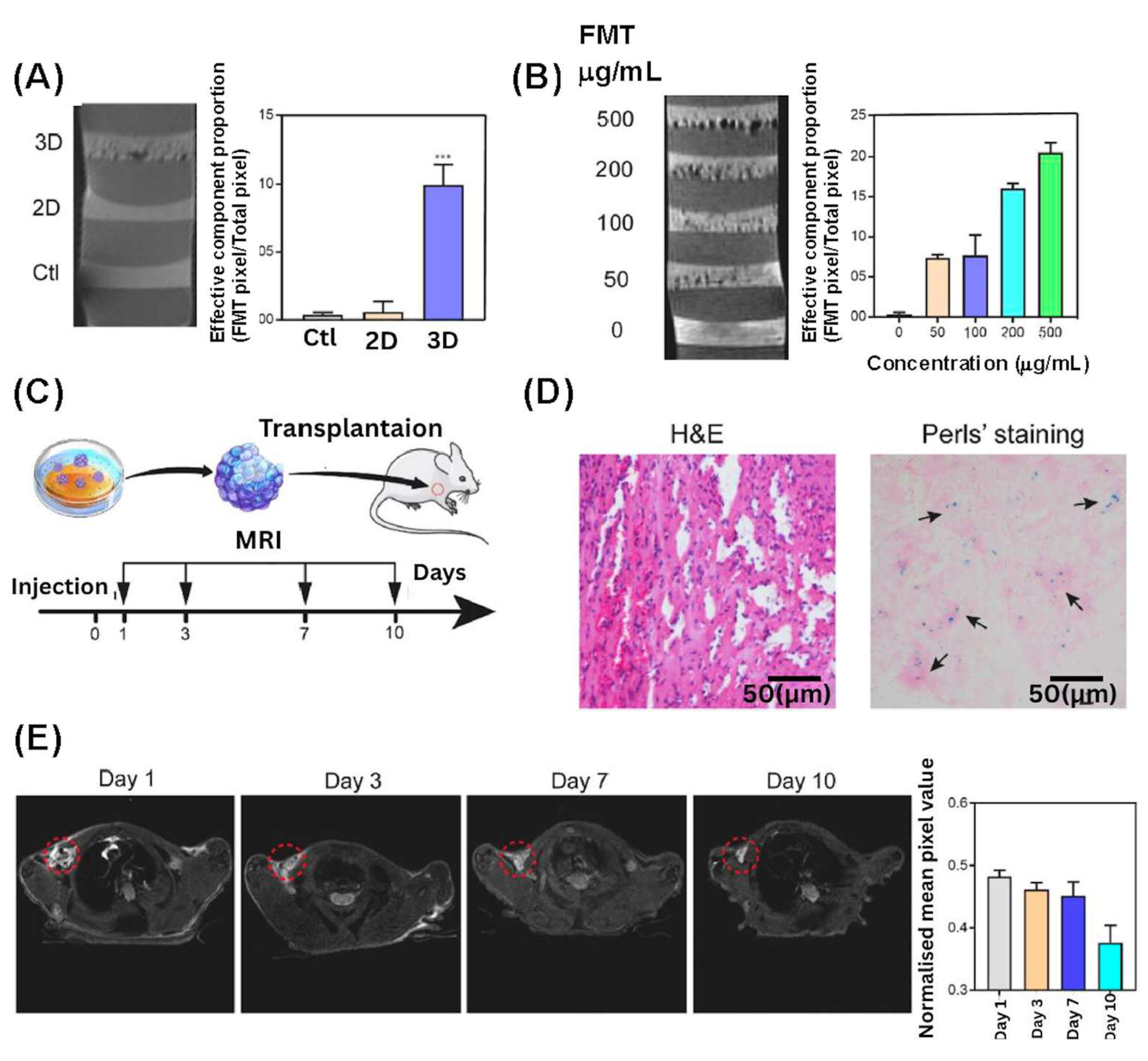
- Yan, S.; Hu, K.; Zhang, M.; Sheng, J.; Xu, X.; Tang, S.; Li, Y.; Yang, S.; Si, G.; Mao, Y.; et al. Extracellular magnetic labeling of biomimetic hydrogel-induced human mesenchymal stem cell spheroids with ferumoxytol for MRI tracking. Bioact. Mater. 2023, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Mistral, J.; Ve Koon, K.T.; Fernando Cotica, L.; Sanguino Dias, G.; Aparecido Santos, I.; Alcouffe, P.; Milhau, N.; Pin, D.; Chapet, O.; Serghei, A.; et al. Chitosan-coated superparamagnetic Fe3O4 nanoparticles for magnetic resonance imaging, magnetic hyperthermia, and drug delivery. ACS Appl. Nano Mater. 2024, 7, 7097–7110. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in hydrogels for skin wound repair. Macromol. Biosci. 2022, 22, 2100475. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and future directions in the antibacterial wound dressings–A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 703–716. [Google Scholar] [CrossRef]
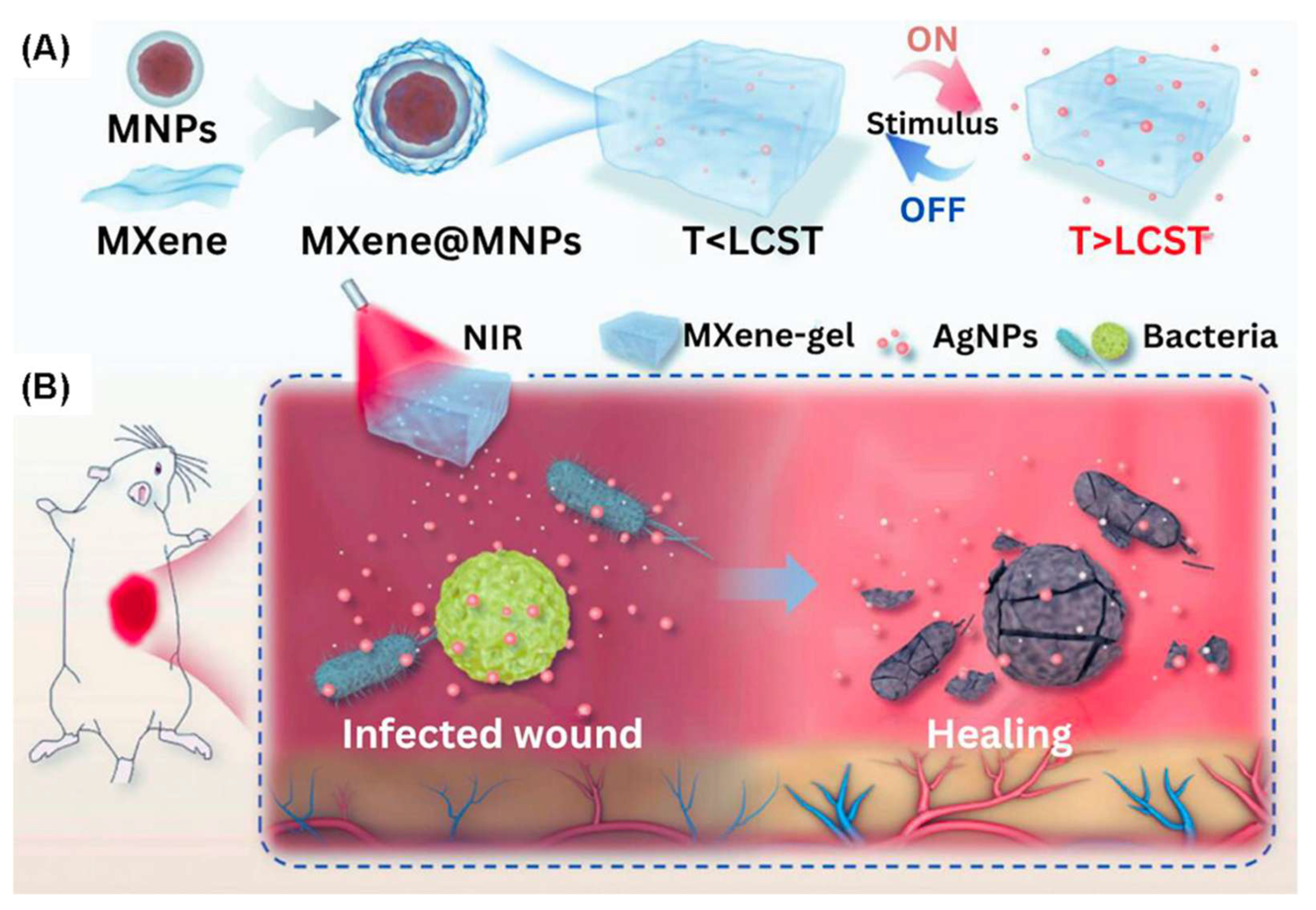
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple stimuli-responsive MXene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef]
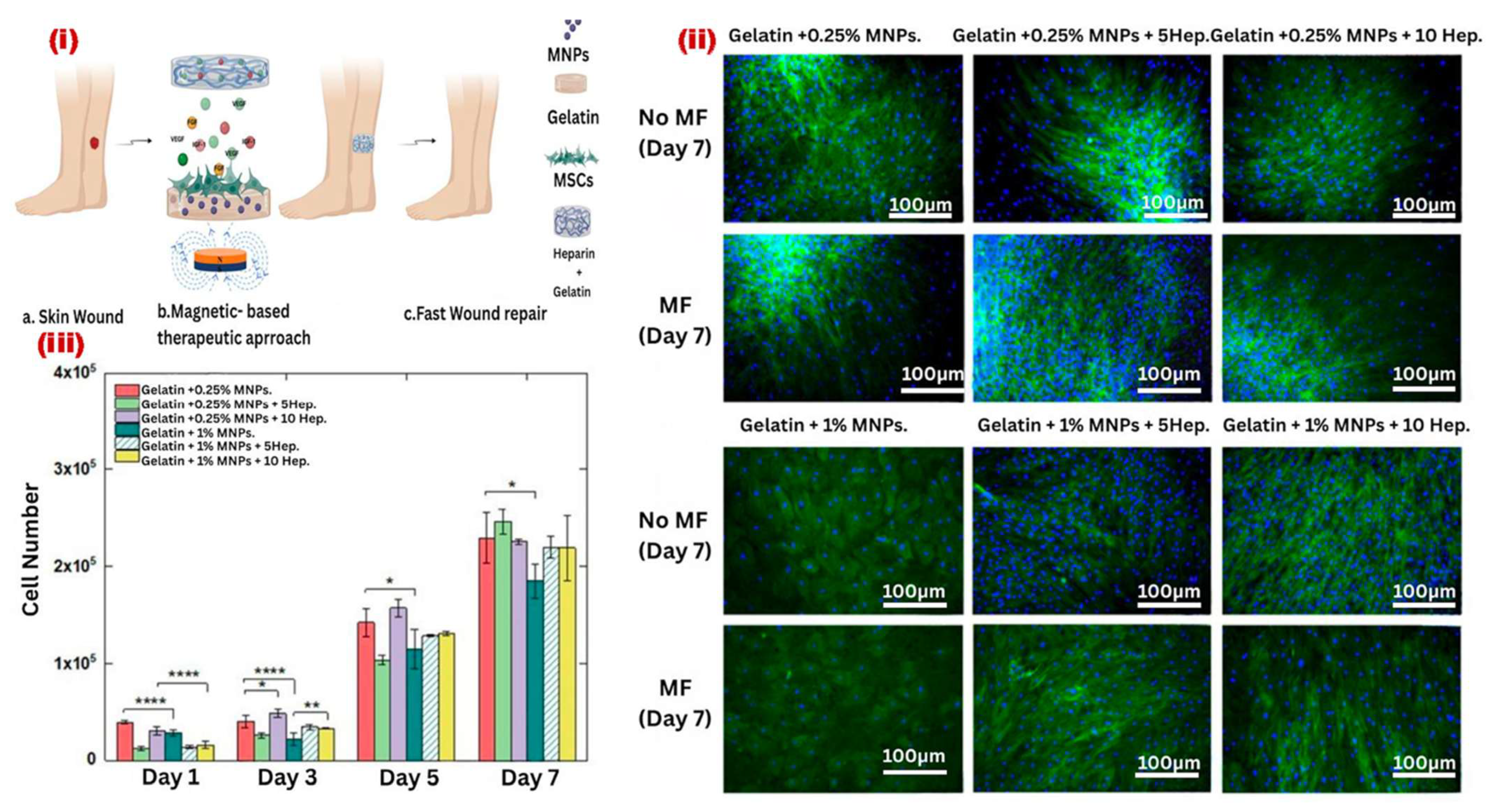
- Pires, F.; Silva, J.C.; Ferreira, F.C.; Portugal, C.A. Heparinized acellular hydrogels for magnetically induced wound healing applications. ACS Appl. Mater. Interfaces 2024, 16, 9908–9924. [Google Scholar] [CrossRef]
- Li, X.; Tan, Z.; Guo, B.; Yu, C.; Yao, M.; Liang, L.; Wu, X.; Zhao, Z.; Yao, F.; Zhang, H.; et al. Magnet-oriented hydrogels with mechanical–electrical anisotropy and photothermal antibacterial properties for wound repair and monitoring. Chem. Eng. J. 2023, 463, 142387. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Z.; Gao, Y.; Wu, P.; Lai, J.; Li, S.; Jin, X.; Liu, H.; Chen, W.; Wu, Y.; et al. Thermoresponsive, magnetic, adhesive and conductive nanocomposite hydrogels for wireless and non-contact flexible sensors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128113. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Hori, T.; Nashimoto, Y.; Shi, X.; Kaji, H. Molecularly imprinted polymer-based sensors for the detection of skeletal- and cardiac-muscle-related analytes. Sensors 2023, 23, 5625. [Google Scholar] [CrossRef] [PubMed]
- Banan Sadeghian, R.; Han, J.; Ostrovidov, S.; Salehi, S.; Bahraminejad, B.; Ahadian, S.; Chen, M.; Khademhosseini, A. Macroporous mesh of nanoporous gold in electrochemical monitoring of superoxide release from skeletal muscle cells. Biosens. Bioelectron. 2017, 88, 41–47. [Google Scholar] [CrossRef]
- Sadeghian, R.B.; Ostrovidov, S.; Han, J.; Salehi, S.; Bahraminejad, B.; Bae, H.; Chen, M.; Khademhosseini, A. Online monitoring of superoxide anions released from skeletal muscle cells using an electrochemical biosensor based on thick-film nanoporous gold. ACS Sens. 2016, 1, 921–928. [Google Scholar] [CrossRef]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional hydrogels and their application in drug delivery, biosensors, and tissue engineering. Int. J. Polym. Sci. 2019, 2019, 3160732. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.S.; Kim, M.I. Poly-γ-glutamic acid/chitosan hydrogel nanoparticles entrapping glucose oxidase and magnetic nanoparticles for glucose biosensing. J. Nanosci. Nanotechnol. 2020, 20, 5333–5337. [Google Scholar] [CrossRef]
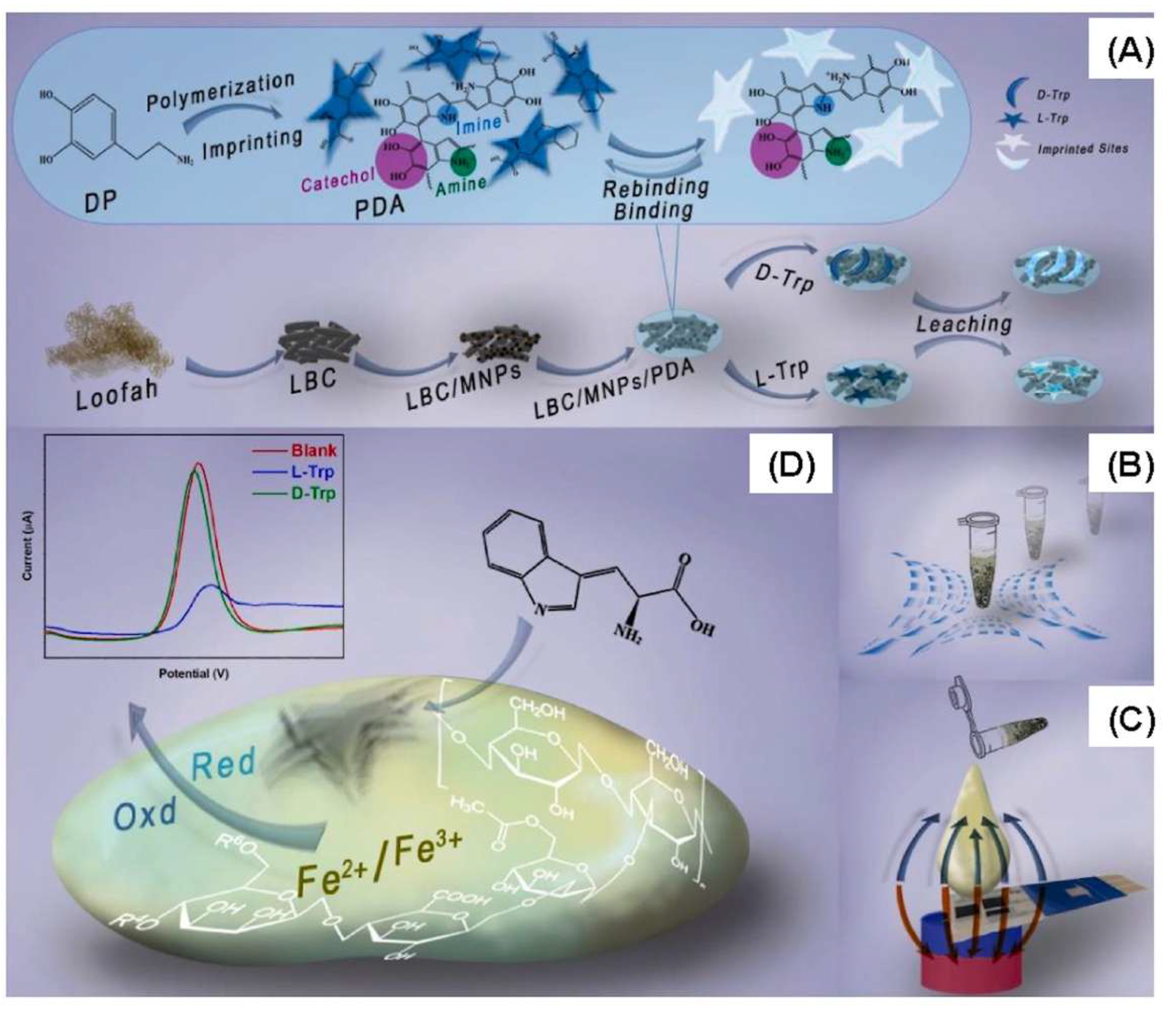
- Hosseini, F.; Dashtian, K.; Golzani, M.; Ejraei, Z.; Zare-Dorabei, R. Remote magnetically stimulated xanthan-biochar-Fe3O4-molecularly imprinted biopolymer hydrogel toward electrochemical enantioselection of l-tryptophan. Anal. Chim. Acta 2024, 1316, 342837. [Google Scholar] [CrossRef]
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for neural tissue engineering. Front. Nanotechnol. 2021, 3, 643507. [Google Scholar] [CrossRef]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Di Carlo, D. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Lacko, C.S.; Singh, I.; Wall, M.A.; Garcia, A.R.; Porvasnik, S.L.; Rinaldi, C.; Schmidt, C.E. Magnetic particle templating of hydrogels: Engineering naturally derived hydrogel scaffolds with 3D aligned microarchitecture for nerve repair. J. Neural Eng. 2020, 17, 016057. [Google Scholar] [CrossRef]
- Li, K.; Ye, D.; An, Z.; Xu, J.; Sun, X.; Liu, M.; Li, P. Nerve tissue regeneration based on magnetic and conductive bifunctional hydrogel scaffold. Mater. Today Commun. 2024, 39, 109120. [Google Scholar] [CrossRef]
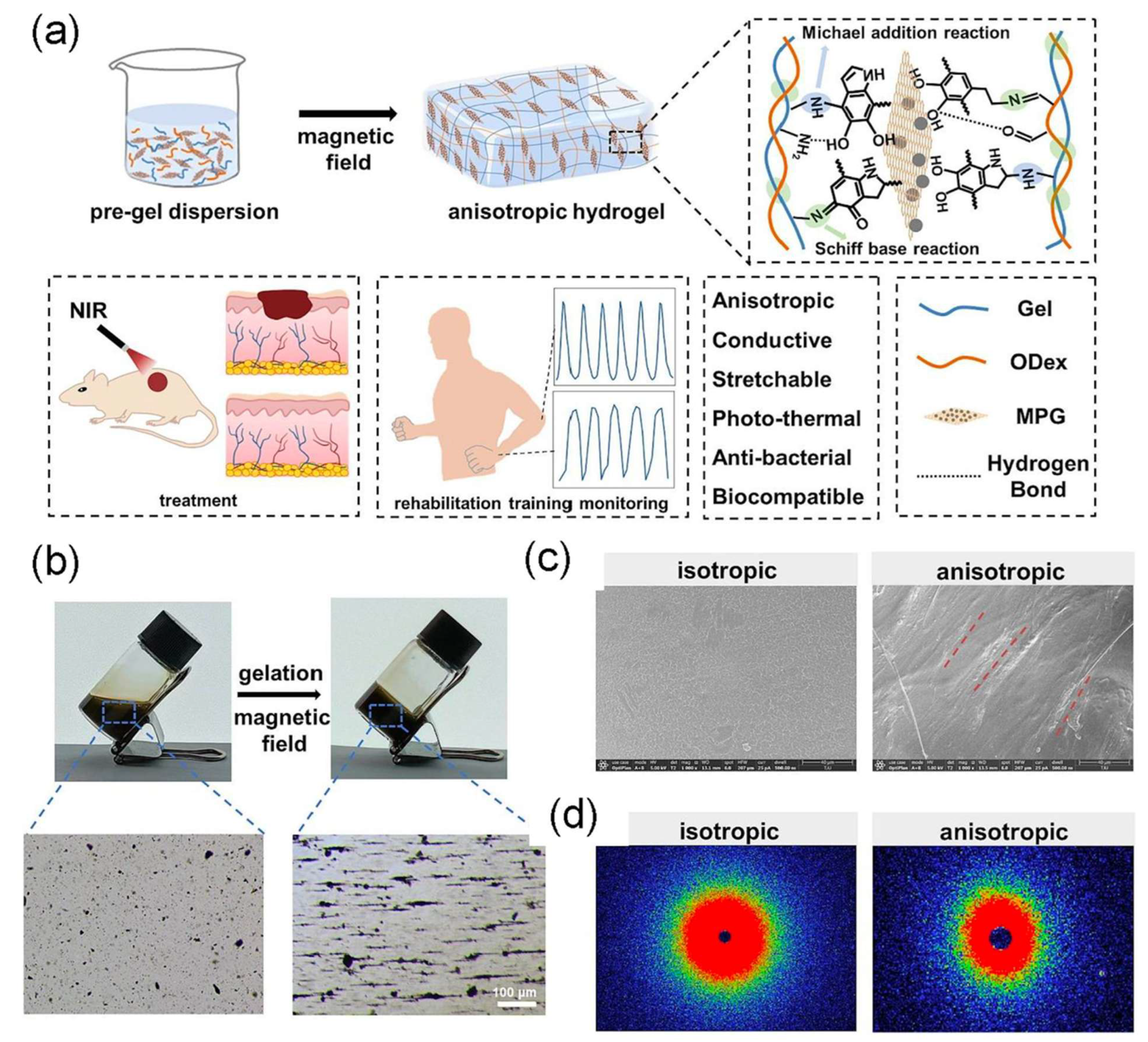
- Han, Q.; Guan, W.; Sun, S.; Zheng, T.; Wu, L.; Gao, H.; Liu, Y.; Yang, Y.; Li, G. Anisotropic topological scaffolds synergizing non-invasive wireless magnetic stimulation for accelerating long-distance peripheral nerve regeneration. Chem. Eng. J. 2024, 496, 153809. [Google Scholar] [CrossRef]
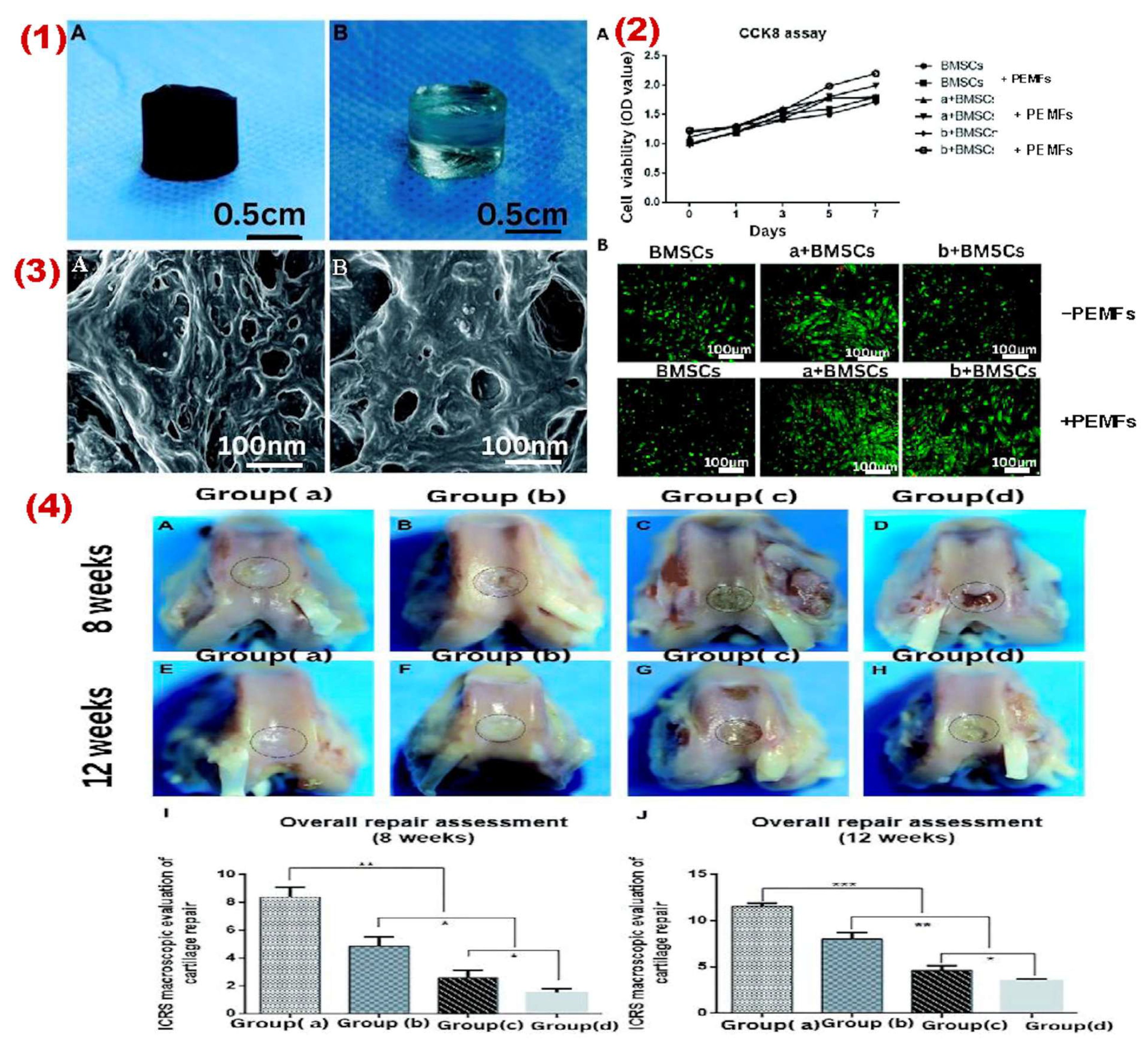
- Taghizadeh, S.; Tayebi, L.; Akbarzadeh, M.; Lohrasbi, P.; Savardashtaki, A. Magnetic hydrogel applications in articular cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2024, 112, 260–275. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, C.; Yan, S.; Liu, Q.; Hou, M.; Xu, Y.; Guo, R. Non-invasive monitoring of in vivo hydrogel degradation and cartilage regeneration by multiparametric MR imaging. Theranostics 2018, 8, 1146–1158. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Huang, H.; Zheng, Y.; Liu, J.; Feng, L.; Guo, H.; Tang, S.; Guo, R. Functionalization of novel theranostic hydrogels with kartogenin-grafted USPIO nanoparticles to enhance cartilage regeneration. ACS Appl. Mater. Interfaces 2019, 11, 34744–34754. [Google Scholar] [CrossRef] [PubMed]
- Zlotnick, H.M.; Clark, A.T.; Gullbrand, S.E.; Carey, J.L.; Cheng, X.M.; Mauck, R.L. Magneto-driven gradients of diamagnetic objects for engineering complex tissues. Adv. Mater. 2020, 32, 2005030. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, C.; Kim, H.S.; Moon, C.; Lee, K.Y. 3D Printing of dynamic tissue scaffold by combining self-healing hydrogel and self-healing ferrogel. Colloids Surf. B Biointerfaces 2021, 208, 112108. [Google Scholar] [CrossRef]
- Alford, A.I.; Kozloff, K.M.; Hankenson, K.D. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 2015, 65, 20–31. [Google Scholar] [CrossRef]
- Katsamenis, O.L.; Chong, H.M.; Andriotis, O.G.; Thurner, P.J. Load-bearing in cortical bone microstructure: Selective stiffening and heterogeneous strain distribution at the lamellar level. J. Mech. Behav. Biomed. Mater. 2013, 17, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, M.; Ardeshirylajimi, A.; Maghsoudi, H.; Azadian, E. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J. Cell Physiol. 2018, 233, 1061–1070. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Zheng, L.; Cai, H.; Yang, X.; Xue, Y.; Wan, Q.; Chen, J.; Li, Y. Magnetic scaffold constructing by micro-injection for bone tissue engineering under static magnetic field. J. Mater. Res. Technol. 2024, 29, 3554–3565. [Google Scholar] [CrossRef]
- Babakhani, A.; Peighambardoust, S.J.; Olad, A. Fabrication of magnetic nanocomposite scaffolds based on polyvinyl alcohol-chitosan containing hydroxyapatite and clay modified with graphene oxide: Evaluation of their properties for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2024, 150, 106263. [Google Scholar] [CrossRef]
- Bolonduro, O.A.; Duffy, B.M.; Rao, A.A.; Black, L.D.; Timko, B.P. From biomimicry to bioelectronics: Smart materials for cardiac tissue engineering. Nano Res. 2020, 13, 1253–1267. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.-T.; et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99A, 376–385. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.-C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Bonfrate, V.; Manno, D.; Serra, A.; Salvatore, L.; Sannino, A.; Buccolieri, A.; Serra, T.; Giancane, G. Enhanced electrical conductivity of collagen films through long-range aligned iron oxide nanoparticles. J. Colloid Interface Sci. 2017, 501, 185–191. [Google Scholar] [CrossRef]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-folded hydrogel tubes for implantable muscular tissue scaffolds. Macromol. Biosci. 2018, 18, 1700377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, K.; Chang, Q.; Darabi, M.A.; Lin, B.; Zhong, W.; Xing, M. Highly flexible and resilient elastin hybrid cryogels with shape memory, injectability, conductivity, and magnetic responsive properties. Adv. Mater. 2016, 28, 7758–7767. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, B.; Shin, J.-Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J.-S.; Lee, Y.; Lee, N.; Hyeon, T.; et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhu, K.; Sun, X.-N.; Liu, C.-G.; Wang, S.-B.; Chen, A.-Z. Cardiac tissue engineering on the nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef]
- Nazari, H.; Heirani-Tabasi, A.; Hajiabbas, M.; Salimi Bani, M.; Nazari, M.; Pirhajati Mahabadi, V.; Rad, I.; Kehtari, M.; Ahmadi Tafti, S.H.; Soleimani, M. Incorporation of SPION-casein core-shells into silk-fibroin nanofibers for cardiac tissue engineering. J. Cell. Biochem. 2020, 121, 2981–2993. [Google Scholar] [CrossRef]
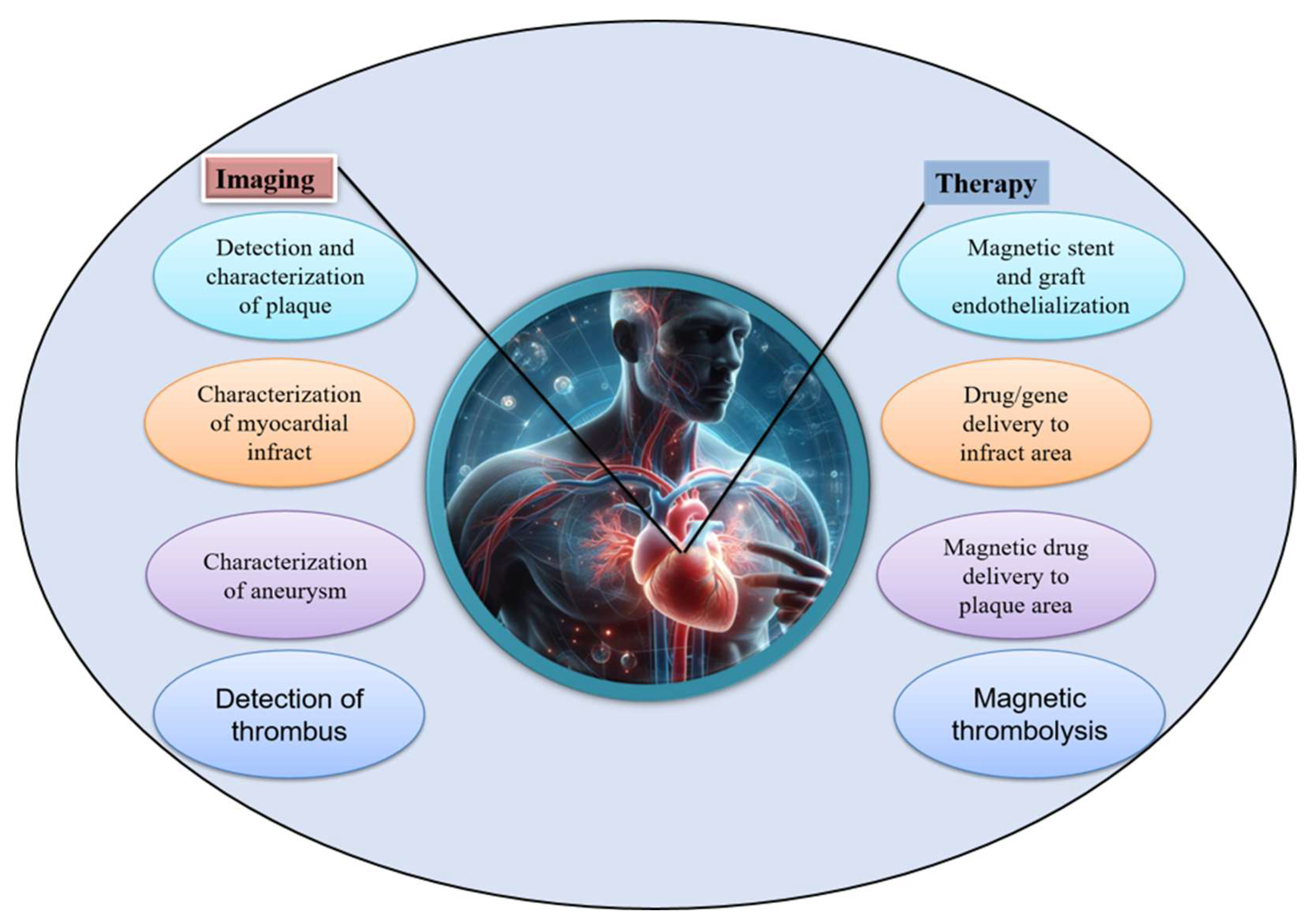
- Cicha, I.; Alexiou, C. Cardiovascular applications of magnetic particles. J. Magn. Magn. Mater. 2021, 518, 167428. [Google Scholar] [CrossRef]
- Ma, H.; Yu, G.; Cheng, J.; Song, L.; Zhou, Z.; Zhao, Y.; Zhao, Q.; Liu, L.; Wei, X.; Yang, M. Design of an injectable magnetic hydrogel based on the tumor microenvironment for multimodal synergistic cancer therapy. Biomacromolecules 2023, 24, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, P.; Zou, Y.; Zhang, S.; Huang, C.; Liu, L.; Yu, J.; Fan, Y. One-step preparation of Fe3O4/nanochitin magnetic hydrogels with remolding ability by ammonia vapor diffusion gelation for osteosarcoma therapy. Biomacromolecules 2022, 23, 1314–1325. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Lu, M.; Etxeberria, A.E.; Zhou, Y.; Gu, P.; Song, P.; Min, L.; Luo, Y.; Nand, A.V.; et al. Dual-cross-linked magnetic hydrogel with programmed release of parathyroid hormone promotes bone healing. ACS Appl. Mater. Interfaces 2023, 15, 35815–35831. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, E.C.; Wickramasinghe, S.; Ju, M.; Uppaluri, S.; Klika, A.; George, J.; Barsoum, W.; Frangiamore, S.J.; Higuera-Rueda, C.A.; Samia, A.C.S. Magnetic glycol chitin-based hydrogel nanocomposite for combined thermal and d-amino-acid-assisted biofilm disruption. ACS Infect. Dis. 2018, 4, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Mañas-Torres, M.C.; Gila-Vilchez, C.; Vazquez-Perez, F.J.; Kuzhir, P.; Momier, D.; Scimeca, J.-C.; Borderie, A.; Goracci, M.; Burel-Vandenbos, F.; Blanco-Elices, C.; et al. Injectable magnetic-responsive short-peptide supramolecular hydrogels: Ex vivo and in vivo evaluation. ACS Appl. Mater. Interfaces 2021, 13, 49692–49704. [Google Scholar] [CrossRef]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic nanocomposite hydrogel for potential cartilage tissue engineering: Synthesis, characterization, and cytocompatibility with bone marrow derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Meng, Z.; Yang, K.; He, Z.; Hou, Z.; Yang, J.; Lu, J.; Cao, Z.; Yang, S.; Chai, Y.; et al. External magnetic field non-invasively stimulates spinal cord regeneration in rat via a magnetic-responsive aligned fibrin hydrogel. Biofabrication 2023, 15, 035022. [Google Scholar] [CrossRef]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Li, K.; Xu, J.; Liu, M.; Fan, Y. The effect of magnetic poly (lactic-co-glycolic acid) microsphere-gelatin hydrogel on the growth of pre-osteoblasts under static magnetic field. J. Biomed. Nanotechnol. 2020, 16, 1658–1666. [Google Scholar] [CrossRef]
- Silva, E.D.; Babo, P.S.; Costa-Almeida, R.; Domingues, R.M.A.; Mendes, B.B.; Paz, E.; Freitas, P.; Rodrigues, M.T.; Granja, P.L.; Gomes, M.E. Multifunctional magnetic-responsive hydrogels to engineer tendon-to-bone interface. Nanomedicine NBM 2018, 14, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, J.; Hu, Y.; Xia, Z.; Cai, K. Osteogenic differentiation of the MSCs on silk fibroin hydrogel loaded Fe3O4@PAA NPs in static magnetic field environment. Colloids Surf. B Biointerfaces 2022, 220, 112947. [Google Scholar] [CrossRef]
- Zou, Y.-P.; Liang, H.-F.; Wang, B.; Zhang, Q.-C.; Su, D.-H.; Lu, S.-Y.; Zhang, Q.-Y.; Wu, T.; Xiao, L.; Xiao, Y.; et al. Precipitation-based silk fibroin fast gelling, highly adhesive, and magnetic nanocomposite hydrogel for repair of irregular bone defects. Adv. Funct. Mater. 2023, 33, 2302442. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Thirupathi, K.; Kumar, S.S.D.; Pandiaraj, S.; Rahaman, M.; Phan, T.T.V.; Kim, S.-C. k-Carrageenan based magnetic@polyelectrolyte complex composite hydrogel for pH and temperature-responsive curcumin delivery. Int. J. Biol. Macromol. 2023, 244, 125467. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Mohammadi, A.; Aghamirza Moghim Aliabadi, H.; Kashtiaray, A.; Bani, M.S.; Karimi, A.H.; Maleki, A.; Mahdavi, M. A novel ternary magnetic nanobiocomposite based on tragacanth-silk fibroin hydrogel for hyperthermia and biological properties. Sci. Rep. 2024, 14, 8166. [Google Scholar] [CrossRef] [PubMed]
- Carrelo, H.; Escoval, A.R.; Vieira, T.; Jiménez-Rosado, M.; Silva, J.C.; Romero, A.; Soares, P.I.P.; Borges, J.P. Injectable thermoresponsive microparticle/hydrogel system with superparamagnetic nanoparticles for drug release and magnetic hyperthermia applications. Gels 2023, 9, 982. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M.; Zhang, J.; Dong, W. Design of a pH-sensitive magnetic composite hydrogel based on salecan graft copolymer and Fe3O4@SiO2 nanoparticles as drug carrier. Int. J. Biol. Macromol. 2018, 107, 1811–1820. [Google Scholar] [CrossRef]







| Strategy | How It Works (for Magnetic Gels) | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|
| In situ gelation | Gel forms directly at target site (pH, temperature, ionic, or light-responsive) with embedded magnetic nanoparticles. | Minimally invasive, injectable, conforms to defects, easy drug loading. | MNP dispersion control is difficult, risk of burst release, uniformity issues. | Injectable depots, localized hyperthermia, image-guided therapies. | [59,60] |
| Freeze-drying (cryogels/aerogels) | Polymer-MNPs slurry frozen; ice templating + sublimation gives porous, magnetic scaffold. | Very high porosity, good permeability, tunable pore size, easy drug loading | Brittle, variable mechanics, sterilization issues, possible MNPs shedding. | Bone/tissue scaffolds, wound dressings, on-demand thermal release. | [61] |
| Electrospinning | Electric field spins MNPs-loaded polymer jets into nanofibers/fiber mats. | High surface area, anisotropy, tunable alignment, good for directional cell growth. | Fabrication is often confined to thin layers, with concerns over solvent biocompatibility and aggregation of magnetic nanoparticles affecting uniformity. | Nerve/muscle/ cartilage scaffolds, guided regeneration. | [62,63] |
| 3D bioprinting | Extrusion/DLP of MNPs-laden bioinks into ordered, complex constructs. | Patient-specific structures, gradient/multi-material capability, good cell viability. | Balancing print accuracy with nanoparticle concentration is challenging, and cells may experience shear stress during extrusion. | Custom tissue scaffolds, drug depots, actuators. | [55,64] |
| Micropatterning | Lithographic or photopatterning techniques generate defined spatial architectures while embedding magnetic domains in targeted regions | Enables high spatial resolution and patterning, compatibility with bioelectronic systems, and delivery of site-specific biological signals | Primarily limited to 2D or thin constructs, with low fabrication throughput and demanding equipment requirements | In vitro models, patterned scaffolds, bioelectronic interfaces. | [65] |
| Microfluidics | Using flow focusing, microfluidic systems can reliably fabricate magnetic microgels and fibers with controlled and consistent sizes. | Highly uniform particles with superior encapsulation capacity and versatile modular assembly. | Challenges include clogging of devices, restricted surfactant compatibility, and difficulties in large-scale translation. | Injectable microgels, targeted drug delivery, modular tissues. | [66,67] |
| Field-assisted templating (ordering) | External magnetic field aligns MNPs/fibers during gelation to create anisotropic gels. | Mimics natural anisotropy, improves guidance cues, enhances actuation. | Risk of MNPs clustering; requires specialized hardware. | Cartilage, tendon, neural scaffolds, actuators. | [36] |
| Hydrogel | MNPs | Additional Properties | Application |
|---|---|---|---|
| Poly (acrylic acid-co-vinyl sulfonic acid) PAAVSA/Fe3O4 Hydrogel | Fe3O4 Magnetic nanoparticles (MNPs) | The pH reversibility of the PAAVSA/Fe3O4 hydrogel was further examined in the views of the swelling/deswelling cycling for the given set of characteristic pH numeric, pH 4.1–7. | pH-responsive hydrogel for healthcare applications including drug delivery systems, diagnosis of diseases, and biosensors [21]. |
| Gelatin methacrylate/Fe3O4 magnetic hydrogel | Fe3O4 MNPs (0–15 mg/mL) | Several passive magnetic-based devices have shown potentials in wireless biomechanical monitoring in terms of high sensitivity and non-contact sensing, but these devices suffer from issues with mechanical properties, biocompatibility, and sensitivity contradictions. | The developed GelMA/Fe3O4 magnetic hydrogels’ mechanical properties are close to natural tissue, and they have a stable sensing capacity for minor strains in ionic solution for long-term monitoring [19]. |
| Oxidized hydroxypropyl cellulose with carboxymethyl chitosan, an injectable hydrogel | Fe3O4 MNPs (15 mg/mL) | The chemotherapeutic agent Artemisinin (ART) was integrated into the three-dimensional network architecture of the Nanoparticle-hydrogel (NP-hydrogel) to fabricate the ARTNP-hydrogel, facilitating targeted cancer therapeutic interventions. | This composite hydrogel has multiple functions, including magnetic targeting, pH sensitivity, chemodynamic therapy, and photothermal response [196]. |
| Magnetize deacetylated chitin nanofibers (M-DEChNs) hydrogel | Fe3O4 (0–89.2 mM/L) | This M-DEChN showed cytocompatibility against ATDC-5 cells and could be heated in AMF to kill osteosarcoma in vitro/in vivo by the temperature. | The gel synthesized in the present study demonstrates remolding capacity, biocompatibility, and exhibits antitumor properties, rendering it applicable as a tumoricidal agent or as an adjunctive treatment following tumor resection [197]. |
| Xanthan gum/Fe3O4-based drug-loaded magnetic nanoparticle composite hydrogel | Fe3O4 MNPs (0 (w/w) and 10% (w/w) of the mass of the polymer) | In addition to an enhanced activity of the drug-loaded hydrogel compared to the free drug, results showed that the application of an alternating magnetic field efficiently stimulated a 3-fold faster release of the encapsulated drug compared to passive conditions, whereas a concentration-dependent shortening of the water protons’ relaxation time at a clinical field of 3 T confirmed this magnetic hydrogel as a T2-MRI contrast enhancer. | XG/Fe3O4 magnetic nanoparticle composite hydrogels represent a novel generation of multifunctional theranostic platforms designed for injection and implantation across various clinical contexts, including postoperative applications in oncology, wound healing in dermatology, and in dental practices, among others [136]. |
| GelMA–PVA magnetic (GPM) | IONs (1, 5, and 10% w/v) | An in vitro cytocompatibility test showed that all formulations were biocompatible and that PTH addition significantly promoted the proliferation of MC3T3-E1 pre-osteoblasts. | This recently formulated GPMP sample facilitates simultaneous osteogenic effects through the controlled release of PTH and magnetically mediated bone regeneration, demonstrating potential in enhancing bone healing and addressing various delayed or non-union conditions without the necessity of daily injections [198]. |
| Magnetic Glycol Chitin-Based Hydrogel | Fe3O4 MNPs (250–750 μg Fe/mL) | The prepared hydrogel nanocomposite was nontoxic toward HeLa cells when exposed for 2 h, in contrast to similar concentrations of antibiotics used in the clinical setting (i.e., vancomycin). | This pioneering treatment methodology, enabled through the utilization of nanocomposites, exhibits substantial potential for addressing chronic infections associated with bacterial biofilm proliferation, which is often correlated with persistent external wounds in bedridden patients and individuals suffering from chronic diabetic foot conditions [199]. |
| Fmoc(fluorenylmethoxycarbonyl)-RGD (arginine–glycine–aspartic acid)/MNP hydrogel | Fe3O4 MNPs (0.1 vol % of sample). | In the current investigation, the conjunction of cellular components and magnetic nanoparticles exhibited a synergistic influence in mitigating degradation within magnetic peptide hydrogels. | This research introduces an innovative methodology aimed at enhancing the physical and mechanical characteristics of supramolecular hydrogels through the integration of magnetic nanoparticles, which provide structural reinforcement and stability, enable remote actuation via magnetic fields, and improve injectability [200]. |
| Hybrid hydrogel containing type II collagen, hyaluronic acid (HA), and polyethylene glycol (PEG) and incorporated magnetic nanoparticles hydrogels (collagen II-HA-PEG hydrogel) | MNPs (10 mg/mL) | In addition, the presence of magnetic nanoparticles did not affect the viability of BMSCs within 24 h of culture when compared with the control group. | This investigation presents a promising magnetically responsive nanocomposite hydrogel for prospective applications in cartilage tissue engineering, warranting further examination of its impact on cellular functions when synergistically combined with electromagnetic stimulation [201]. |
| Magnetic-Responsive PVA Hydrogels | MNPs (0.25% to 1% v/v of PVA solution) | The extent of reversibility in protein sorption–desorption processes was observed to enhance with a reduction in magnetic field intensity to 0.45 Tesla. | The advancement of bioseparation systems characterized by superior performance, specifically those exhibiting decreased susceptibility to biofouling, as well as the design of magnetically controlled drug delivery systems, biosensors, and tissue engineering devices endowed with enhanced efficiency [10]. |
| Magnetic-responsive aligned fibrin hydrogel (MAFG) | Fe3O4 MNPs (10 mg/mL) | A comparative analysis revealed a diminished cell proliferation rate within the initial three days of culture for the MAFG group relative to the AFG group, while the cell counts for MAFG and AFG after five days of culture exhibited no statistically significant difference. | MAFG@MF facilitates axonal regrowth and promotes functional neuronal regeneration, thereby significantly contributing to the restoration of motor function following spinal cord injury [202]. |
| Alginate-magnetic short nanofibers 3D composite hydrogel | SPIONs 10% w/w of polymer | The magnetic SNF/hydrogels demonstrated a notably elevated expression of the neuron-like cell marker β-tubulin III in comparison to their non-magnetic counterparts, indicating that the magnetic characteristics of the composite hydrogel can foster neural-like differentiation of Olfactory Epithelial–Mesenchymal Stem Cells (OE-MSCs). | The alginate-magnetic short nanofibers 3D composite hydrogel enhances the bioactivity of encapsulated human olfactory mucosa stem cells, presenting promising prospects for nerve regeneration applications [203]. |
| Magnetic PLGA Microsphere-Gelatin Hydrogel | Fe3O4 MNPs (200, 400, and 800 mg/L) | GelFe3O4-400 had the best effect on promoting the growth of pre-osteoblasts under 20 mT static magnetic field in this experiment. | The magnetic poly(lactic-co-glycolic acid) microsphere-gelatin hydrogel exhibits remarkable application potential in promoting osteogenesis and facilitating bone repair [204]. |
| Methacrylate–chondroitin sulfate magnetic nanoparticles (MA-CS MNPs) hydrogel | Fe3O4 MNPs (2% (w/v)) | The impact of electromagnetic field (EMF) stimulation was also evaluated, revealing its capacity to modulate cellular responses, thereby demonstrating the feasibility of generating gradient tissue constructs through magnetic responsive hydrogels. | The proposed hydrogel system facilitated the development of a tendon-to-bone interface model for the investigation of cellular crosstalk [205]. |
| Silk Fibroin hydrogel-loaded Fe3O4@PAA NPs | Fe3O4 NPs(0.8 mg/mL) | Fe3O4@PAA Silk Fibroin (SF) hydrogel exhibits hydrogen peroxide scavenging activity. | Silk fibroin hydrogel incorporated with Fe3O4@PAA nanoparticles in a static magnetic field environment promotes osteogenic differentiation [206]. |
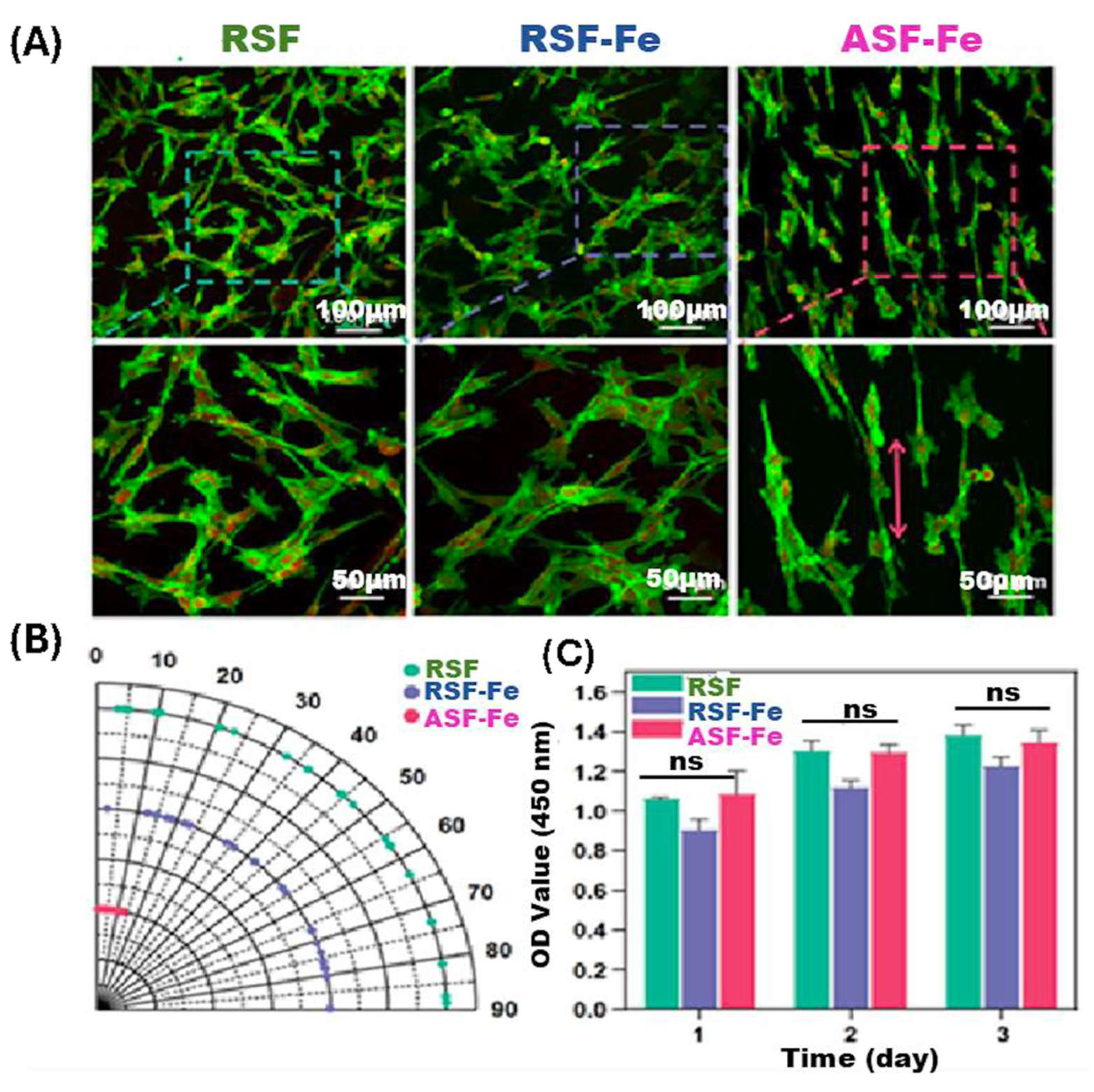
| RSF/TA/Fe3O4 Hydrogel | Fe3O4 (1%–5% w/v) | The RSF/TA/Fe3O4 hydrogel demonstrates adequate adhesion within biological microenvironments and exhibits a robust osteogenic effect both in vitro and in vivo when subjected to an external static magnetic field (SMF), thereby rendering it applicable for the repair of critical-sized bone defects. | A methodical approach for the development of a rapid-gelling, shape-adaptive, highly adhesive, and magnetically responsive nanocomposite hydrogel via the precipitation technique has been established, presenting a promising biomaterial for tissue engineering aimed at facilitating the repair of irregular bone defects in the foreseeable future [207]. |
| Alginate/poly-l-ornithine/gelatin (alginate-PLO-gelatin) hydrogel | Fe3O4 (16.67 μg/mL) | The differentiation of endothelial progenitor cells (EPCs) into endothelial cells was substantiated, and their capacity to secrete pro-angiogenic growth factors was found to significantly enhance both cell migration and vascularization. | The augmented regeneration of blood vessels in the injured region through the administration of EPCs affixed to the hydrogel sheet indicates that the proposed system possesses substantial potential as a therapeutic modality for tissue regeneration [78]. |
| k-Carrageenan based magnetic@polyelectrolyte complex composite hydrogel | FeNP (0.05 wt% of total volume of PHMG) | Under the synergistic conditions of pH and temperature stimuli (pH 5.0/42 °C), the formulated hydrogel system exhibited remarkable drug loading efficacy (~ 68%) alongside improved drug release characteristics. | The magnetic polyelectrolyte complex-based hydrogel (MPEC) is deemed appropriate for application in pH- and temperature-responsive controlled drug delivery systems pertinent to cancer therapy [208]. |
| Tragacanth-silk fibroin hydrogel (TG/SF/Fe3O4) | Fe3O4 | The efficacy of hyperthermia using the hybrid (TG/SF/Fe3O4) scaffold was assessed, revealing a maximum specific absorption rate (SAR) value of 41.2 W/g recorded during the initial interval. | The resulting TG hydrogel/SF hybrid was magnetized with Fe3O4 MNPs for hyperthermia application [209]. |
| Pluronic thermoresponsive hydrogel | SPIONs 5 mg/mL | The developed hydrogel/microparticle system demonstrated a lower drug release rate compared to the microparticles utilized in isolation. | The Pluronic thermoresponsive hydrogel represents a viable thermoresponsive drug delivery system (DDS) suitable for magnetic hyperthermia applications, thereby facilitating a synergistic approach to cancer treatment [210]. |
| Salecan-g-PCH/Fe3O4@SiO2 composite hydrogels | Fe3O4@SiO2 nanoparticles (2%, w/v) | Salecan-g-PCH/Fe3O4@SiO2 composite hydrogels exhibit potential as carriers for anticancer drugs, particularly within the context of magnetically targeted drug delivery applications [211]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, U.N.; Ostrovidov, S.; Datta, S.; Kaji, H. Overview of Magnetic Hydrogel Fabrication, Its Basic Characteristics, and Potential Uses in Biomedical Engineering. Bioengineering 2025, 12, 1142. https://doi.org/10.3390/bioengineering12111142
Sharma UN, Ostrovidov S, Datta S, Kaji H. Overview of Magnetic Hydrogel Fabrication, Its Basic Characteristics, and Potential Uses in Biomedical Engineering. Bioengineering. 2025; 12(11):1142. https://doi.org/10.3390/bioengineering12111142
Chicago/Turabian StyleSharma, Udit Narayan, Serge Ostrovidov, Sudipto Datta, and Hirokazu Kaji. 2025. "Overview of Magnetic Hydrogel Fabrication, Its Basic Characteristics, and Potential Uses in Biomedical Engineering" Bioengineering 12, no. 11: 1142. https://doi.org/10.3390/bioengineering12111142
APA StyleSharma, U. N., Ostrovidov, S., Datta, S., & Kaji, H. (2025). Overview of Magnetic Hydrogel Fabrication, Its Basic Characteristics, and Potential Uses in Biomedical Engineering. Bioengineering, 12(11), 1142. https://doi.org/10.3390/bioengineering12111142








