Design and Performance Assessment of a High-Resolution Small-Animal PET System
Abstract
1. Introduction
2. Materials and Methods
2.1. PET System Description
2.2. Performance Test
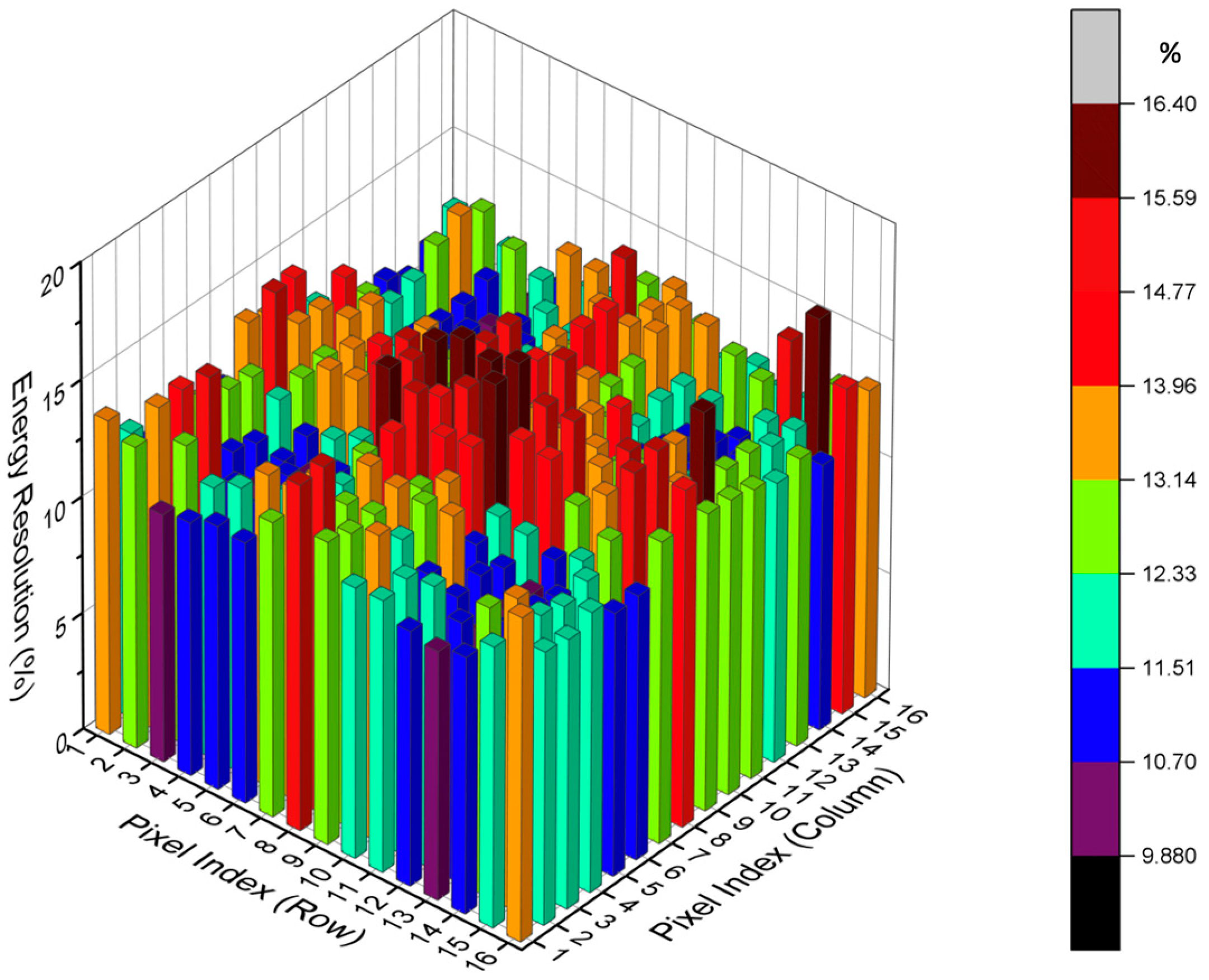
2.2.1. Energy Resolution
2.2.2. Spatial Resolution
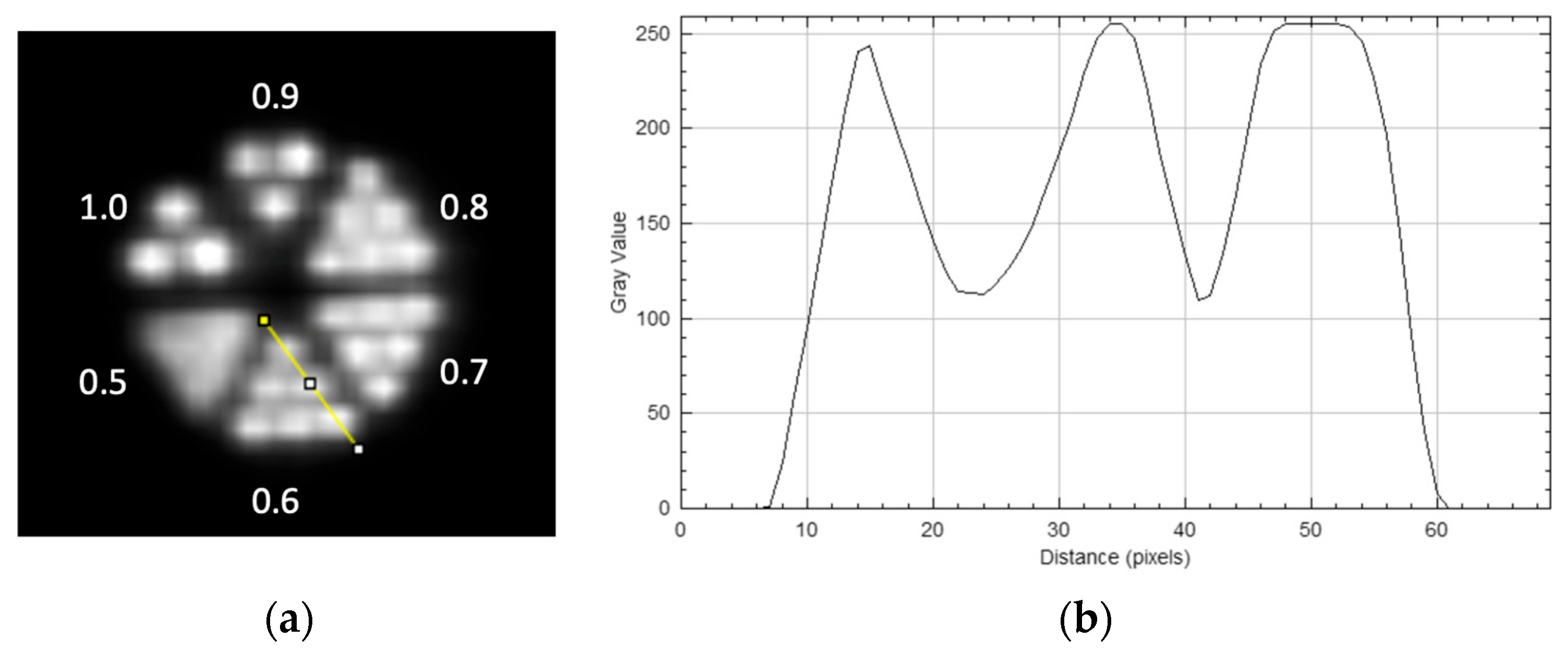
2.2.3. Micro-Derenzo Phantom
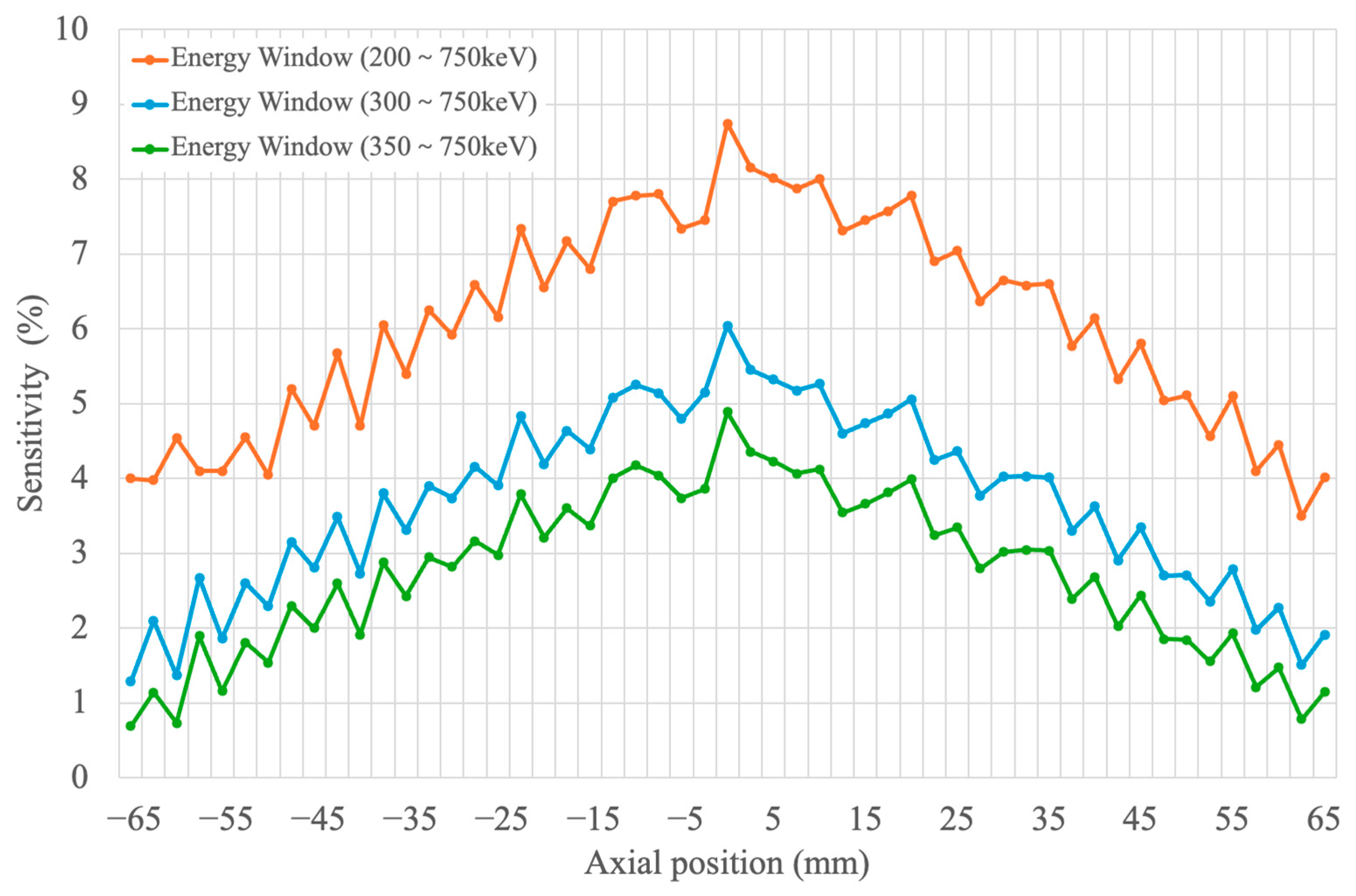
2.2.4. Sensitivity
2.2.5. Scatter Fraction and Count Rate Performance
2.2.6. Image Quality
2.2.7. Animal Image Studies
3. Results
3.1. Energy Resolution
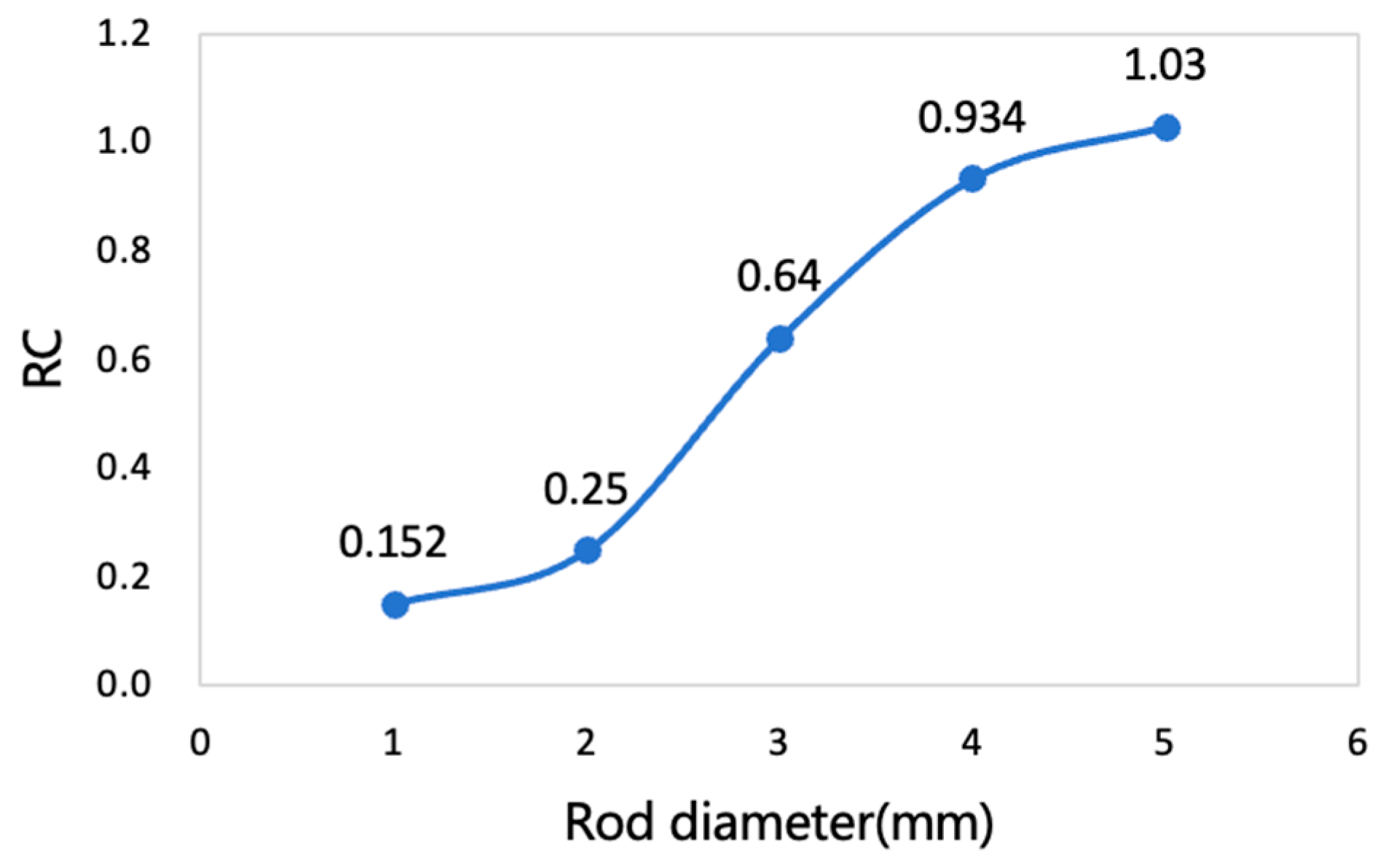
3.2. Spatial Resolution
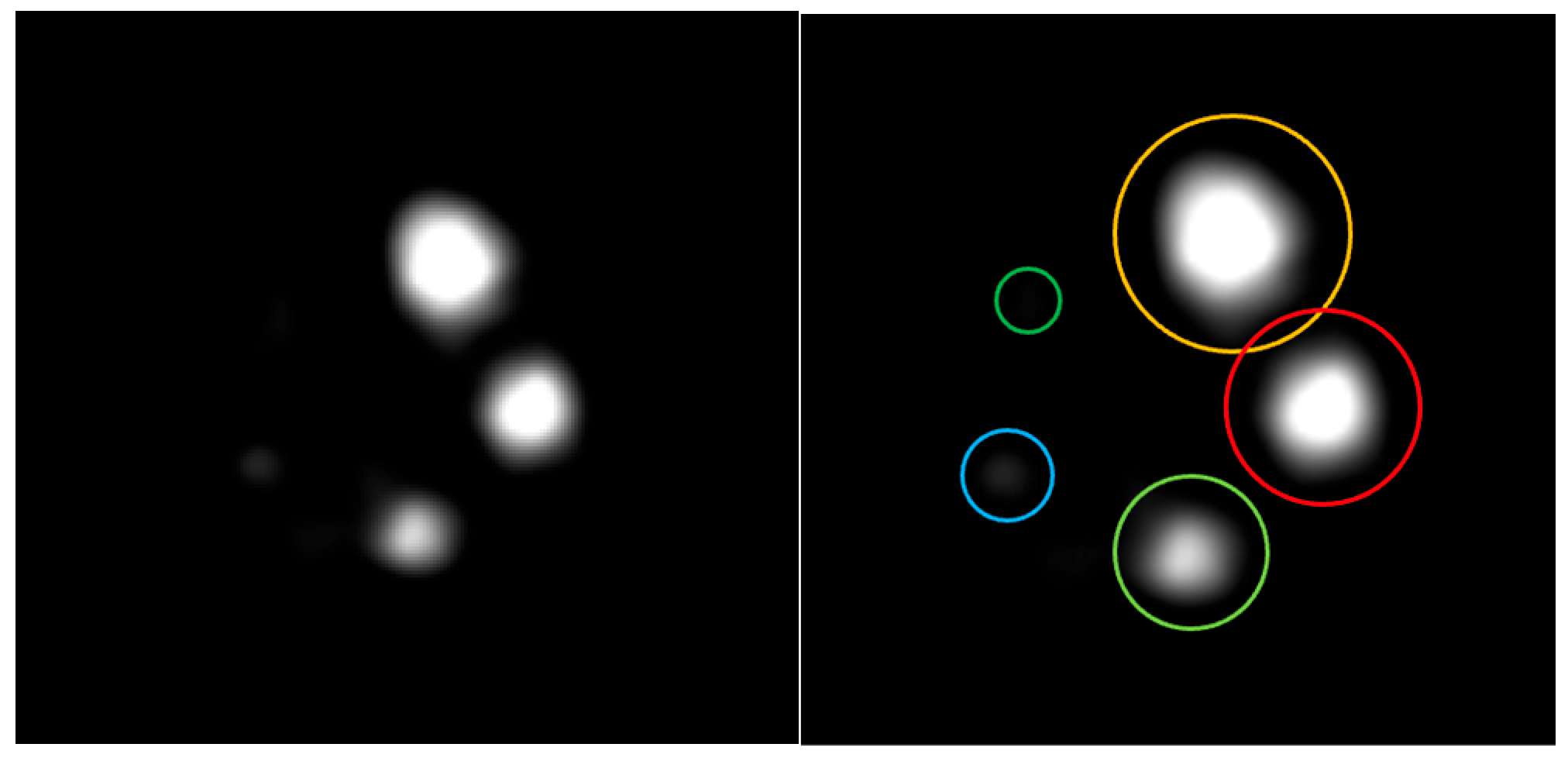
3.3. Micro Derenzo Phantom
3.4. Sensitivity
3.5. Scatter Fraction and Count Rate Performance
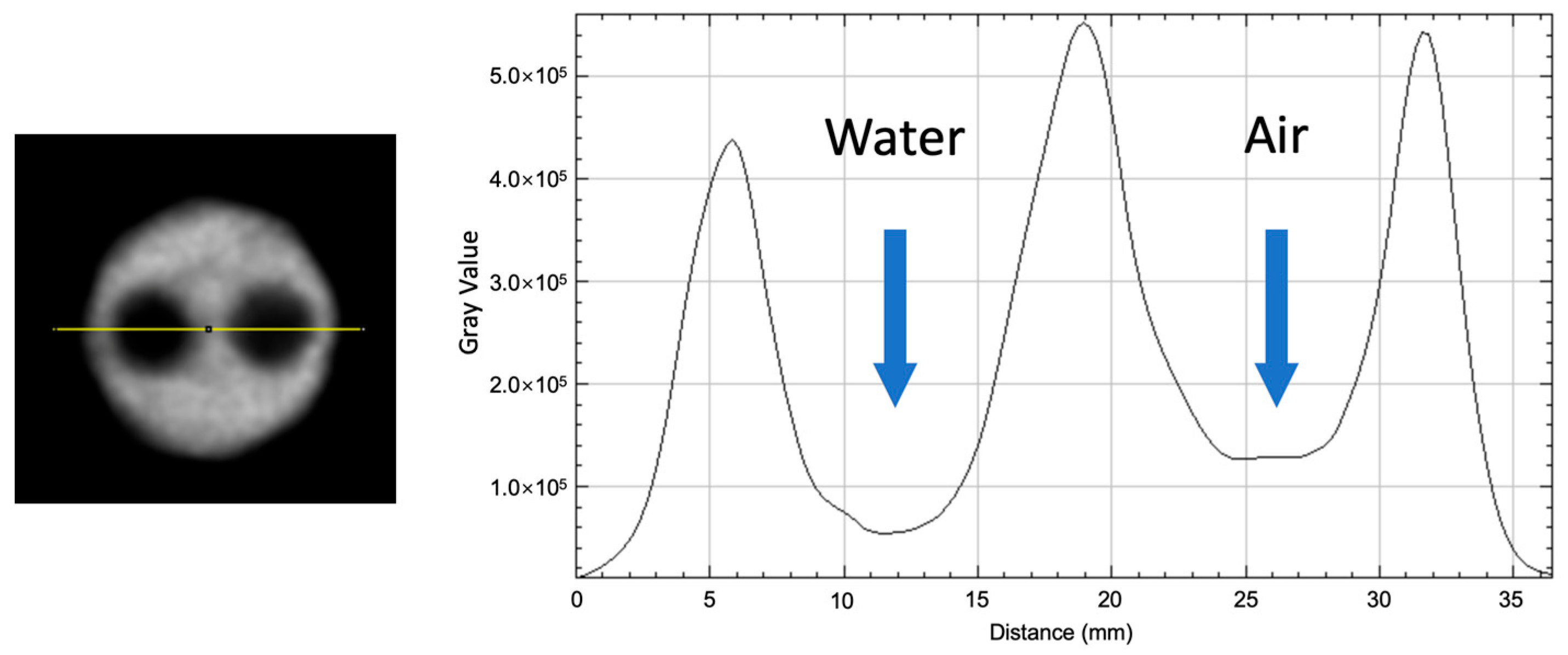
3.6. Image Quality
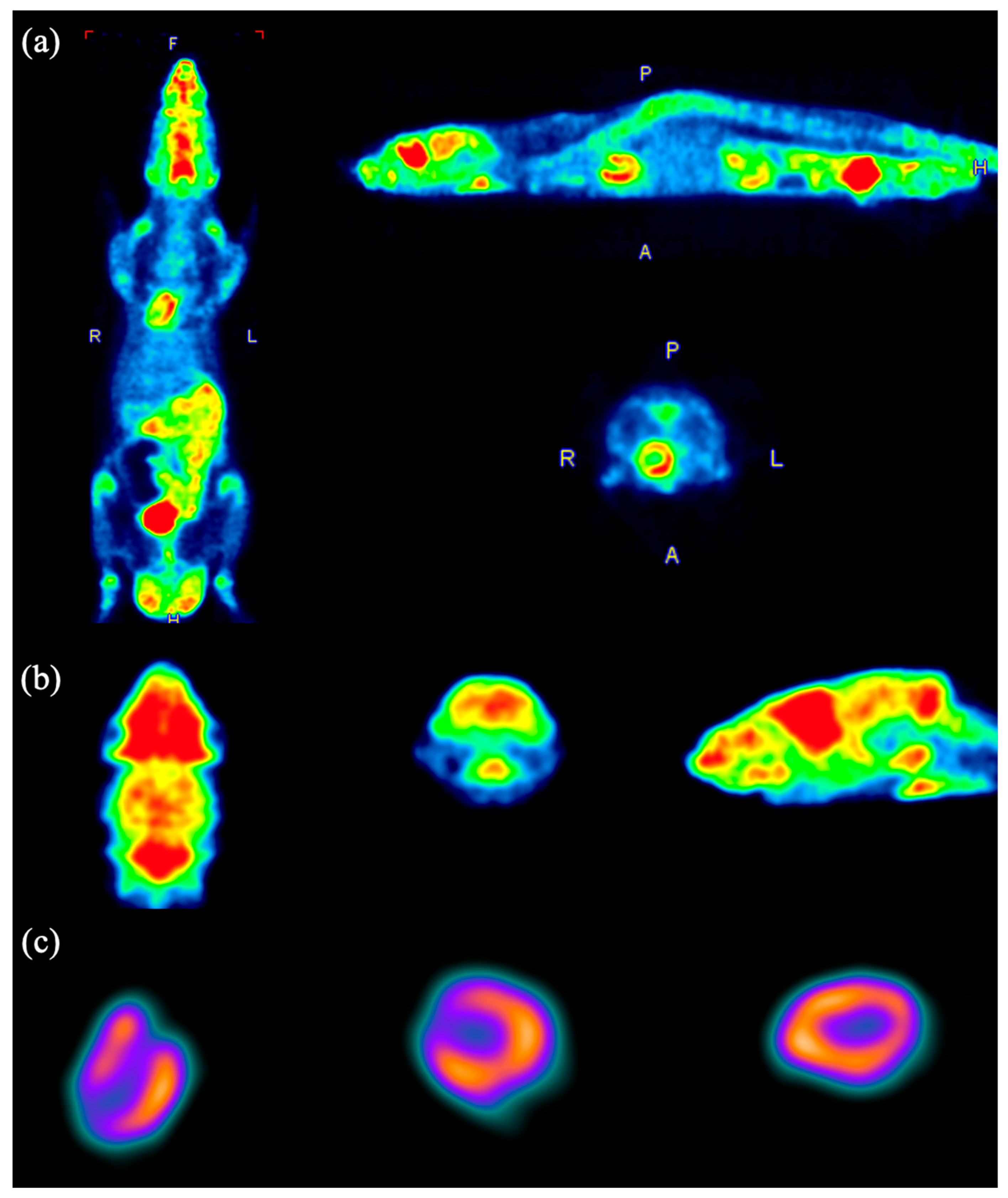
3.7. Animal Image Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitson, S.; Cuccurullo, V.; Ciarmiello, A.; Salvo, D.; Mansi, L. Clinical applications of positron emission tomography (PET) imaging in medicine: Oncology, brain diseases and cardiology. Curr. Radiopharm. 2009, 2, 224–253. [Google Scholar] [CrossRef]
- Galldiks, N.; Lohmann, P.; Albert, N.L.; Tonn, J.C.; Langen, K.-J. Current status of PET imaging in neuro-oncology. Neuro-Oncology Adv. 2019, 1, vdz010. [Google Scholar] [CrossRef]
- Anand, S.; Singh, H.; Dash, A. Clinical applications of PET and PET-CT. Med. J. Armed Forces India 2009, 65, 353–358. [Google Scholar] [CrossRef]
- Schnöckel, U.; Hermann, S.; Stegger, L.; Law, M.; Kuhlmann, M.; Schober, O.; Schäfers, K.; Schäfers, M. Small-animal PET: A promising, non-invasive tool in pre-clinical research. Eur. J. Pharm. Biopharm. 2010, 74, 50–54. [Google Scholar] [CrossRef]
- Fine, E.J.; Herbst, L.; Jelicks, L.A.; Koba, W.; Theele, D. Small-animal research imaging devices. in Seminars in nuclear medicine. Semin. Nucl. Med. 2014, 44, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, R.S.; Lehnert, A.L. Small animal PET: A review of what we have done and where we are going. Phys. Med. Biol. 2020, 65, 24TR04. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Rubello, D.; Fanti, S. Role of small animal PET for molecular imaging in pre-clinical studies. Eur. J. Nucl. Med. 2007, 34, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Yamamura, K.; Sato, K.; Ota, T.; Suzuki, H.; Ohsuka, S. Development of multi-pixel photon counter (MPPC). In Proceedings of the 2006 IEEE Nuclear Science Symposium Conference Record, San Diego, CA, USA, 29 October–1 November 2006. [Google Scholar]
- Lecoq, P.; Gundacker, S. SiPM applications in positron emission tomography: Toward ultimate PET time-of-flight resolution. Eur. Phys. J. Plus 2021, 136, 292. [Google Scholar] [CrossRef]
- Goertzen, A.L.; Van Elburg, D. Performance characterization of MPPC modules for TOF-PET applications. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 3, 475–482. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhang, H.; Peng, H.; Ren, Q.; Xu, J.; Peng, Q.; Xie, S. Requirements of scintillation crystals with the development of PET scanners. Crystals 2022, 12, 1302. [Google Scholar] [CrossRef]
- Freire, M.; Echegoyen, S.; Gonzalez-Montoro, A.; Sanchez, F.; Gonzalez, A.J. Performance evaluation of side-by-side optically coupled monolithic LYSO crystals. Med. Phys. 2022, 49, 5616–5626. [Google Scholar] [CrossRef]
- Cucarella, N.; Barrio, J.; Lamprou, E.; Valladares, C.; Benlloch, J.M.; Gonzalez, A.J. Timing evaluation of a PET detector block based on semi-monolithic LYSO crystals. Med Phys. 2021, 48, 8010–8023. [Google Scholar] [CrossRef]
- Hu, P.; Hua, Z.; Ma, L.; Qian, S.; Wu, Q.; Wang, Z. Study on the optimized energy resolution of scintillator detectors based on SiPMs and LYSO: Ce. J. Instrum. 2022, 17, T09010. [Google Scholar] [CrossRef]
- Xie, Z.; Li, S.; Zhou, K.; Vuletic, I.; Meng, X.; Zhu, S.; Xu, H.; Yang, K.; Xu, B.; Zhang, J.; et al. PKU-PET-II: A novel SiPM-based PET imaging system for small animals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 877, 104–111. [Google Scholar] [CrossRef]
- NEMA. Performance Measurements for Small Animal Positron Emission Tomographs; NEMA Standards Publication NU 4-2008; National Electrical Manufacturers Association: Rosslyn, VA, USA, 2008; pp. 1–23. [Google Scholar]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E.; ScienceDirect (Online service). Physics in Nuclear Medicine; Saunders: Philadelphia, PA, USA, 2003; Volume 3. [Google Scholar]
- Hamill, J.J. 2D energy histograms for scatter estimation in an SiPM PET scanner. In Proceedings of the 2019 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Manchester, UK, 26 October–2 November 2019. [Google Scholar]
- Cherry, S.R.; Shao, Y.; Silverman, R.; Meadors, K.; Siegel, S.; Chatziioannou, A.; Young, J.; Jones, W.; Moyers, J.; Newport, D.; et al. MicroPET: A high resolution PET scanner for imaging small animals. IEEE Trans. Nucl. Sci. 1997, 44, 1161–1166. [Google Scholar] [CrossRef]
- Doss, K.K.M.; Mion, P.E.; Kao, Y.-C.J.; Kuo, T.-T.; Chen, J.-C. Performance evaluation of a PET of 7T Bruker Micro-PET/MR based on NEMA NU 4-2008 standards. Electronics 2022, 11, 2194. [Google Scholar] [CrossRef]
- Farquhar, T.; Chatziioannou, A.; Cherry, S. An evaluation of exact and approximate 3-D reconstruction algorithms for a high-resolution, small-animal PET scanner. IEEE Trans. Med. Imaging 2002, 17, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.C.; Mukherjee, J. Performance evaluation of an Inveon PET preclinical scanner. Phys. Med. Biol. 2009, 54, 2885. [Google Scholar] [CrossRef] [PubMed]
- Szanda, I.; Mackewn, J.; Patay, G.; Major, P.; Sunassee, K.; Mullen, G.E.; Nemeth, G.; Haemisch, Y.; Blower, P.J.; Marsden, P.K. National Electrical Manufacturers Association NU-4 performance evaluation of the PET component of the NanoPET/CT preclinical PET/CT scanner. J. Nucl. Med. 2011, 52, 1741–1747. [Google Scholar] [CrossRef]
- Liu, Q.; Li, C.; Liu, J.; Krish, K.; Fu, X.; Zhao, J.; Chen, J.C. Performance evaluation of a small-animal PET/CT system based on NEMA NU 4–2008 standards. Med. Phys. 2021, 48, 5272–5282. [Google Scholar] [CrossRef] [PubMed]
- Stickel, J.R.; Cherry, S.R. High-resolution PET detector design: Modelling components of intrinsic spatial resolution. Phys. Med. Biol. 2004, 50, 179. [Google Scholar] [CrossRef] [PubMed]
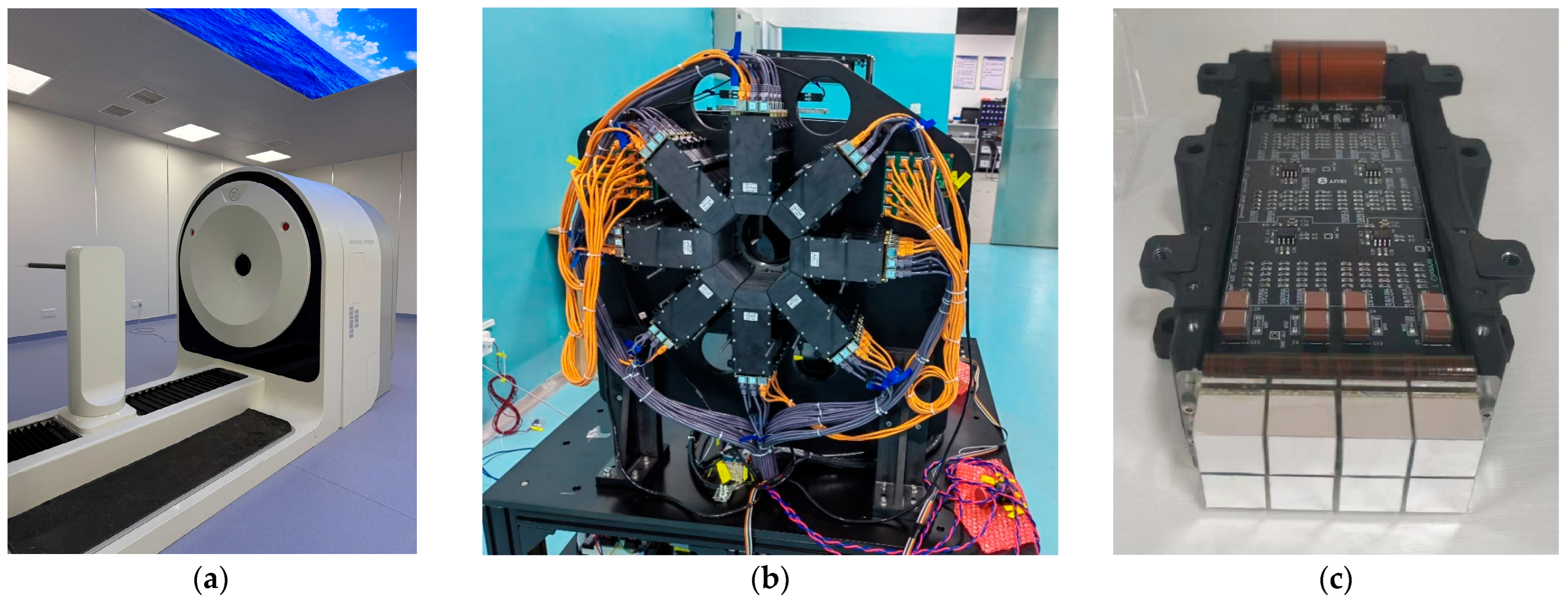





| Parameters | PKU-PET-II | PKU-PET-III | |
|---|---|---|---|
| Detector level | Crystal material | LYSO | LYSO |
| No. of crystals per block | 8 × 8 | 8 × 8 | |
| Crystal size (length × width × thickness, mm3) | 2.0 × 2.0 × 15.0 | 1.457 × 1.457 × 12 | |
| Crystal rings | 16 | 64 | |
| Photodetector | SensL ArrayC-10035-64P | Hamamatsu S14160-4075HS | |
| No. of pixels per SiPM/MPPC array | 8 × 8 | 4 × 4 | |
| Size of SiPM/MPPC pixel (mm2) | 2 × 2 | 3 × 3 | |
| System level | No. of detector modules | 22 | 32 |
| No. of blocks per module | 2 | 8 | |
| Ring diameter (mm) | 100 | 129 | |
| Transaxial FOV (mm) | 60 | 81 | |
| Axial FOV (mm) | 32 | 122 | |
| Coincidence window (ns) | 6 | 6 | |
| Energy window (keV) | 300–700 | 200–750/300-750 | |
| Reconstruction algorithm | 3D OSEM | 3D OSEM |
| Mean (kBq/cc) | Maximum (kBq/cc) | Minimum (kBq/cc) | %STD | |
|---|---|---|---|---|
| Uniformity | 724.38 | 913.26 | 548.85 | 7.97 |
| Mean of SOR | STD of SOR | |
|---|---|---|
| Water | 0.284 | 27.9% |
| Air | 0.282 | 39.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Xi, P.; Liu, J.; Xu, X.; Xie, Z.; Lu, Y.; Meng, X.; Ren, Q. Design and Performance Assessment of a High-Resolution Small-Animal PET System. Bioengineering 2025, 12, 1119. https://doi.org/10.3390/bioengineering12101119
Liu W, Xi P, Liu J, Xu X, Xie Z, Lu Y, Meng X, Ren Q. Design and Performance Assessment of a High-Resolution Small-Animal PET System. Bioengineering. 2025; 12(10):1119. https://doi.org/10.3390/bioengineering12101119
Chicago/Turabian StyleLiu, Wei, Peng Xi, Jiguo Liu, Xilong Xu, Zhaoheng Xie, Yanye Lu, Xiangxi Meng, and Qiushi Ren. 2025. "Design and Performance Assessment of a High-Resolution Small-Animal PET System" Bioengineering 12, no. 10: 1119. https://doi.org/10.3390/bioengineering12101119
APA StyleLiu, W., Xi, P., Liu, J., Xu, X., Xie, Z., Lu, Y., Meng, X., & Ren, Q. (2025). Design and Performance Assessment of a High-Resolution Small-Animal PET System. Bioengineering, 12(10), 1119. https://doi.org/10.3390/bioengineering12101119






