Control Deficits and Compensatory Mechanisms in Individuals with Chronic Ankle Instability During Dual-Task Stair-to-Ground Transition
Abstract
1. Introduction
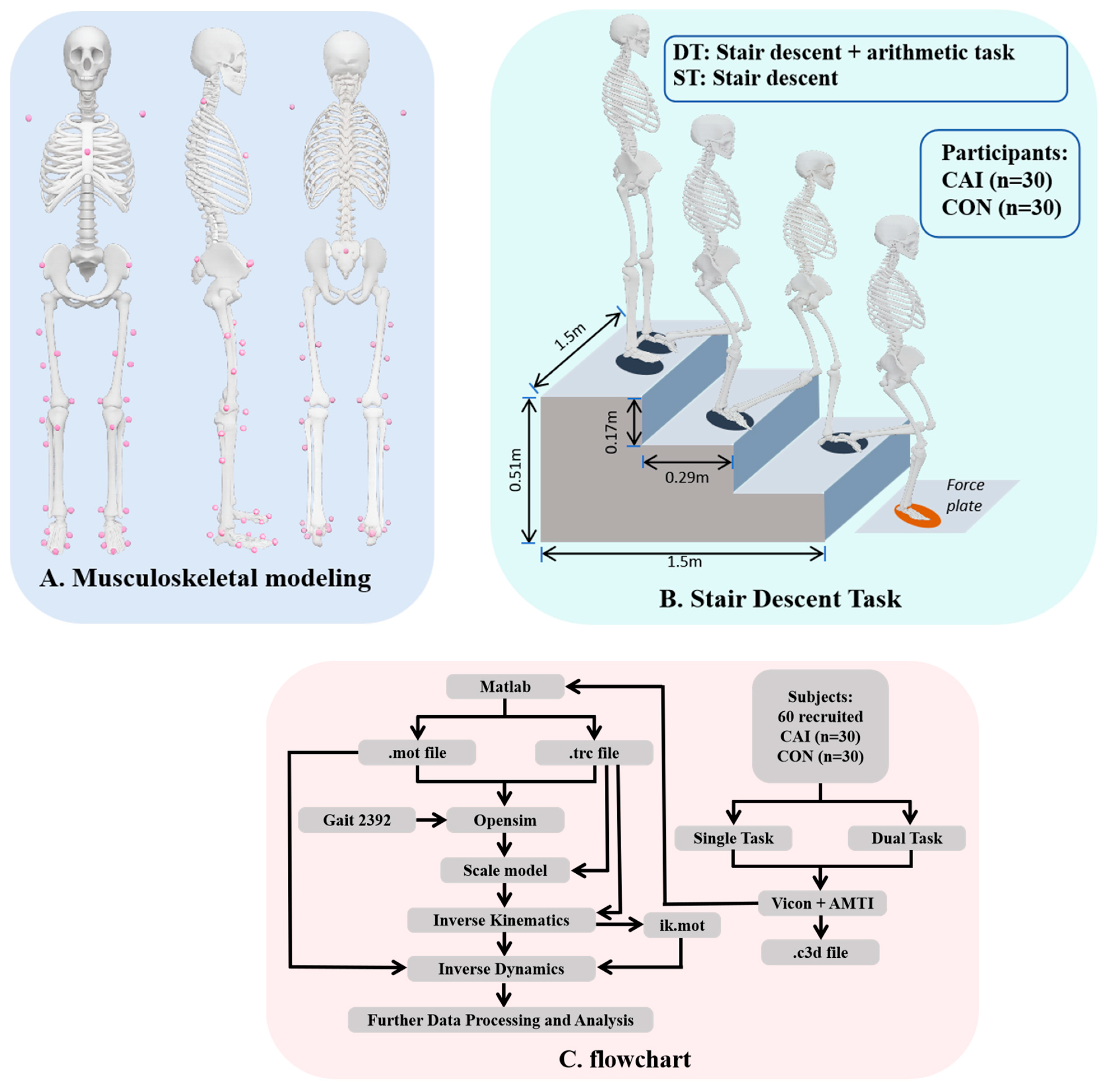
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Data Collection and Processing
2.4. Statistical Analysis
3. Results
3.1. Balance and Stability
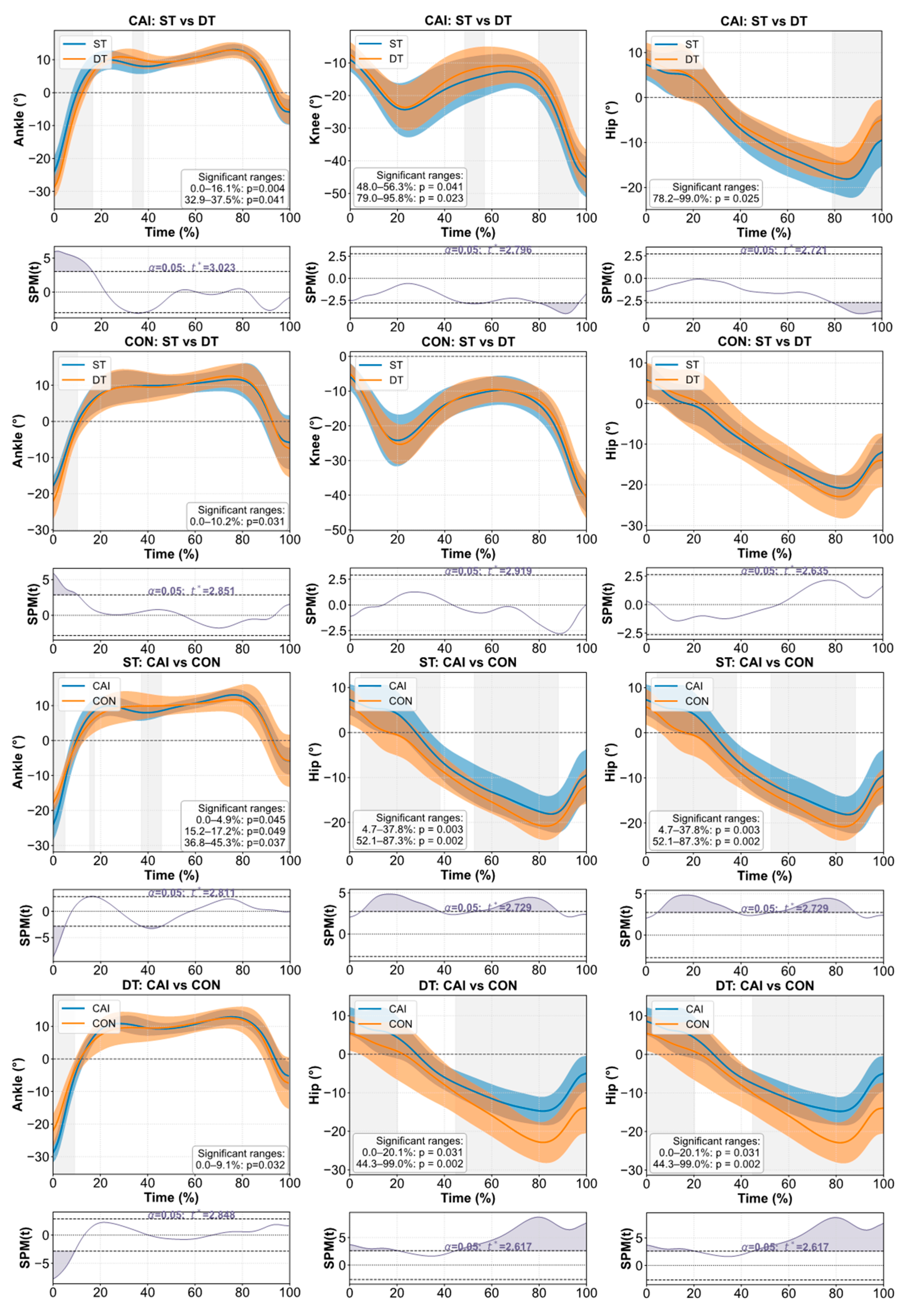
3.2. Joint Kinematics and Kinetics
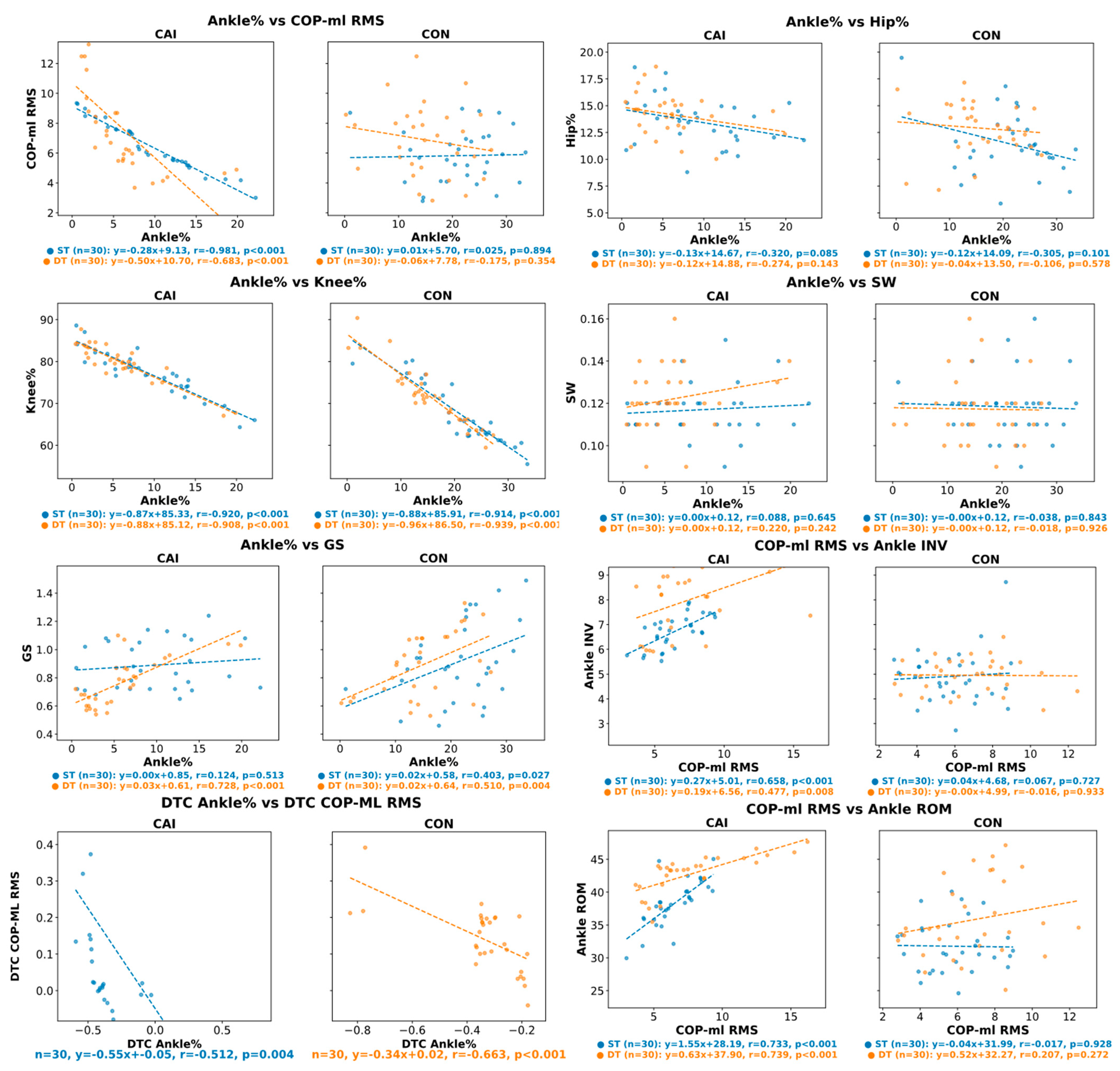
3.3. Predictive Relationships Between Ankle% and Lower-Limb Biomechanics
3.4. Dual-Task Cost
4. Discussion
4.1. Postural Stability and Attentional Demands Under Dual-Task Conditions
4.2. Distal Suppression and Proximal Compensation
4.3. Regulatory Role of Ankle Function in Motor Control
4.4. Clinical Implications
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAI | Chronic ankle instability |
| CON | Controls |
| ST | Single-task/single task |
| DT | Dual-task/dual task |
| DTC | Dual-task cost |
| COP-ml | Total mediolateral displacement of the center of pressure |
| COP-ml RMS | Root mean square of mediolateral Center of Pressure displacement |
| GS | Gait speed |
| SW | Step width |
| ROM | Range of motion |
| Ankle% | Relative joint work contribution of the ankle |
| Knee% | Relative joint work contribution of the knee |
| Hip% | Relative joint work contribution of the hip |
| Ankle INV | Ankle inversion angle |
| Hip ABD | Hip abduction |
| Hip FM | Hip flexion moment |
| Hip EM | Hip extension moment |
| KE M1 | First peak knee extension moment |
| KE M2 | Second peak knee extension moment |
| PF M1 | First peak ankle plantarflexion moment |
| PF M2 | Second peak ankle plantarflexion moment |
| ATS | Arithmetic task score |
| SPM | Statistical parametric mapping |
Appendix A
| Indicator | CAI-ST | CAI-DT | CON-ST | CON-DT | |
|---|---|---|---|---|---|
| Ankle ROM | W | 0.98 | 0.93 | 0.96 | 0.94 |
| P | 0.81 | 0.05 | 0.25 | 0.11 | |
| Knee ROM | W | 0.96 | 0.98 | 0.98 | 0.95 |
| P | 0.30 | 0.86 | 0.82 | 0.20 | |
| Hip ROM | W | 0.97 | 0.97 | 0.95 | 0.97 |
| P | 0.51 | 0.59 | 0.15 | 0.001 | |
| Ankle inversion | W | 0.96 | 0.92 | 0.92 | 0.98 |
| P | 0.32 | 0.02 | 0.02 | 0.83 | |
| Knee inversion | W | 0.86 | 0.94 | 0.96 | 0.90 |
| P | 0.001 | 0.08 | 0.40 | 0.01 | |
| Hip abduction | W | 0.93 | 0.92 | 0.92 | 0.92 |
| P | 0.04 | 0.03 | 0.03 | 0.03 | |
| PF M1 | W | 0.96 | 0.96 | 0.93 | 0.97 |
| P | 0.40 | 0.35 | 0.06 | 0.64 | |
| PF M2 | W | 0.96 | 0.87 | 0.94 | 0.94 |
| P | 0.28 | 0.002 | 0.08 | 0.09 | |
| KE M1 | W | 0.96 | 0.94 | 0.94 | 0.92 |
| P | 0.26 | 0.08 | 0.09 | 0.03 | |
| KE M2 | W | 0.97 | 0.95 | 0.97 | 0.95 |
| P | 0.50 | 0.17 | 0.51 | 0.18 | |
| Hip FM | W | 0.92 | 0.96 | 0.94 | 0.93 |
| P | 0.03 | 0.38 | 0.11 | 0.06 | |
| Hip EM | W | 0.92 | 0.96 | 0.94 | 0.93 |
| P | 0.03 | 0.38 | 0.11 | 0.06 | |
| COP-ml | W | 0.93 | 0.94 | 0.98 | 0.96 |
| P | 0.05 | 0.07 | 0.89 | 0.40 | |
| COP-ml RMS | W | 0.96 | 0.87 | 0.97 | 0.97 |
| P | 0.40 | 0.002 | 0.58 | 0.64 | |
| GS | W | 0.90 | 0.94 | 0.97 | 0.95 |
| P | 0.01 | 0.08 | 0.48 | 0.13 | |
| SW | W | 0.96 | 0.97 | 0.88 | 0.95 |
| P | 0.35 | 0.55 | 0.003 | 0.22 | |
| ATS | W | 0.99 | 0.99 | 0.98 | 0.98 |
| P | 0.98 | 0.94 | 0.81 | 0.84 | |
| Hip% | W | 0.98 | 0.98 | 0.97 | 0.95 |
| P | 0.92 | 0.78 | 0.42 | 0.18 | |
| Knee% | W | 0.98 | 0.90 | 0.88 | 0.9 |
| P | 0.86 | 0.005 | 0.003 | 0.31 | |
| Ankle% | W | 0.97 | 0.84 | 0.96 | 0.96 |
| P | 0.44 | 0.001 | 0.40 | 0.40 | |
| Indicator | ST | DT | |
|---|---|---|---|
| Ankle ROM | F | 0.45 | 0.86 |
| P | 0.50 | 0.003 | |
| Knee ROM | F | 0.46 | 2.23 |
| P | 0.50 | 0.14 | |
| Hip ROM | F | 2.58 | 0.03 |
| P | 0.11 | 0.87 | |
| Ankle inversion | F | 2.50 | 11.53 |
| P | 0.12 | 0.001 | |
| Knee inversion | F | 0.25 | 11.43 |
| P | 0.62 | 0.001 | |
| Hip abduction | F | 4.67 | 0.03 |
| P | 0.48 | 0.49 | |
| PFM1 | F | 29.54 | 5.52 |
| P | <0.001 | 0.022 | |
| PFM2 | F | 0.37 | 13.41 |
| P | 0.54 | 0.001 | |
| KE M1 | F | 4.38 | 1.27 |
| P | 0.04 | 0.26 | |
| KE M2 | F | 0.005 | 3.62 |
| P | 0.95 | 0.06 | |
| HIP FM | F | 1.21 | 12.97 |
| P | 0.28 | 0.001 | |
| HIP EM | F | 1.21 | 12.97 |
| P | 0.28 | 0.001 | |
| COP-ml | F | 0.36 | 5.49 |
| P | 0.55 | 0.02 | |
| COP-ml RMS | F | 0.004 | 0.95 |
| P | 0.65 | 0.42 | |
| GS | F | 3.54 | 4.50 |
| P | 0.07 | 0.04 | |
| SW | F | 0.42 | 5.76 |
| P | 0.52 | 0.02 | |
| ATS | F | 0.15 | 0.30 |
| P | 0.70 | 0.58 | |
| Hip% | F | 0.62 | 0.43 |
| P | 1.89 | 0.17 | |
| Knee% | F | 1.38 | 4.06 |
| P | 0.24 | 0.05 | |
| Ankle% | F | 0.62 | 4.17 |
| P | 0.44 | 0.05 | |
| Indicator | CAI | CON | |
|---|---|---|---|
| Ankle inversion DTC | W | 0.86 | 0.79 |
| P | 0.001 | 0.001 | |
| SW DTC | W | 0.90 | 0.90 |
| P | 0.01 | 0.005 | |
| GS DTC | W | 0.95 | 0.94 |
| P | 0.19 | 0.09 | |
| COP-ml DTC | W | 0.96 | 0.96 |
| P | 0.41 | 0.28 | |
| COP-ml RMS DTC | W | 0.70 | 0.92 |
| P | 0.001 | 0.003 | |
| Ankle% DTC | W | 0.91 | 0.71 |
| P | 0.02 | 0.001 | |
| Knee% DTC | W | 0.83 | 0.94 |
| P | 0.001 | 0.11 | |
| Hip% DTC | W | 0.93 | 0.95 |
| P | 0.05 | 0.20 | |
| ATS DTC | W | 0.93 | 0.96 |
| P | 0.04 | 0.001 | |
| PF M1 DTC | W | 0.91 | 0.90 |
| P | 0.01 | <0.01 | |
| PF M2 DTC | W | 0.94 | 0.93 |
| P | 0.10 | 0.06 | |
| KE M1 DTC | W | 0.92 | 0.60 |
| P | 0.02 | 0.000 | |
| KE M2 DTC | W | 0.51 | 0.75 |
| P | 0.000 | 0.000 | |
| Hip FM DTC | W | 0.88 | 0.92 |
| P | 0.003 | 0.03 | |
| Hip EM DTC | W | 0.70 | 0.86 |
| P | 0.000 | 0.001 | |
| Ankle ROM DTC | W | 0.98 | 0.95 |
| P | 0.77 | 0.14 | |
| Knee ROM DTC | W | 0.58 | 0.001 |
| P | 0.91 | 0.01 | |
| Hip ROM DTC | W | 0.95 | 0.14 |
| P | 0.70 | 0.001 | |
| Indicator | F | P |
|---|---|---|
| Ankle inversion DTC | 2.89 | 0.009 |
| SW DTC | 2.39 | 0.13 |
| GS DTC | 0.32 | 0.57 |
| COP-ml DTC | 15.30 | 0.001 |
| COP-ml RMS DTC | 4.64 | 0.004 |
| Ankle% DTC | 0.41 | 0.52 |
| Knee% DTC | 72.27 | 0.001 |
| Hip% DTC | 8.25 | 0.006 |
| ATS DTC | 0.13 | 0.72 |
| PF M1 DTC | 44.31 | 0.000 |
| PF M2 DTC | 35.83 | 0.000 |
| KE M1 DTC | 0.27 | 0.60 |
| KE M2 DTC | 1.58 | 0.21 |
| Hip FM DTC | 5.84 | 0.02 |
| Hip EM DTC | 1.60 | 0.21 |
| Ankle ROM DTC | 2.70 | 0.11 |
| Knee ROM DTC | 5.33 | 0.02 |
| Hip ROM DTC | 4.44 | 0.04 |
References
- Chang, H.; Cen, X. Can running technique modification benefit patellofemoral pain improvement in runners? A systematic review and meta-analysis. Int. J. Biomed. Eng. Technol. 2024, 45, 83–101. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, C.; Song, W.; Gao, J.; Tian, H.; Li, H.; Ke, X.; Jiang, C.; Lin, Z. Gait variability and biomechanical distinctions in individuals with functional ankle instability: A case–control study based on three-dimensional motion analysis. Eur. J. Med. Res. 2025, 30, 493. [Google Scholar] [CrossRef]
- Doherty, C.; Bleakley, C.; Hertel, J.; Caulfield, B.; Ryan, J.; Delahunt, E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: A prospective cohort analysis. Am. J. Sports Med. 2016, 44, 995–1003. [Google Scholar] [CrossRef]
- Herzog, M.M.; Kerr, Z.Y.; Marshall, S.W.; Wikstrom, E.A. Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 2019, 54, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.A. Evaluating and differentiating ankle instability. J. Athl. Train. 2019, 54, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.A.; Delahunt, E.; Bleakley, C.; Caulfield, B.; Docherty, C.; Fourchet, F.; Fong, D.; Hertel, J.; Hiller, C.; Kaminski, T. Selection criteria for patients with chronic ankle instability in controlled research: A position statement of the International Ankle Consortium. J. Orthop. Sports Phys. Ther. 2013, 43, 585–591. [Google Scholar] [CrossRef]
- Chamorro-Moriana, G.; Perez-Cabezas, V.; Benitez-Lugo, M. Effectiveness of functional or biomechanical bandages with athletic taping and kinesiotaping in subjects with chronic ankle instability: A systematic review and meta-analysis. EFORT Open Rev. 2024, 9, 94–106. [Google Scholar] [CrossRef]
- Witchalls, J.B.; Waddington, G.; Adams, R.; Blanch, P. Chronic ankle instability affects learning rate during repeated proprioception testing. Phys. Ther. Sport 2014, 15, 106–111. [Google Scholar] [CrossRef]
- Jolman, S.; Robbins, J.; Lewis, L.; Wilkes, M.; Ryan, P. Comparison of magnetic resonance imaging and stress radiographs in the evaluation of chronic lateral ankle instability. Foot Ankle Int. 2017, 38, 397–404. [Google Scholar] [CrossRef]
- Ghanavati, T.; Salavati, M.; Karimi, N.; Negahban, H.; Takamjani, I.E.; Mehravar, M.; Hessam, M. Intra-limb coordination while walking is affected by cognitive load and walking speed. J. Biomech. 2014, 47, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, B. Dual-task methodology and motor skills research: Some applications and methodological constraints. J. Hum. Mov. Stud. 1988, 14, 101–132. [Google Scholar]
- Beurskens, R.; Bock, O. Age-related deficits of dual-task walking: A review. Neural Plast. 2012, 2012, 131608. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Tan, C.W.; Mukherjee, M.; Davidson, A.J.; Stergiou, N. Biomechanical analyses of stair-climbing while dual-tasking. J. Biomech. 2015, 48, 921–929. [Google Scholar] [CrossRef]
- Lempke, L.B.; Oh, J.; Johnson, R.S.; Schmidt, J.D.; Lynall, R.C. Single-versus dual-task functional movement paradigms: A biomechanical analysis. J. Sport. Rehabil. 2021, 30, 774–785. [Google Scholar] [CrossRef]
- Hamacher, D.; Hamacher, D.; Schega, L. A cognitive dual task affects gait variability in patients suffering from chronic low back pain. Exp. Brain Res. 2014, 232, 3509–3513. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Zhong, C.; Luo, X.; Gao, H.; Zhang, T.; Zhu, X.; Huang, X.; Shen, P. Effects of dual-task paradigm on the injury potential during landing among individuals with chronic ankle instability. Front. Physiol. 2024, 15, 1473844. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yoo, T.; Burcal, C.J.; Rosen, A.B. Dual-task differences in individuals with chronic ankle instability: A systematic review with meta-analysis. Gait Posture 2023, 106, 28–33. [Google Scholar] [CrossRef]
- Yang, J.; Yan, S.; Cao, C. Different Dual-Task Paradigm Reduce Postural Control Ability and Dynamic Stability of Healthy Young Adults during Stair Descent. Appl. Bionics Biomech. 2024, 2024, 9942042. [Google Scholar] [CrossRef]
- Springer, S.; Gottlieb, U. Effects of dual-task and walking speed on gait variability in people with chronic ankle instability: A cross-sectional study. BMC Musculoskelet. Disord. 2017, 18, 316. [Google Scholar] [CrossRef]
- Watson, E.L.; Bearden, A.C.; Doughton, J.H.; Needle, A.R. The effects of multiple modalities of cognitive loading on dynamic postural control in individuals with chronic ankle instability. Gait Posture 2020, 79, 10–15. [Google Scholar] [CrossRef]
- Kwak, K.-I.; Choi, B.-J. Effects of dual task training on balance and functional performance in high school soccer players with functional ankle instability. J. Korean Phys. Ther. 2016, 28, 254–258. [Google Scholar] [CrossRef]
- Asai, T.; Misu, S.; Doi, T.; Yamada, M.; Ando, H. Effects of dual-tasking on control of trunk movement during gait: Respective effect of manual-and cognitive-task. Gait Posture 2014, 39, 54–59. [Google Scholar] [CrossRef]
- Wang, L.; Yu, G.; Chen, Y. Effects of dual-task training on chronic ankle instability: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 814. [Google Scholar] [CrossRef]
- Goh, H.-T.; Pearce, M.; Vas, A. Task matters: An investigation on the effect of different secondary tasks on dual-task gait in older adults. BMC Geriatr. 2021, 21, 510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Li, Q.; Liu, H.; Wan, X. Comparative effects of arithmetic, speech, and motor dual-task walking on gait in stroke survivors: A cross-sectional study. Front. Hum. Neurosci. 2025, 19, 1587153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Adams, R.; Ganderton, C.; Lyu, J.; Han, J. Ankle inversion proprioception measured during stair descent can identify chronic ankle instability. Musculoskelet. Sci. Pract. 2024, 72, 102958. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; McFadyen, B.J.; Malouin, F. Frontal and sagittal plane analyses of the stair climbing task in healthy adults aged over 40 years: What are the challenges compared to level walking? Clin. Biomech. 2003, 18, 950–959. [Google Scholar] [CrossRef]
- Protopapadaki, A.; Drechsler, W.I.; Cramp, M.C.; Coutts, F.J.; Scott, O.M. Hip, knee, ankle kinematics and kinetics during stair ascent and descent in healthy young individuals. Clin. Biomech. 2007, 22, 203–210. [Google Scholar] [CrossRef]
- Templer, J. The Staircase: Studies of Hazards, Falls, and Safer Design; MIT Press: Cambridge, MA, USA, 1995; Volume 2. [Google Scholar]
- Chou, L.-S.; Lee, H.-J. Balance control during stair negotiation. J. Biomech. 2007, 40, S207. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Adams, R.; Gao, Y.; Lyu, J.; Han, J. Effects of stair riser height on ankle proprioception in individuals with and without chronic ankle stability. Front. Bioeng. Biotechnol. 2025, 13, 1457233. [Google Scholar] [CrossRef] [PubMed]
- Son, S.J.; Kim, H.; Seeley, M.K.; Hopkins, J.T. Movement Strategies among Groups of Chronic Ankle Instability, Coper, and Control. Med. Sci. Sports Exerc. 2017, 49, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.-C.; Chui, K.K.; Corkery, M.B.; Allen, E.A.; Cloonan, C.M. Hip-ankle coordination during gait in individuals with chronic ankle instability. Gait Posture 2017, 53, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, M.; Kong, L.; Meng, L.; Xue, J.; Zheng, Y.; Zhang, Q. Impact of Cognitive Tasks on Biomechanical Adjustments During Single-Leg Drop Landings in Individuals with Functional Ankle Instability. Appl. Sci. 2024, 14, 10297. [Google Scholar] [CrossRef]
- James, B.; Parker, A. Electromyography of stair locomotion in elderly men and women. Electromyogr. Clin. Neurophysiol. 1989, 29, 161–168. [Google Scholar]
- Song, Q.; Li, L.; Zhang, C.; Sun, W.; Mao, D. Long-term Tai Chi practitioners have superior body stability under dual task condition during stair ascent. Gait Posture 2018, 66, 124–129. [Google Scholar] [CrossRef]
- Cen, X.; Yu, P.; Song, Y.; Sun, D.; Liang, M.; Bíró, I.; Gu, Y. Influence of medial longitudinal arch flexibility on lower limb joint coupling coordination and gait impulse. Gait Posture 2024, 114, 208–214. [Google Scholar] [CrossRef]
- Gao, S.; Song, Y.; Sun, D.; Cen, X.; Wang, M.; Lu, Z.; Gu, Y. Impact of Becker muscular dystrophy on gait patterns: Insights from biomechanical analysis. Gait Posture 2025, 121, 160–165. [Google Scholar] [CrossRef]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Hertel, J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J. Athl. Train. 2002, 37, 364. [Google Scholar]
- Hiller, C.E.; Kilbreath, S.L.; Refshauge, K.M. Chronic ankle instability: Evolution of the model. J. Athl. Train. 2011, 46, 133–141. [Google Scholar] [CrossRef]
- Wickens, C.D. Multiple resources and performance prediction. Theor. Issues Ergon. Sci. 2002, 3, 159–177. [Google Scholar] [CrossRef]
- Delahunt, E.; Monaghan, K.; Caulfield, B. Altered neuromuscular control and ankle joint kinematics during walking in subjects with functional instability of the ankle joint. Am. J. Sports Med. 2006, 34, 1970–1976. [Google Scholar] [CrossRef]
- McVey, E.D.; Palmieri, R.M.; Docherty, C.L.; Zinder, S.M.; Ingersoll, C.D. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005, 26, 1055–1061. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Jacobs, J.; Horak, F. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Attentional demands and postural control: The effect of sensorycontext. J. Gerontol. Ser. A 2000, 55, M10–M16. [CrossRef] [PubMed]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central nervous system adaptation after ligamentous injury: A summary of theories, evidence, and clinical interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin. Sports Med. 2008, 27, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Witchalls, J.; Blanch, P.; Waddington, G.; Adams, R. Intrinsic functional deficits associated with increased risk of ankle injuries: A systematic review with meta-analysis. Br. J. Sports Med. 2012, 46, 515–523. [Google Scholar] [CrossRef]
- Brockett, C.L.; Chapman, G.J. Biomechanics of the ankle. Orthop. Trauma 2016, 30, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.T.; Ingersoll, C.D.; Edwards, J.; Klootwyk, T.E. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J. Athl. Train. 2002, 37, 25. [Google Scholar] [PubMed]
- Chuter, V.H.; de Jonge, X.A.J. Proximal and distal contributions to lower extremity injury: A review of the literature. Gait Posture 2012, 36, 7–15. [Google Scholar] [CrossRef]
- DeJong, A.F.; Koldenhoven, R.M.; Hertel, J. Proximal Adaptations in Chronic Ankle Instability: Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 1563–1575. [Google Scholar] [CrossRef]
- Moisan, G.; Descarreaux, M.; Cantin, V. Effects of chronic ankle instability on kinetics, kinematics and muscle activity during walking and running: A systematic review. Gait Posture 2017, 52, 381–399. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Lepley, L.K. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am. J. Sports Med. 2015, 43, 1662–1669. [Google Scholar] [CrossRef]
- Winter, D.A. Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin. Orthop. Relat. Res. 1983, 175, 147–154. [Google Scholar] [CrossRef]
- Feger, M.A.; Donovan, L.; Hart, J.M.; Hertel, J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J. Athl. Train. 2015, 50, 350–357. [Google Scholar] [CrossRef]
- Barton, C.J.; Bonanno, D.; Carr, J.; Neal, B.; Malliaras, P.; Franklyn-Miller, A.; Menz, H. Running retraining to treat lower limb injuries: A mixed-methods study of current evidence synthesised with expert opinion. Br. J. Sports Med. 2016, 50, 513–526. [Google Scholar] [CrossRef]
- Jaiswal, S.; Rishi, P.; Sen, S. Efficacy of Dual Task Training on Ankle Stability in Chronic Ankle Sprain. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ivanov, I.; Tchorbadjieff, A.; Hristov, O.; Peev, P.; Gutev, G.; Ivanova, S. Spine Kinematic Alterations in Nordic Walking Under Two Different Speeds of 3 and 5 km/h—A Pilot Study. J. Funct. Morphol. Kinesiol. 2025, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cen, X.; Wang, M.; Gao, Z.; Tan, Q.; Sun, D.; Gu, Y.; Wang, Y.; Zhang, M. A Systematic Review of Finite Element Analysis in Running Footwear Biomechanics: Insights for Running-Related Musculoskeletal Injuries. J. Sports Sci. Med. 2025, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cen, X.; Sun, D.; Bálint, K.; Wang, Y.; Chen, H.; Gao, S.; Bíró, I.; Zhang, M.; Gu, Y. Curved carbon-plated shoe may further reduce forefoot loads compared to flat plate during running. Sci. Rep. 2024, 14, 13215. [Google Scholar] [CrossRef]
- Song, Y.; Cen, X.; Wang, M.; Bálint, K.; Tan, Q.; Sun, D.; Gao, S.; Li, F.; Gu, Y.; Wang, Y. The influence of simulated worn shoe and foot inversion on heel internal biomechanics during running impact: A subject-specific finite element analysis. J. Biomech. 2025, 180, 112517. [Google Scholar] [CrossRef]


| Indicator | CAI | CON | P1 | F1 | η21 | P2 | F2 | η22 | P3 | F3 | η23 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | DT | ST | DT | ||||||||||
| ATS (point) | 0.59 ± 0.05 | 0.51 ± 0.06 | 0.59 ± 0.05 | 0.64 ± 0.07 | <0.001 | 40.38 | 0.41 | 0.11 | 2.60 | 0.04 | <0.001 | 44.71 | 0.44 |
| COP-ml (mm) | 80.54 (73.77, 94.07) | 101.31 (81.51, 109.49) | 94.04 (81.59, 99.67) | 96.63 (87.48, 109.92) | 0.47 | 0.53 | 0.009 | <0.001 | 75.17 | 0.56 | <0.001 | 14.40 | 0.20 |
| COP-ml RMS (mm) | 6.15 (5.40, 7.60) | 6.31 (5.48, 8.69) | 5.62 (4.22, 6.91) | 6.30 (4.98, 8.28) | 0.26 | 1.31 | 0.02 | <0.001 | 36.04 | 0.38 | 0.03 | 4.95 | 0.08 |
| GS (m/s) | 0.862 (0.73, 1.07) | 0.74 (0.62, 0.87) | 0.89 (0.76, 1.11) | 0.91 (0.68, 1.08) | 0.20 | 1.69 | 0.03 | <0.001 | 168.05 | 0.74 | <0.001 | 118.16 | 0.67 |
| SW (m) | 6.15 (5.40, 7.60) | 6.31 (5.48, 8.69) | 5.62 (4.22, 6.91) | 6.30 (4.98, 8.28) | 0.68 | 0.17 | 0.003 | <0.001 | 139.14 | 0.71 | 0.64 | 0.22 | 0.004 |
| Indicator | CAI | CON | P1 | F1 | η21 | P2 | F2 | η22 | P3 | F3 | η23 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | DT | ST | DT | ||||||||||
| Ankle ROM | 38.41 (36.14, 40.18) | 43.45 (41.37, 44.45) | 30.75 (28.78, 34.01) | 34.47 (32.03, 41.17) | <0.001 | 39.88 | 0.41 | <0.001 | 334.68 | 0.85 | 0.07 | 3.45 | 0.06 |
| Knee ROM | 36.42 ± 3.80 | 36.92 ± 4.04 | 34.08 ± 4.17 | 35.13 ± 4.64 | 0.04 | 4.63 | 0.07 | 0.12 | 2.51 | 0.04 | 0.57 | 0.32 | 0.006 |
| Hip ROM | 26.50 (25.77, 27.34) | 23.70 (22.52, 25.21) | 26.828 (26.04, 27.97) | 27.39 (25.99, 28.28) | <0.001 | 32.48 | 0.36 | <0.001 | 13.80 | 0.19 | <0.001 | 24.04 | 0.29 |
| Ankle Inversion | 6.80 (6.32, 7.19) | 8.37 (6.96, 9.09) | 4.83 (4.23, 5.44) | 5.02 (4.44, 5.50) | <0.001 | 185.48 | 0.76 | 0.002 | 10.57 | 0.15 | 0.003 | 9.70 | 0.14 |
| Knee Varus | 1.15 (0.88, 1.87) | 2.29 (1.98, 3.97) | 2.18 (1.57, 2.80) | 2.09 (1.71, 2.51) | 0.23 | 1.45 | 0.02 | 0.004 | 8.92 | 0.13 | 0.01 | 6.72 | 0.10 |
| Hip Abduction | 6.42 (2.95, 7.98) | 8.01 (3.29, 9.38) | 4.67 (3.22, 6.80) | 4.60 (2.29, 8.38) | 0.03 | 5.04 | 0.08 | 0.52 | 0.42 | 0.007 | 0.37 | 0.84 | 0.01 |
| Hip FM | 0.32 (0.23, 0.36) | 0.33 (0.26, 0.39) | 0.34 (0.25, 0.37) | 0.35 (0.27, 0.40) | 0.48 | 0.51 | 0.009 | <0.001 | 60.15 | 0.51 | 0.006 | 8.11 | 0.12 |
| Hip EM | 0.65 ± 0.10 | 0.51 ± 0.07 | 0.60 ± 0.09 | 0.52 ± 0.11 | 0.37 | 0.80 | 0.01 | <0.001 | 100.32 | 0.63 | 0.003 | 9.93 | 0.15 |
| KE M1 | 0.27 (0.15, 0.44) | 0.52 (0.31, 0.65) | 0.35 (0.30, 0.42) | 0.43 (0.40, 0.55) | 0.46 | 0.56 | 0.01 | <0.001 | 150.90 | 0.72 | 0.002 | 5.93 | 0.009 |
| KE M2 | 0.10 (0.07, 0.14) | 0.08 (0.06, 0.09) | 0.09 (0.08, 0.11) | 0.08 (0.07, 0.10) | 0.69 | 0.17 | 0.003 | <0.001 | 116.63 | 0.67 | 0.43 | 0.63 | 0.01 |
| PF M1 | 1.18 (1.15, 1.25) | 1.127 (1.074, 1.191) | 1.30 (1.11, 1.45) | 1.256 (1.19, 1.33) | <0.001 | 13.80 | 0.19 | 0.002 | 10.96 | 0.16 | 0.004 | 8.85 | 0.13 |
| PF M2 | 1.13 (1.09, 1.27) | 1.04 (0.92 1.169) | 1.29 (1.25, 1.41) | 1.40 (1.35, 1.46) | <0.001 | 92.99 | 0.62 | 0.41 | 0.70 | 0.01 | <0.001 | 17.33 | 0.23 |
| Hip% | 13.49 ± 2.28 | 14.20 ± 1.96 | 11.41 ± 2.98 | 12.94 ± 2.47 | <0.001 | 17.15 | 0.23 | 0.02 | 5.30 | 0.08 | 0.41 | 0.69 | 0.01 |
| Knee% | 77.02 (74.14, 79.78) | 80.13 (78.45, 83.24) | 64.59 (62.39, 73.93) | 71.68 (66.63, 75.21) | <0.001 | 74.77 | 0.56 | 0.001 | 11.99 | 0.17 | 0.95 | 0.004 | 7.20 |
| Ankle% | 8.53 (5.29, 13.61) | 5.34 (2.30, 7.16) | 22.62 (18.11, 26.30) | 14.33 (11.82, 20.50) | <0.001 | 95.83 | 0.62 | <0.001 | 15.67 | 0.21 | 0.61 | 0.56 | 0.004 |
| Indicator | CAI | CON |
|---|---|---|
| Ankle inversion DTC | 23.07 (9.99, 25.61) | 16.12 (9.69, 28.33) |
| SW DTC | 10.10 (8.51, 11.05) | 11.82 (8.57, 13.00) |
| GS DTC | 14.83 ± 4.00 | 7.68 ± 4.27 a |
| COP-ml DTC | 17.94 ± 8.65 | 5.21 ± 3.02 a |
| COP-ml RMS DTC | 7.95 (1.83, 14.92) | 14.36 (10.03, 19.84) |
| Ankle% DTC | 44.05 (36.16, 49.02) | 33.22 (22.53, 35.12) a |
| Knee% DTC | 1.24 (1.19, 1.26) | 7.95 (4.75, 10.23) a |
| Hip% DTC | 4.995 (2.51, 9.66) | 16.33 (11.92, 22.17) a |
| ATS DTC | 14.295 (10.09, 22.08) | 8.05 (3.46, 20.12) a |
| PF M1 DTC | 5.435 (4.78, 6.63) | 8.69 (2.12, 13.09) |
| PF M2 DTC | 15.37 ± 10.92 | 7.90 ± 2.83 a |
| KE M1 DTC | 78.02 (48.09, 95.07) | 24.19 (17.15, 33.50) a |
| KE M2 DTC | 21.01 (13.67, 29.97) | 13.10 (11.26, 14.61) a |
| Hip FM DTC | 8.44 (3.74, 11.60) | 6.28 (2.35, 8.34) |
| Hip EM DTC | 22.18 (20.04, 23.45) | 22.23 (18.98, 23.33) |
| Ankle ROM DTC | 12.17 ± 3.43 | 12.26 ± 5.50 |
| Knee ROM DTC | 5.62 (3.57, 14.32) | 2.63 (1.36, 5.03) a |
| Hip ROM DTC | 9.94 (8.16, 12.45) | 7.45 (2.81, 11.37) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Cen, X.; Hu, X.; Xu, D.; Tu, L.; Jemni, M.; Fekete, G.; Sun, D.; Song, Y. Control Deficits and Compensatory Mechanisms in Individuals with Chronic Ankle Instability During Dual-Task Stair-to-Ground Transition. Bioengineering 2025, 12, 1120. https://doi.org/10.3390/bioengineering12101120
Zhong Y, Cen X, Hu X, Xu D, Tu L, Jemni M, Fekete G, Sun D, Song Y. Control Deficits and Compensatory Mechanisms in Individuals with Chronic Ankle Instability During Dual-Task Stair-to-Ground Transition. Bioengineering. 2025; 12(10):1120. https://doi.org/10.3390/bioengineering12101120
Chicago/Turabian StyleZhong, Yilin, Xuanzhen Cen, Xiaopan Hu, Datao Xu, Lei Tu, Monèm Jemni, Gusztáv Fekete, Dong Sun, and Yang Song. 2025. "Control Deficits and Compensatory Mechanisms in Individuals with Chronic Ankle Instability During Dual-Task Stair-to-Ground Transition" Bioengineering 12, no. 10: 1120. https://doi.org/10.3390/bioengineering12101120
APA StyleZhong, Y., Cen, X., Hu, X., Xu, D., Tu, L., Jemni, M., Fekete, G., Sun, D., & Song, Y. (2025). Control Deficits and Compensatory Mechanisms in Individuals with Chronic Ankle Instability During Dual-Task Stair-to-Ground Transition. Bioengineering, 12(10), 1120. https://doi.org/10.3390/bioengineering12101120









