Artificial Intelligence in Cardiac Electrophysiology: A Clinically Oriented Review with Engineering Primers
Abstract
1. Introduction
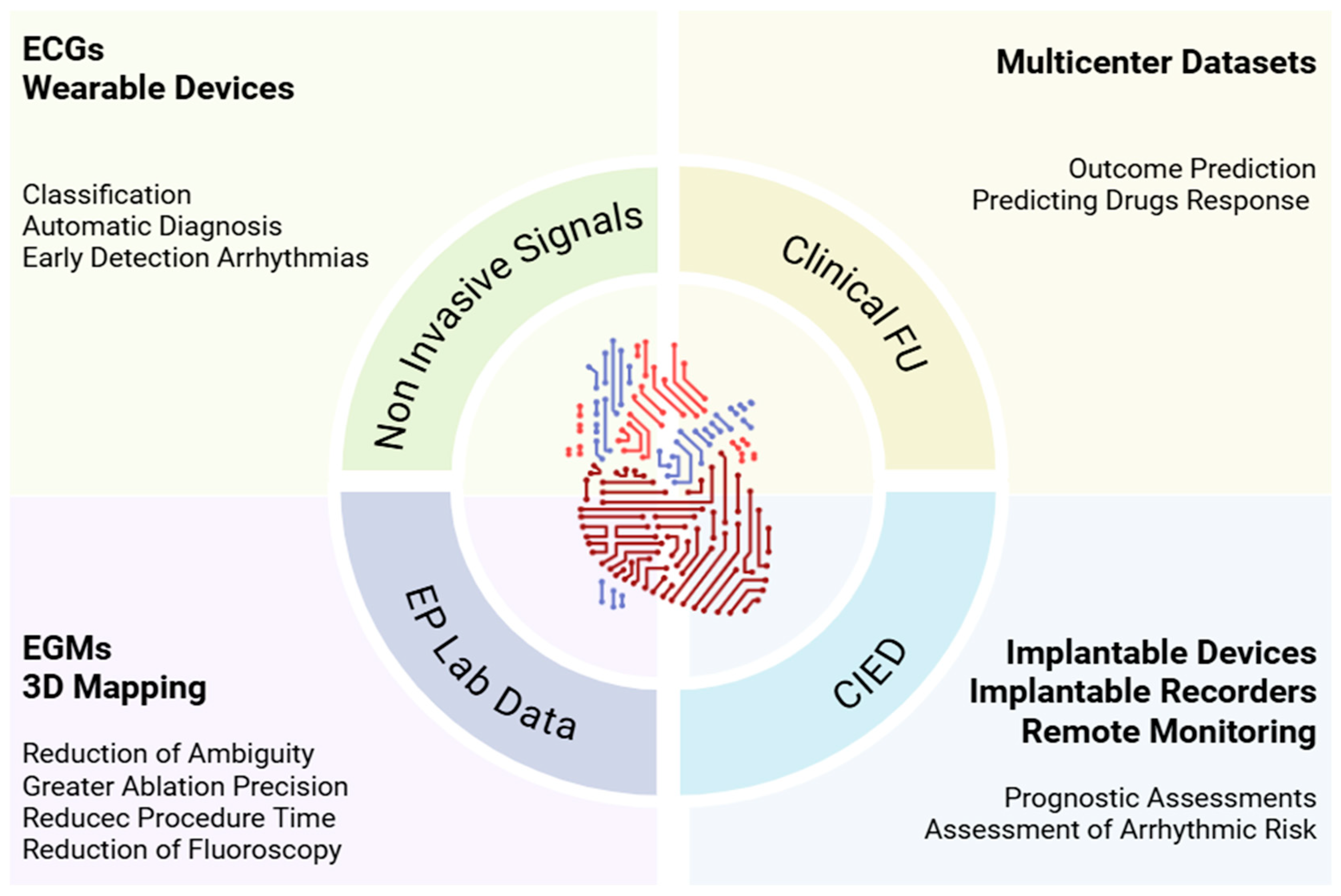
2. Clinical Landscape of Artificial Intelligence in Electrophysiology
2.1. Non-Invasive Electrocardiographic Signals
2.2. Electrophysiology Laboratory Data
2.3. Longitudinal Information from Clinical Follow-Up and Implantable Cardiac Electronic Devices (CIEDs)
3. Technical Foundations and Engineering Considerations
3.1. Supervised Learning
3.1.1. Least-Squares Regression (LSR)
3.1.2. Support Vector Machines (SVMs)
3.1.3. Supervised Neural Networks
3.1.4. Random Forest
3.2. Unsupervised Learning
3.2.1. Clustering
3.2.2. Dimensionality Reduction
3.2.3. Association Rule Learning
3.2.4. Unsupervised Deep Learning
4. Emerging Technologies
4.1. Digital Twin
4.2. Physics-Informed Neural Networks
- Eikonal equation (activation times). Guides reconstruction of activation-time maps: where conduction is slow (scar/fibrosis), activation must occur later. The PINN prevents unrealistic “jumps” of the wavefront.
- Monodomain/bidomain models (current propagation). Describe how current spreads in an anisotropic myocardium (easier along fibers, harder across) and how it depends on ionic currents (e.g., Hodgkin–Huxley, ten Tusscher–Panfilov, O’Hara–Rudy). The PINN enforces charge conservation, anisotropy, and consistency with ionic models, reducing non-physiologic solutions.
- Boundary/initial conditions. At tissue borders (e.g., chambers or non-conductive scar) current does not cross the boundary (no-flux/Neumann). The PINN respects these anatomical “walls”.
- Physiological constraints. Clinical guardrails: conduction velocity ≥ 0, diffusivity ≥ 0, APD within plausible ranges, anisotropy aligned with fiber orientation. The PINN penalizes solutions outside these ranges.
4.3. Deep Learning for Cardiac Imaging
4.4. Graph-Based AI and Convolutional Models for Sparse Data
4.5. Advanced Wearables and On-Device AI
5. Ethical, Legal, and Regulatory Considerations for AI in Cardiac Electrophysiology
5.1. Ethical Considerations
5.2. Regulatory and Legislative Challenges
5.3. Clinical Validation and Generalizability
5.4. Integration and Application
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three Dimensional |
| AF | Atrial Fibrillation |
| AFib | Atrial Fibrillation |
| AIx | Ablation Index |
| AI | Artificial Intelligence |
| AI-ECG | Artificial-Intelligence Electrocardiography |
| APD | Action Potential Duration |
| ARL | Association Rule Learning |
| BPV | Blood-Pressure Variability |
| CABANA | Catheter ABlation vs. ANtiarrhythmic Drug Therapy for Atrial Fibrillation (trial) |
| CDSS | Clinical Decision Support Systems |
| CE | Conformité Européenne |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| CIED | Cardiac Implantable Electronic Device |
| CMR | Cardiovascular Magnetic Resonance |
| CNN | Convolutional Neural Network |
| CRT | Cardiac Resynchronization Therapy |
| CT | Computed Tomography |
| CV | Conduction Velocity |
| CVAE | Conditional Variational Autoencoder |
| DBSCAN | Density-Based Spatial Clustering of Applications with Noise |
| DL | Deep Learning |
| DPIA | Data Protection Impact Assessment |
| DT | Digital Twin |
| EAM | Electroanatomical Mapping |
| EAT | Epicardial Adipose Tissue |
| ECG | Electrocardiogram |
| ECGi | Electrocardiographic Imaging |
| EGM | Intracardiac Electrogram |
| edge-AI | On-device/edge Artificial Intelligence |
| EHR | Electronic Health Record |
| EU | European Union |
| FDA | Food and Drug Administration |
| FU | Follow Up |
| GCNN | Graph Convolutional Neural Network |
| GDPR | General Data Protection Regulation |
| GMLP | Good Machine Learning Practice |
| HF | Heart Failure |
| HIPAA | Health Insurance Portability and Accountability Act |
| HRV | Heart-Rate Variability |
| ICD | Implantable Cardioverter-Defibrillator |
| ICE | Intracardiac Echocardiography |
| inFAT | CT-derived intramyocardial/epicardial fat marker |
| LA | Left Atrium |
| LGE-CMR | Late Gadolinium Enhancement Cardiac Magnetic Resonance |
| LSI | Lesion Size Index |
| LSR | Least-Squares Regression |
| MDR | Medical Device Regulation |
| MLOps | Machine Learning Operations |
| NN | Neural Network(s) |
| OOD | Out-Of-Distribution |
| PCA | Principal Component Analysis |
| PCCP | Predetermined Change Control Plan |
| PINN | Physics-Informed Neural Network |
| PMA | Premarket Approval |
| PPG | Photoplethysmography |
| PTB-XL | Public ECG dataset “PTB-XL” |
| PTB-XL+ | Extended release of PTB-XL |
| PVI | Pulmonary Vein Isolation |
| RA | Right Atrium |
| RF | Radiofrequency |
| RVI | Reentry Vulnerability Index |
| SaMD | Software as a Medical Device |
| SSI | Systolic Stretch Index |
| t-SNE | t-Distributed Stochastic Neighbor Embedding |
| TinyML | Tiny/embedded Machine Learning |
| U-Net | U-Net convolutional architecture |
| UMAP | Uniform Manifold Approximation and Projection |
| VF | Ventricular Fibrillation |
| VT | Ventricular Tachycardia |
| XAI | Explainable Artificial Intelligence |
References
- Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention. Biomedicines 2025, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bacoyannis, T.; Ly, B.; Cedilnik, N.; Cochet, H.; Sermesant, M. Deep learning formulation of electrocardiographic imaging integrating image and signal information with data-driven regularization. EP Eur. 2021, 23, i55–i62. [Google Scholar] [CrossRef]
- Kabra, R.; Israni, S.; Vijay, B.; Baru, C.; Mendu, R.; Fellman, M.; Sridhar, A.; Mason, P.; Cheung, J.W.; DiBiase, L.; et al. Emerging role of artificial intelligence in cardiac electrophysiology. Cardiovasc. Digit. Health J. 2022, 3, 263–275. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Singh, J.P.; Ghanbari, H.; McManus, D.D.; Deering, T.F.; Silva, A.J.N.; Mittal, S.; Krahn, A.; Hurwitz, J.L. The potential of artificial intelligence to revolutionize health care delivery, research, and education in cardiac electrophysiology. Heart Rhythm 2024, 21, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.H.-V.; Thu, A.; Twayana, A.R.; Fuertes, A.; Gonzalez, M.; Harrison, J.L.; Mehta, K.A.; James, M.; Basta, M.; Frishman, W.H.; et al. Artificial Intelligence in Cardiac Electrophysiology: Enhancing Mapping and Ablation Precision. Cardiol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.-Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Yao, X.; Lopez-Jimenez, F.; Mohan, T.L.; Pellikka, P.A.; Carter, R.E.; Shah, N.D.; Friedman, P.A.; Noseworthy, P.A. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019, 30, 668–674. [Google Scholar] [CrossRef]
- Wagner, P.; Strodthoff, N.; Bousseljot, R.-D.; Kreiseler, D.; Lunze, F.I.; Samek, W.; Schaeffter, T. PTB-XL, a large publicly available electrocardiography dataset. Sci. Data 2020, 7, 154. [Google Scholar] [CrossRef]
- Strodthoff, N.; Mehari, T.; Nagel, C.; Aston, P.J.; Sundar, A.; Graff, C.; Kanters, J.K.; Haverkamp, W.; Dössel, O.; Loewe, A.; et al. PTB-XL+, a comprehensive electrocardiographic feature dataset. Sci. Data 2023, 10, 279. [Google Scholar] [CrossRef]
- Deisenhofer, I.; Albenque, J.-P.; Busch, S.; Gitenay, E.; Mountantonakis, S.E.; Roux, A.; Horvilleur, J.; Bakouboula, B.; Oza, S.; Abbey, S.; et al. Artificial intelligence for individualized treatment of persistent atrial fibrillation: A randomized controlled trial. Nat. Med. 2025, 31, 1286–1293. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Sammut-Powell, C.; Martin, G.P.; Callan, P.; Cunnington, C.; Kale, M.; Gerritse, B.; Lanctin, D.; Soken, N.; Campbell, N.G.; et al. Use of a device-based remote management heart failure care pathway is associated with reduced hospitalization and improved patient outcomes: TriageHF Plus real-world evaluation. Eur. Heart J.—Digit. Health 2022, 3, ztac076.2814. [Google Scholar] [CrossRef]
- Boehmer, J.; Sauer, A.J.; Gardner, R.; Stolen, C.M.; Kwan, B.; Wariar, R.; Ruble, S. PRecision Event Monitoring for PatienTs with Heart Failure using HeartLogic (PREEMPT-HF) study design and enrolment. ESC Heart Fail. 2023, 10, 3690–3699. [Google Scholar] [CrossRef]
- Kuo, L.; Wang, G.-J.; Su, P.-H.; Chang, S.-L.; Lin, Y.-J.; Chung, F.-P.; Lo, L.-W.; Hu, Y.-F.; Lin, C.-Y.; Chang, T.-Y.; et al. Deep learning-based workflow for automatic extraction of atria and epicardial adipose tissue on cardiac computed tomography in atrial fibrillation. J. Chin. Med. Assoc. 2024, 87, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jamart, K.; Xiong, Z.; Maso Talou, G.D.; Stiles, M.K.; Zhao, J. Mini Review: Deep Learning for Atrial Segmentation from Late Gadolinium-Enhanced MRIs. Front. Cardiovasc. Med. 2020, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Maurer, M.H.; Thomas, R.P. Explainable Artificial Intelligence in Radiological Cardiovascular Imaging—A Systematic Review. Diagnostics 2025, 15, 1399. [Google Scholar] [CrossRef]
- Zolotarev, A.M.; Hansen, B.J.; Ivanova, E.A.; Helfrich, K.M.; Li, N.; Janssen, P.M.L.; Mohler, P.J.; Mokadam, N.A.; Whitson, B.A.; Fedorov, M.V.; et al. Optical Mapping-Validated Machine Learning Improves Atrial Fibrillation Driver Detection by Multi-Electrode Mapping. Circ. Arrhythmia Electrophysiol. 2020, 13, 1199–1212. [Google Scholar] [CrossRef]
- Whitaker, J.; Baum, T.E.; Qian, P.; Prassl, A.J.; Plank, G.; Blankstein, R.; Cochet, H.; Sauer, W.H.; Bishop, M.J.; Tedrow, U. Frequency Domain Analysis of Endocardial Electrograms for Detection of Nontransmural Myocardial Fibrosis in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2023, 9, 923–935. [Google Scholar] [CrossRef]
- Baldazzi, G.; Orrù, M.; Viola, G.; Pani, D. Computer-aided detection of arrhythmogenic sites in post-ischemic ventricular tachycardia. Sci. Rep. 2023, 13, 6906. [Google Scholar] [CrossRef]
- Seitz, J.; Durdez, T.M.; Albenque, J.P.; Pisapia, A.; Gitenay, E.; Durand, C.; Monteau, J.; Moubarak, G.; Théodore, G.; Lepillier, A.; et al. Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 2250–2260. [Google Scholar] [CrossRef]
- Sakata, K.; Bradley, R.P.; Prakosa, A.; Yamamoto, C.A.P.; Ali, S.Y.; Loeffler, S.; Tice, B.M.; Boyle, P.M.; Kholmovski, E.G.; Yadav, R.; et al. Assessing the arrhythmogenic propensity of fibrotic substrate using digital twins to inform a mechanisms-based atrial fibrillation ablation strategy. Nat. Cardiovasc. Res. 2024, 3, 857–868. [Google Scholar] [CrossRef]
- Martin, C.H.; Oved, A.; Chowdhury, R.A.; Ullmann, E.; Peters, N.S.; Bharath, A.A.; Varela, M. EP-PINNs: Cardiac Electrophysiology Characterisation Using Physics-Informed Neural Networks. Front. Cardiovasc. Med. 2022, 8, 768419. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Faranesh, A.Z.; Selvaggi, C.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar] [CrossRef]
- Svennberg, E.; Han, J.K.; Caiani, E.G.; Engelhardt, S.; Ernst, S.; Friedman, P.; Garcia, R.; Ghanbari, H.; Hindricks, G.; Man, S.H.; et al. State of the Art of Artificial Intelligence in Clinical Electrophysiology in 2025: A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC Working Group on E-Cardiology. EP Eur. 2025, 27, euaf071. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spaccarotella, C.A.M.; Esposito, G.; Indolfi, C. An Artificial Intelligence Analysis of Electrocardiograms for the Clinical Diagnosis of Cardiovascular Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Scientific Data Curation Team. Metadata Record for: PTB-XL, a Large Publicly Available Electrocardiography Dataset. Figshare. 2020, p. 3235 Bytes. Available online: https://springernature.figshare.com/articles/dataset/Metadata_record_for_PTB-XL_a_large_publicly_available_Electrocardiography_Dataset/12098055 (accessed on 10 October 2025).
- Ribeiro, A.H.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Bjerkén, L.V.; Rønborg, S.N.; Jensen, M.T.; Ørting, S.N.; Nielsen, O.W. Artificial intelligence enabled ECG screening for left ventricular systolic dysfunction: A systematic review. Heart Fail. Rev. 2022, 28, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mannhart, D.; Lischer, M.; Knecht, S.; Lavallaz, J.d.F.d.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 232–242. [Google Scholar] [CrossRef]
- Shahid, S.; Iqbal, M.; Saeed, H.; Hira, S.; Batool, A.; Khalid, S.; Tahirkheli, N.K. Diagnostic Accuracy of Apple Watch Electrocardiogram for Atrial Fibrillation. JACC Adv. 2025, 4, 101538. [Google Scholar] [CrossRef]
- Scholten, J.; Jansen, W.P.J.; Horsthuis, T.; Mahes, A.D.; Winter, M.M.; Zwinderman, A.H.; Keijer, J.T.; Minneboo, M.; De Groot, J.R.; Bokma, J.P. Six-lead device superior to single-lead smartwatch ECG in atrial fibrillation detection. Am. Heart J. 2022, 253, 53–58. [Google Scholar] [CrossRef]
- Pengel, L.K.D.; Robbers-Visser, D.; Groenink, M.; Winter, M.M.; Schuuring, M.J.; Bouma, B.J.; Bokma, J.P. A comparison of ECG-based home monitoring devices in adults with CHD. Cardiol. Young 2023, 33, 1129–1135. [Google Scholar] [CrossRef]
- Wijesurendra, R.; Pessoa-Amorim, G.; Buck, G.; Harper, C.; Bulbulia, R.; Jones, N.R.; A’Court, C.; Kurien, R.; Taylor, K.; Casadei, B.; et al. Active Monitoring for AtriaL FIbrillation (AMALFI): Rationale, protocol, and pilot for a pragmatic, randomized, controlled trial of remote screening for asymptomatic atrial fibrillation. Am. Heart J. 2025, 290, 310–324. [Google Scholar] [CrossRef]
- Proesmans, T.; Mortelmans, C.; Van Haelst, R.; Verbrugge, F.; Vandervoort, P.; Vaes, B. Mobile Phone–Based Use of the Photoplethysmography Technique to Detect Atrial Fibrillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR mHealth uHealth 2019, 7, e12284. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Chang, S.-L.; Yeh, Y.-H.; Chung, F.-P.; Hu, Y.-F.; Chou, C.-C.; Hung, K.-C.; Chang, P.-C.; Liao, J.-N.; Chan, Y.-H.; et al. Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int. J. Cardiol. 2021, 332, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.Y.; Cho, M.S.; Kim, M.; Kim, J.; Oh, I.-Y.; Cho, Y.; Lee, J.H. Artificial intelligence predicts undiagnosed atrial fibrillation in patients with embolic stroke of undetermined source using sinus rhythm electrocardiograms. Heart Rhythm 2024, 21, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Saluja, D.; Huang, Z.; Majumder, J.; Zeldin, L.; Yarmohammadi, H.; Biviano, A.; Wan, E.Y.; Ciaccio, E.J.; Hendon, C.P.; Garan, H. Automated prediction of isthmus areas in scar-related atrial tachycardias using artificial intelligence. J. Cardiovasc. Electrophysiol. 2024, 35, 1401–1411. [Google Scholar] [CrossRef]
- Rodrigo, M.; Alhusseini, M.I.; Rogers, A.J.; Krittanawong, C.; Thakur, S.; Feng, R.; Ganesan, P.; Narayan, S.M. Atrial fibrillation signatures on intracardiac electrograms identified by deep learning. Comput. Biol. Med. 2022, 145, 105451. [Google Scholar] [CrossRef]
- Alhusseini, M.I.; Abuzaid, F.; Rogers, A.J.; Zaman, J.A.B.; Baykaner, T.; Clopton, P.; Bailis, P.; Zaharia, M.; Wang, P.J.; Rappel, W.-J.; et al. Machine Learning to Classify Intracardiac Electrical Patterns During Atrial Fibrillation: Machine Learning of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e008160. [Google Scholar] [CrossRef]
- Fox, S.R.; Toomu, A.; Gu, K.; Kang, J.; Sung, K.; Han, F.T.; Hoffmayer, K.S.; Hsu, J.C.; Raissi, F.; Feld, G.K.; et al. Impact of artificial intelligence arrhythmia mapping on time to first ablation, procedure duration, and fluoroscopy use. J. Cardiovasc. Electrophysiol. 2024, 35, 916–928. [Google Scholar] [CrossRef]
- Park, J. Machine Learning for Predicting Atrial Fibrillation Recurrence After Cardioversion: A Modest Leap Forward. Korean Circ. J. 2023, 53, 690–692. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, E.; Ju, H.; Ahn, H.-J.; Lee, S.-R.; Choi, E.-K.; Suh, J.; Oh, S.; Rhee, W. Machine Learning Prediction for the Recurrence After Electrical Cardioversion of Patients with Persistent Atrial Fibrillation. Korean Circ. J. 2023, 53, 677–689. [Google Scholar] [CrossRef]
- De La Nava, A.M.S.; Arenal, Á.; Fernández-Avilés, F.; Atienza, F. Artificial Intelligence-Driven Algorithm for Drug Effect Prediction on Atrial Fibrillation: An in silico Population of Models Approach. Front. Physiol. 2021, 12, 768468. [Google Scholar] [CrossRef] [PubMed]
- Gruwez, H.; Barthels, M.; Dhont, S.; Meekers, E.; Wouters, F.; Pierlet, N.; Nuyens, D.; Rivero-Ayerza, M.; Van Herendael, H.; Pison, L.; et al. Predicting atrial fibrillation recurrence after catheter ablation using an artificial intelligence-enabled electrocardiogram algorithm. EP Eur. 2024, 26, euae102.167. [Google Scholar] [CrossRef]
- Razeghi, O.; Kapoor, R.; Alhusseini, M.I.; Fazal, M.; Tang, S.; Roney, C.H.; Rogers, A.J.; Lee, A.; Wang, P.J.; Clopton, P.; et al. Atrial fibrillation ablation outcome prediction with a machine learning fusion framework incorporating cardiac computed tomography. J. Cardiovasc. Electrophysiol. 2023, 34, 1164–1174. [Google Scholar] [CrossRef]
- Saglietto, A.; Gaita, F.; Blomstrom-Lundqvist, C.; Arbelo, E.; Dagres, N.; Brugada, J.; Maggioni, A.P.; Tavazzi, L.; Kautzner, J.; De Ferrari, G.M.; et al. AFA-Recur: An ESC EORP AFA-LT registry machine-learning web calculator predicting atrial fibrillation recurrence after ablation. EP Eur. 2023, 25, 92–100. [Google Scholar] [CrossRef]
- Brahier, M.S.; Zou, F.; Abdulkareem, M.; Kochi, S.; Migliarese, F.; Thomaides, A.; Ma, X.; Wu, C.; Sandfort, V.; Bergquist, P.J.; et al. Using machine learning to enhance prediction of atrial fibrillation recurrence after catheter ablation. J. Arrhythmia 2023, 39, 868–875. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Albert, N.M.; Allen, L.A.; Ahmed, R.; Averina, V.; Boehmer, J.P.; Cowie, M.R.; Chien, C.V.; Galvao, M.; Klein, L.; et al. Multiple cArdiac seNsors for mAnaGEment of Heart Failure (MANAGE-HF)—Phase I Evaluation of the Integration and Safety of the HeartLogic Multisensor Algorithm in Patients with Heart Failure. J. Card. Fail. 2022, 28, 1245–1254. [Google Scholar] [CrossRef]
- Virani, S.A.; Sharma, V.; McCann, M.; Koehler, J.; Tsang, B.; Zieroth, S. Prospective evaluation of integrated device diagnostics for heart failure management: Results of the TRIAGE-HF study. ESC Heart Fail. 2018, 5, 809–817. [Google Scholar] [CrossRef]
- Zanotto, G.; Capucci, A. HeartInsight: From SELENE HF to implementation in clinical practice. Eur. Heart J. Suppl. 2023, 25, C337–C343. [Google Scholar] [CrossRef]
- Ginder, C.; Li, J.; Halperin, J.L.; Akar, J.G.; Martin, D.T.; Chattopadhyay, I.; Upadhyay, G.A. Predicting Malignant Ventricular Arrhythmias Using Real-Time Remote Monitoring. J. Am. Coll. Cardiol. 2023, 81, 949–961. [Google Scholar] [CrossRef]
- Varma, N.; Braunschweig, F.; Burri, H.; Hindricks, G.; Linz, D.; Michowitz, Y.; Ricci, R.P.; Nielsen, J.C. Remote monitoring of cardiac implantable electronic devices and disease management. EP Eur. 2023, 25, euad233. [Google Scholar] [CrossRef]
- Loeffler, S.E.; Trayanova, N. Primer on Machine Learning in Electrophysiology. Arrhythmia Electrophysiol. Rev. 2023, 12, e06. [Google Scholar] [CrossRef]
- Missel, R.; Gyawali, P.K.; Murkute, J.V.; Li, Z.; Zhou, S.; AbdelWahab, A.; Davis, J.; Warren, J.; Sapp, J.L.; Wang, L. A hybrid machine learning approach to localizing the origin of ventricular tachycardia using 12-lead electrocardiograms. Comput. Biol. Med. 2020, 126, 104013. [Google Scholar] [CrossRef]
- Sprenger, L.; Moser, F.; Maslova, V.; Zaman, A.; Nonnenmacher, M.; Willert, S.; Frank, D.; Lian, E. Prediction of Ablation Index and Lesion Size Index for Local Impedance Drop-Guided Ablation. J. Clin. Med. 2025, 14, 832. [Google Scholar] [CrossRef]
- Giffard-Roisin, S.; Jackson, T.; Fovargue, L.; Lee, J.; Delingette, H.; Razavi, R.; Ayache, N.; Sermesant, M. Noninvasive Personalization of a Cardiac Electrophysiology Model from Body Surface Potential Mapping. IEEE Trans. Biomed. Eng. 2017, 64, 2206–2218. [Google Scholar] [CrossRef]
- Budzianowski, J.; Kaczmarek-Majer, K.; Rzeźniczak, J.; Słomczyński, M.; Wichrowski, F.; Hiczkiewicz, D.; Musielak, B.; Grydz, Ł.; Hiczkiewicz, J.; Burchardt, P. Machine learning model for predicting late recurrence of atrial fibrillation after catheter ablation. Sci. Rep. 2023, 13, 15213. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, T.; Wang, W.; Han, X.; Liu, M.; Zhang, S.; Feng, W.; Wang, Y.; Chen, Y. Machine learning-based prediction model for recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Front. Cardiovasc. Med. 2025, 12, 1642409. [Google Scholar] [CrossRef]
- Jia, S.; Mou, H.; Wu, Y.; Lin, W.; Zeng, Y.; Chen, Y.; Chen, Y.; Zhang, Q.; Wang, W.; Feng, C.; et al. A Simple Logistic Regression Model for Predicting the Likelihood of Recurrence of Atrial Fibrillation Within 1 Year After Initial Radio-Frequency Catheter Ablation Therapy. Front. Cardiovasc. Med. 2022, 8, 819341. [Google Scholar] [CrossRef]
- Sánchez, J.; Luongo, G.; Nothstein, M.; Unger, L.A.; Saiz, J.; Trenor, B.; Luik, A.; Dössel, O.; Loewe, A. Using Machine Learning to Characterize Atrial Fibrotic Substrate from Intracardiac Signals with a Hybrid in silico and in vivo Dataset. Front. Physiol. 2021, 12, 699291. [Google Scholar] [CrossRef]
- Corrado, C.; Williams, S.; Roney, C.; Plank, G.; O’nEill, M.; Niederer, S. Using machine learning to identify local cellular properties that support re-entrant activation in patient-specific models of atrial fibrillation. EP Eur. 2021, 23 (Suppl. S1), i12–i20. [Google Scholar] [CrossRef]
- Pander, T. An Improved Approach for Atrial Fibrillation Detection in Long-Term ECG Using Decomposition Transforms and Least-Squares Support Vector Machine. Appl. Sci. 2023, 13, 12187. [Google Scholar] [CrossRef]
- Rogers, A.J.; Selvalingam, A.; Alhusseini, M.I.; Krummen, D.E.; Corrado, C.; Abuzaid, F.; Baykaner, T.; Meyer, C.; Clopton, P.; Giles, W.; et al. Machine Learned Cellular Phenotypes in Cardiomyopathy Predict Sudden Death. Circ. Res. 2021, 128, 172–184. [Google Scholar] [CrossRef]
- Melgani, F.; Bazi, Y. Classification of Electrocardiogram Signals with Support Vector Machines and Particle Swarm Optimization. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 667–677. [Google Scholar] [CrossRef]
- Smisek, R.; Hejc, J.; Ronzhina, M.; Nemcova, A.; Marsanova, L.; Kolarova, J.; Smital, L.; Vitek, M. Multi-stage SVM approach for cardiac arrhythmias detection in short single-lead ECG recorded by a wearable device. Physiol. Meas. 2018, 39, 094003. [Google Scholar] [CrossRef]
- Rad, A.B.; Galloway, C.; Treiman, D.; Xue, J.; Li, Q.; Sameni, R.; Albert, D.; Clifford, G.D. Atrial fibrillation detection in outpatient electrocardiogram monitoring: An algorithmic crowdsourcing approach. PLoS ONE 2021, 16, e0259916. [Google Scholar] [CrossRef]
- Mäkynen, M.; Ng, G.A.; Li, X.; Schlindwein, F.S. Wearable Devices Combined with Artificial Intelligence—A Future Technology for Atrial Fibrillation Detection? Sensors 2022, 22, 8588. [Google Scholar] [CrossRef]
- Martinez-Alanis, M.; Bojorges-Valdez, E.; Wessel, N.; Lerma, C. Prediction of Sudden Cardiac Death Risk with a Support Vector Machine Based on Heart Rate Variability and Heartprint Indices. Sensors 2020, 20, 5483. [Google Scholar] [CrossRef]
- Rodriguez, J.; Schulz, S.; Giraldo, B.F.; Voss, A. Risk Stratification in Idiopathic Dilated Cardiomyopathy Patients Using Cardiovascular Coupling Analysis. Front. Physiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- He, K.; Liang, W.; Liu, S.; Bian, L.; Xu, Y.; Luo, C.; Li, Y.; Yue, H.; Yang, C.; Wu, Z. Long-term single-lead electrocardiogram monitoring to detect new-onset postoperative atrial fibrillation in patients after cardiac surgery. Front. Cardiovasc. Med. 2022, 9, 1001883. [Google Scholar] [CrossRef]
- Manohar, A.; Colvert, G.M.; Yang, J.; Chen, Z.; Ledesma-Carbayo, M.J.; Kronborg, M.B.; Sommer, A.; Nørgaard, B.L.; Nielsen, J.C.; McVeigh, E.R. Prediction of Cardiac Resynchronization Therapy Response Using a Lead Placement Score Derived from 4-Dimensional Computed Tomography. Circ. Cardiovasc. Imaging 2022, 15, 603–613. [Google Scholar] [CrossRef]
- Raghunath, S.; Pfeifer, J.M.; Ulloa-Cerna, A.E.; Nemani, A.; Carbonati, T.; Jing, L.; vanMaanen, D.P.; Hartzel, D.N.; Ruhl, J.A.; Lagerman, B.F.; et al. Deep Neural Networks Can Predict New-Onset Atrial Fibrillation from the 12-Lead ECG and Help Identify Those at Risk of Atrial Fibrillation–Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Mahara, K.; Beussink-Nelson, L.; Ikura, H.; Katsumata, Y.; Endo, J.; Gaggin, H.K.; Shah, S.J.; Itabashi, Y.; MacRae, C.A.; et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat. Commun. 2021, 12, 2726. [Google Scholar] [CrossRef]
- Agliari, E.; Barra, A.; Barra, O.A.; Fachechi, A.; Franceschi Vento, L.; Moretti, L. Detecting cardiac pathologies via machine learning on heart-rate variability time series and related markers. Sci. Rep. 2020, 10, 8845. [Google Scholar] [CrossRef]
- O’Brien, H.; Whitaker, J.; Singh Sidhu, B.; Gould, J.; Kurzendorfer, T.; O’Neill, M.D.; Rajani, R.; Grigoryan, K.; Rinaldi, C.A.; Taylor, J.; et al. Automated Left Ventricle Ischemic Scar Detection in CT Using Deep Neural Networks. Front. Cardiovasc. Med. 2021, 8, 655252. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Sun, L.; Cai, J.; Wang, S.; Zeng, L.; Sun, S. Application of machine learning to predict the occurrence of arrhythmia after acute myocardial infarction. BMC Med. Inform. Decis. Mak. 2021, 21, 301. [Google Scholar] [CrossRef]
- Howell, S.J.; Stivland, T.; Stein, K.; Ellenbogen, K.A.; Tereshchenko, L.G. Using Machine-Learning for Prediction of the Response to Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2021, 7, 1505–1515. [Google Scholar] [CrossRef]
- Gallard, A.; Hubert, A.; Smiseth, O.; Voigt, J.-U.; Le Rolle, V.; Leclercq, C.; Bidaut, A.; Galli, E.; Donal, E.; Hernandez, A.I. Prediction of response to cardiac resynchronization therapy using a multi-feature learning method. Int. J. Cardiovasc. Imaging 2021, 37, 989–998. [Google Scholar] [CrossRef]
- Komlósi, F.; Tóth, P.; Bohus, G.; Vámosi, P.; Tokodi, M.; Szegedi, N.; Salló, Z.; Piros, K.; Perge, P.; Osztheimer, I.; et al. Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease. Bioengineering 2023, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D.; De Deus, L.F.; Sehgal, N. Evaluation of Atrial Fibrillation Detection in Short-Term Photoplethysmography (PPG) Signals Using Artificial Intelligence. Cureus 2023, 15, e45111. [Google Scholar] [CrossRef]
- Rad, A.B.; Kirsch, M.; Li, Q.; Xue, J.; Sameni, R.; Albert, D.; Clifford, G.D. A Crowdsourced AI Framework for Atrial Fibrillation Detection in Apple Watch and Kardia Mobile ECGs. Sensors 2024, 24, 5708. [Google Scholar] [CrossRef]
- Donoso, F.I.; Figueroa, R.L.; Lecannelier, E.A.; Pino, E.J.; Rojas, A.J. Clustering of atrial fibrillation based on surface ECG measurements. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 4203–4206. [Google Scholar]
- Li, X.; Almeida, T.P.; Dastagir, N.; Guillem, M.S.; Salinet, J.; Chu, G.S.; Stafford, P.J.; Schlindwein, F.S.; Ng, G.A. Standardizing Single-Frame Phase Singularity Identification Algorithms and Parameters in Phase Mapping During Human Atrial Fibrillation. Front. Physiol. 2020, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Li, Y.; Li, Q.; Liu, X.; Weng, S.; Yin, Y.; Liang, Z.; Zhang, T.; Wang, Y. Atrial fibrillation phenotypes identified through cluster analysis in the CABANA study. Int. J. Cardiol. 2025, 438, 133606. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.P.; Soriano, D.C.; Mase, M.; Ravelli, F.; Bezerra, A.S.; Li, X.; Chu, G.S.; Salinet, J.; Stafford, P.J.; Andre Ng, G.; et al. Unsupervised Classification of Atrial Electrograms for Electroanatomic Mapping of Human Persistent Atrial Fibrillation. IEEE Trans. Biomed. Eng. 2021, 68, 1131–1141. [Google Scholar] [CrossRef]
- Kong, X.; Ravikumar, V.; Mulpuru, S.K.; Roukoz, H.; Tolkacheva, E.G. A Data-Driven Preprocessing Framework for Atrial Fibrillation Intracardiac Electrocardiogram Analysis. Entropy 2023, 25, 332. [Google Scholar] [CrossRef]
- Yang, W.; Deo, R.; Guo, W. Functional feature extraction and validation from twelve-lead electrocardiograms to identify atrial fibrillation. Commun. Med. 2025, 5, 32. [Google Scholar] [CrossRef]
- Sánchez-Carballo, E.; Melgarejo-Meseguer, F.M.; Vijayakumar, R.; Sánchez-Muñoz, J.J.; García-Alberola, A.; Rudy, Y.; Rojo-Álvarez, J.L. Interpretable manifold learning for T-wave alternans assessment with electrocardiographic imaging. Eng. Appl. Artif. Intell. 2025, 143, 109996. [Google Scholar] [CrossRef]
- Jung, S.-J.; Son, C.-S.; Kim, M.-S.; Kim, D.-J.; Park, H.-S.; Kim, Y.-N. Association Rules to Identify Complications of Cerebral Infarction in Patients with Atrial Fibrillation. Healthc. Inform. Res. 2013, 19, 25–32. [Google Scholar] [CrossRef]
- Loring, Z.; Holmes, D.N.; Matsouaka, R.A.; Curtis, A.B.; Day, J.D.; Desai, N.; Ellenbogen, K.A.; Feld, G.K.; Fonarow, G.C.; Frankel, D.S.; et al. Procedural Patterns and Safety of Atrial Fibrillation Ablation: Findings from Get with The Guidelines-Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e007944. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, C. Deep learning and electrocardiography: Systematic review of current techniques in cardiovascular disease diagnosis and management. Biomed. Eng. OnLine 2025, 24, 23. [Google Scholar] [CrossRef]
- Goettling, M.; Hammer, A.; Malberg, H.; Schmidt, M. xECGArch: A trustworthy deep learning architecture for interpretable ECG analysis considering short-term and long-term features. Sci. Rep. 2024, 14, 13122. [Google Scholar] [CrossRef]
- Salih, A.; Boscolo Galazzo, I.; Gkontra, P.; Lee, A.M.; Lekadir, K.; Raisi-Estabragh, Z.; Petersen, S.E. Explainable Artificial Intelligence and Cardiac Imaging: Toward More Interpretable Models. Circ. Cardiovasc. Imaging 2023, 16, e014519. [Google Scholar] [CrossRef]
- Antman, E.M.; Loscalzo, J. Precision medicine in cardiology. Nat. Rev. Cardiol. 2016, 13, 591–602. [Google Scholar] [CrossRef]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, Y.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef]
- Seemann, G.; Höper, C.; Sachse, F.B.; Dössel, O.; Holden, A.V.; Zhang, H. Heterogeneous three-dimensional anatomical and electrophysiological model of human atria. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2006, 364, 1465–1481. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational modeling of cardiac electrophysiology and arrhythmogenesis: Toward clinical translation. Physiol. Rev. 2024, 104, 1265–1333. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Kwon, S.-S.; Wi, J.; Park, M.; Lee, H.-S.; Park, J.-S.; Lee, Y.-S.; Shim, E.B.; Pak, H.-N. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: Comparison with clinical catheter ablation. Prog. Biophys. Mol. Biol. 2014, 116, 40–47. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Hocini, M.; Shah, A.J.; Derval, N.; Sacher, F.; Jais, P.; Dubois, R. Noninvasive Panoramic Mapping of Human Atrial Fibrillation Mechanisms: A Feasibility Report. J. Cardiovasc. Electrophysiol. 2013, 24, 711–717. [Google Scholar] [CrossRef]
- Prakosa, A.; Arevalo, H.J.; Deng, D.; Boyle, P.M.; Nikolov, P.P.; Ashikaga, H.; Blauer, J.J.E.; Ghafoori, E.; Park, C.J.; Blake, R.C.; et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat. Biomed. Eng. 2018, 2, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Hill, Y.R.; Child, N.; Hanson, B.; Wallman, M.; Coronel, R.; Plank, G.; Rinaldi, C.A.; Gill, J.; Smith, N.P.; Taggart, P.; et al. Investigating a Novel Activation-Repolarisation Time Metric to Predict Localised Vulnerability to Reentry Using Computational Modelling. PLoS ONE 2016, 11, e0149342. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.; Prakosa, A.; Aronis, K.N.; Zhou, S.; Zimmerman, S.L.; Tandri, H.; Nazarian, S.; Berger, R.D.; Chrispin, J.; Trayanova, N.A. Personalized Digital-Heart Technology for Ventricular Tachycardia Ablation Targeting in Hearts with Infiltrating Adiposity. Circ. Arrhythmia Electrophysiol. 2020, 13, e008912. [Google Scholar] [CrossRef]
- Koopsen, T.; Gerrits, W.; Van Osta, N.; Van Loon, T.; Wouters, P.; Prinzen, F.W.; Vernooy, K.; Delhaas, T.; Teske, A.J.; Meine, M.; et al. Virtual pacing of a patient’s digital twin to predict left ventricular reverse remodelling after cardiac resynchronization therapy. EP Eur. 2023, 26, euae009. [Google Scholar] [CrossRef]
- Niederer, S.A.; Plank, G.; Chinchapatnam, P.; Ginks, M.; Lamata, P.; Rhode, K.S.; Rinaldi, C.A.; Razavi, R.; Smith, N.P. Length-dependent tension in the failing heart and the efficacy of cardiac resynchronization therapy. Cardiovasc. Res. 2011, 89, 336–343. [Google Scholar] [CrossRef]
- Lumens, J.; Tayal, B.; Walmsley, J.; Delgado-Montero, A.; Huntjens, P.R.; Schwartzman, D.; Althouse, A.D.; Delhaas, T.; Prinzen, F.W.; Gorcsan, J. Differentiating Electromechanical from Non–Electrical Substrates of Mechanical Discoordination to Identify Responders to Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2015, 8, e003744. [Google Scholar] [CrossRef]
- Arnold, R.; Prassl, A.J.; Neic, A.; Thaler, F.; Augustin, C.M.; Gsell, M.A.F.; Gillette, K.; Manninger, M.; Scherr, D.; Plank, G. pyCEPS: A cross-platform electroanatomic mapping data to computational model conversion platform for the calibration of digital twin models of cardiac electrophysiology. Comput. Methods Programs Biomed. 2024, 254, 108299. [Google Scholar] [CrossRef]
- Plank, G.; Loewe, A.; Neic, A.; Augustin, C.; Huang, Y.-L.; Gsell, M.A.F.; Karabelas, E.; Nothstein, M.; Prassl, A.J.; Sánchez, J.; et al. The openCARP simulation environment for cardiac electrophysiology. Comput. Methods Programs Biomed. 2021, 208, 106223. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, P.; Strocchi, M.; Bishop, M.J.; Boyle, P.M.; Plank, G. From bits to bedside: Entering the age of digital twins in cardiac electrophysiology. EP Eur. 2024, 26, euae295. [Google Scholar] [CrossRef]
- Karniadakis, G.E.; Kevrekidis, I.G.; Lu, L.; Perdikaris, P.; Wang, S.; Yang, L. Physics-informed machine learning. Nat. Rev. Phys. 2021, 3, 422–440. [Google Scholar] [CrossRef]
- Sahli Costabal, F.; Yang, Y.; Perdikaris, P.; Hurtado, D.E.; Kuhl, E. Physics-Informed Neural Networks for Cardiac Activation Mapping. Front. Phys. 2020, 8, 42. [Google Scholar] [CrossRef]
- Grandits, T.; Pezzuto, S.; Costabal, F.S.; Perdikaris, P.; Pock, T.; Plank, G.; Krause, R. Learning Atrial Fiber Orientations and Conductivity Tensors from Intracardiac Maps Using Physics-Informed Neural Networks. In Functional Imaging and Modeling of the Heart; Ennis, D.B., Perotti, L.E., Wang, V.Y., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12738, pp. 650–658. [Google Scholar] [CrossRef]
- Herrera, C.R.; Grandits, T.; Plank, G.; Perdikaris, P.; Costabal, F.S.; Pezzuto, S. Physics-informed neural networks to learn cardiac fiber orientation from multiple electroanatomical maps. arXiv 2022, arXiv:2201.12362. [Google Scholar] [CrossRef]
- Dermul, N.; Dierckx, H. Reconstruction of excitation waves from mechanical deformation using physics-informed neural networks. Sci. Rep. 2024, 14, 16975. [Google Scholar] [CrossRef]
- Ghanem, R.N.; Jia, P.; Ramanathan, C.; Ryu, K.; Markowitz, A.; Rudy, Y. Noninvasive Electrocardiographic Imaging (ECGI): Comparison to intraoperative mapping in patients. Heart Rhythm 2005, 2, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bilchick, K.; Xie, J. Physics-informed residual learning with spatiotemporal local support for inverse ECG reconstruction. Sci. Rep. 2025, 15, 31747. [Google Scholar] [CrossRef]
- Xiong, Z.; Fedorov, V.V.; Fu, X.; Cheng, E.; Macleod, R.; Zhao, J. Fully Automatic Left Atrium Segmentation from Late Gadolinium Enhanced Magnetic Resonance Imaging Using a Dual Fully Convolutional Neural Network. IEEE Trans. Med. Imaging 2019, 38, 515–524. [Google Scholar] [CrossRef]
- Borra, D.; Andalò, A.; Paci, M.; Fabbri, C.; Corsi, C. A fully automated left atrium segmentation approach from late gadolinium enhanced magnetic resonance imaging based on a convolutional neural network. Quant. Imaging Med. Surg. 2020, 10, 1894–1907. [Google Scholar] [CrossRef]
- Di Biase, L.; Zou, F.; Lin, A.N.; Grupposo, V.; Marazzato, J.; Tarantino, N.; Della Rocca, D.; Mohanty, S.; Natale, A.; Alhuarrat, M.A.D.; et al. Feasibility of three-dimensional artificial intelligence algorithm integration with intracardiac echocardiography for left atrial imaging during atrial fibrillation catheter ablation. EP Eur. 2023, 25, euad211. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, S.Y.; Gala, D.; Makaryus, A.N. Integration of artificial intelligence into cardiac ultrasonography practice. Expert Rev. Med. Devices 2025, 22, 869–879. [Google Scholar] [CrossRef]
- Puyol-Antón, E.; Sidhu, B.S.; Gould, J.; Porter, B.; Elliott, M.K.; Mehta, V.; Rinaldi, C.A.; King, A.P. A multimodal deep learning model for cardiac resynchronisation therapy response prediction. Med. Image Anal. 2022, 79, 102465. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Zhao, C.; Ling, X.; Malhotra, S.; Qian, Z.; Wang, Y.; Hou, X.; Zou, J.; Zhou, W. A method using deep learning to discover new predictors from left-ventricular mechanical dyssynchrony for CRT response. J. Nucl. Cardiol. 2023, 30, 201–213. [Google Scholar] [CrossRef]
- Ogbomo-Harmitt, S.; Muffoletto, M.; Zeidan, A.; Qureshi, A.; King, A.P.; Aslanidi, O. Exploring interpretability in deep learning prediction of successful ablation therapy for atrial fibrillation. Front. Physiol. 2023, 14, 1054401. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.; Passerini, T.; Audigier, C.; Lluch, È.; Mihalef, V.; Ashikaga, H.; Maier, A.; Halperin, H.; Mansi, T. Extrapolation of Ventricular Activation Times from Sparse Electroanatomical Data Using Graph Convolutional Neural Networks. Front. Physiol. 2021, 12, 694869. [Google Scholar] [CrossRef] [PubMed]
- Verardo, G.; Perez-Ramirez, D.F.; Bruchfeld, S.; Boman, M.; Chiesa, M.; Koch, S.; Maguire, G.Q.; Kostic, D. Reducing the Number of Leads for ECG Imaging with Graph Neural Networks and Meaningful Latent Space. In Statistical Atlases and Computational Models of the Heart. Workshop, CMRxRecon and MBAS Challenge Papers; Camara, O., Puyol-Antón, E., Sermesant, M., Suinesiaputra, A., Zhao, J., Wang, C., Tao, Q., Young, A., Eds.; Lecture Notes in Computer Science; Springer Nature: Cham, Switzerland, 2025; Volume 15448, pp. 301–312. ISBN 978-3-031-87755-1. [Google Scholar] [CrossRef]
- Neri, L.; Oberdier, M.T.; Van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef]
- Heydari, S.; Mahmoud, Q.H. Tiny Machine Learning and On-Device Inference: A Survey of Applications, Challenges, and Future Directions. Sensors 2025, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Wasserlauf, J.; Vogel, K.; Whisler, C.; Benjamin, E.; Helm, R.; Steinhaus, D.A.; Yousuf, O.; Passman, R.S. Accuracy of the Apple watch for detection of AF: A multicenter experience. J. Cardiovasc. Electrophysiol. 2023, 34, 1103–1107. [Google Scholar] [CrossRef]
- Isenegger, C.; Mannhart, D.; Arnet, R.; Jordan, F.; Du Fay De Lavallaz, J.; Krisai, P.; Knecht, S.; Kühne, M.; Sticherling, C.; Badertscher, P. Accuracy of Smartwatches for Atrial Fibrillation Detection Over Time. JACC Clin. Electrophysiol. 2024, 10, 2735–2737. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.-I.; Oh, I.-Y.; Jeong, J.H.; Lee, H.S.; Choi, Y.Y.; Lee, J.H.; Cho, Y.; Shim, J.; Son, H.S.; et al. A Patch-Type Electrocardiography Is Superior to Holter Monitoring for Detecting Paroxysmal Cardiac Arrhythmias. J. Korean Med. Sci. 2025, 40, e168. [Google Scholar] [CrossRef]
- Sibomana, O.; Hakayuwa, C.M.; Obianke, A.; Gahire, H.; Munyantore, J.; Chilala, M.M. Diagnostic accuracy of ECG smart chest patches versus PPG smartwatches for atrial fibrillation detection: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2025, 25, 132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Choi, E.K.; Lee, S.R.; Ahn, H.J.; Lee, B.; Oh, S.; Lip, G.Y.H. Atrial fibrillation detection in ambulatory patients using a smart ring powered by deep learning analysis of continuous photoplethysmography monitoring. Eur. Heart J. 2022, 43, ehac544.415. [Google Scholar] [CrossRef]
- Van Steijn, N.J.; Pepplinkhuizen, S.; Postema, P.G.; Knops, R.E.; Winter, M.M. Ventricular arrhythmia detection with a wearable ring-type photoplethysmography sensor: A feasibility study. Heart Rhythm 2025, S1547527125025548. [Google Scholar] [CrossRef] [PubMed]
- Avanu, A.E.; Dodi, G. Wear Your Heart on Your Sleeve: Smart Textile ECG Wearables for Comfort, Integration, Signal Quality and Continuous Monitoring in Paroxysmal Atrial Fibrillation. Sensors 2025, 25, 676. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Eldesouky, M.; Antoun, I.; Lau, E.Y.M.; Koya, A.; Vali, Z.; Suleman, S.A.; Donaldson, J.; Ng, G.A. Wearable Devices for Arrhythmia Detection: Advancements and Clinical Implications. Sensors 2025, 25, 2848. [Google Scholar] [CrossRef]
- Ghassemi, M.; Oakden-Rayner, L.; Beam, A.L. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit. Health 2021, 3, e745–e750. [Google Scholar] [CrossRef]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Liu, X.; Chan, A.-W.; Denniston, A.K.; Calvert, M.J.; SPIRIT-AI and CONSORT-AI Working Group; SPIRIT-AI and CONSORT-AI Steering Group; SPIRIT-AI and CONSORT-AI Consensus Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Nat. Med. 2020, 26, 1351–1363. [Google Scholar] [CrossRef]
- Tjoa, E.; Guan, C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4793–4813. [Google Scholar] [CrossRef] [PubMed]
- Vasey, B.; Nagendran, M.; Campbell, B.; Clifton, D.A.; Collins, G.S.; Denaxas, S.; Denniston, A.K.; Faes, L.; Geerts, B.; Ibrahim, M.; et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat. Med. 2022, 28, 924–933. [Google Scholar] [CrossRef]
- Melvin, T. The European Medical Device Regulation-What Biomedical Engineers Need to Know. IEEE J. Transl. Eng. Health Med. 2022, 10, 4800105. [Google Scholar] [CrossRef]
- Brady, A.P.; Allen, B.; Chong, J.; Kotter, E.; Kottler, N.; Mongan, J.; Oakden-Rayner, L.; Dos Santos, D.P.; Tang, A.; Wald, C.; et al. Developing, purchasing, implementing and monitoring AI tools in radiology: Practical considerations. A multi-society statement from the ACR, CAR, ESR, RANZCR & RSNA. Insights Into Imaging 2024, 15, 16. [Google Scholar] [CrossRef]
- Conduah, A.K.; Ofoe, S.; Siaw-Marfo, D. Data privacy in healthcare: Global challenges and solutions. Digit. Health 2025, 11, 20552076251343959. [Google Scholar] [CrossRef]
- Williams, P.; Woodward, A. Cybersecurity vulnerabilities in medical devices: A complex environment and multifaceted problem. Med. Devices: Evid. Res. 2015, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Tangari, G.; Ikram, M.; Ijaz, K.; Kaafar, M.A.; Berkovsky, S. Mobile health and privacy: Cross sectional study. BMJ 2021, 373, n1248. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, C.; Lee, I.; Lee, K.; Ong, K.-L. A survey on security and privacy issues in wearable health monitoring devices. Comput. Secur. 2025, 155, 104453. [Google Scholar] [CrossRef]
- Leone, D.M.; O’sullivan, D.; Bravo-Jaimes, K. Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review. Children 2024, 12, 25. [Google Scholar] [CrossRef]
- Apfelbacher, T.; Koçman, S.E.; Prokosch, H.-U.; Christoph, J. A Governance Framework for the Implementation and Operation of AI Applications in a University Hospital. Stud. Health Technol. Inform. 2024, 316, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Wessler, B.S.; Nelson, J.; Park, J.G.; McGinnes, H.; Gulati, G.; Brazil, R.; Van Calster, B.; van Klaveren, D.; Venema, E.; Steyerberg, E.; et al. External Validations of Cardiovascular Clinical Prediction Models: A Large-Scale Review of the Literature. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007858. [Google Scholar] [CrossRef]
- Okada, N.; Kubota, A.; Imamura, T.; Suwa, H.; Kawaguchi, Y.; Ohshio, G.; Seino, Y.; Imamura, M. Evaluation of cholecystokinin, gastrin, CCK-A receptor, and CCK-B/gastrin receptor gene expressions in gastric cancer. Cancer Lett. 1996, 106, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Sinha, S.; Zhai, B.; Fudulu, D.; Chan, J.; Narayan, P.; Judge, A.; Caputo, M.; Dimagli, A.; Benedetto, U.; et al. Performance Drift in Machine Learning Models for Cardiac Surgery Risk Prediction: Retrospective Analysis. JMIRx Med. 2024, 5, e45973. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022, 25, 3–9. [Google Scholar] [CrossRef]
- Voets, M.M.; Veltman, J.; Slump, C.H.; Siesling, S.; Koffijberg, H. Systematic Review of Health Economic Evaluations Focused on Artificial Intelligence in Healthcare: The Tortoise and the Cheetah. Value Health 2022, 25, 340–349. [Google Scholar] [CrossRef]
- El Arab, R.A.; Al Moosa, O.A. Systematic review of cost effectiveness and budget impact of artificial intelligence in healthcare. NPJ Digit. Med. 2025, 8, 548. [Google Scholar] [CrossRef]
- Wu, H.; Jin, K.; Yip, C.C.; Koh, V.; Ye, J. A systematic review of economic evaluation of artificial intelligence-based screening for eye diseases: From possibility to reality. Surv. Ophthalmol. 2024, 69, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, N.; Laviola, D.; Mariani, M.V.; Nardini, A.; Adamo, F.; Mahfouz, K.; Colaiaco, C.; Ammirati, F.; Santini, L.; Lavalle, C. Remote monitoring and heart failure. Eur. Heart J. Suppl. 2025, 27, i126–i131. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Sahiner, B.; Chen, W.; Samala, R.K.; Petrick, N. Data drift in medical machine learning: Implications and potential remedies. Br. J. Radiol. 2023, 96, 20220878. [Google Scholar] [CrossRef]
- Andersen, E.S.; Birk-Korch, J.B.; Hansen, R.S.; Fly, L.H.; Röttger, R.; Arcani, D.M.C.; Brasen, C.L.; Brandslund, I.; Madsen, J.S. Monitoring performance of clinical artificial intelligence in health care: A scoping review. JBI Evid. Synth. 2024, 22, 2423–2446. [Google Scholar] [CrossRef] [PubMed]

| Device | Core Performance Indicators | Regulatory Status |
|---|---|---|
| Apple Watch (iECG + IHRN PPG) | AF detection sensitivity 94.8%; specificity 95%; AUC 0.96 [32] | FDA/CE |
| AliveCor KardiaMobile (iECG) | AF detection sensitivity 100%; specificity 97% [33] | |
| Withings ScanWatch (iECG) | AF detection sensitivity 78%, specificity 80% [34] | |
| iRhythm Zio® services (patch for continuous ECG) | Diagnostic yield vs. Holter in AF diagnosis 6.8% vs. 5.4%; median time-to-diagnosis 103 vs. 530 days [35] | |
| Rooti Rx (patch for continuous ECG) | Diagnostic yield vs. Holter in overall arrhythmia detection 59.5% vs. 19.0% (p < 0.001); in AF/AFL 9.5% vs. 3.8% [38] | |
| Fitbit (IHRN PPG) | AF detection PPV 98.2%; sensitivity 67.6% at episode level; specificity 98.4% [23] | FDA |
| Samsung Galaxy Watch (iECG + IHRN PPG) | AF detection sensitivity 85%; specificity 75% [31] | |
| FibriCheck (IHRN PPG) | AF detection sensitivity 96%; specificity 97% [36] | CE |
| Algorithm | Core Inputs (CIED-Derived) | Weights Availability |
|---|---|---|
| HeartLogic (Boston Scientific) |
| Composite index; weights not public [51,52] |
| TriageHF/HFRS (Medtronic) |
| Rule/Bayesian tiers; no numeric weights [53] |
| HeartInsight (Biotronik) |
| Single score; no fixed % weights [54,55] |
| Algorithm | Description | Applicable Scenarios | Data Requirements | Computational Resources |
|---|---|---|---|---|
| Least-squares regression | Models the relationship between a continuous outcome and inputs by minimizing the sum of squared residuals. |
|
| CPU only |
| Support vector machine | Classifies responses by learning a separating hyperplane between groups. |
|
| CPU/GPU optional |
| Neural networks | Learn patterns in data via layered processing of inputs to produce predicted outputs. |
|
| GPU required |
| Random forest | Builds an ensemble of decision trees from bootstrapped samples to predict outcomes. |
|
| CPU sufficient |
| Algorithm | Description | Applicable Scenarios | Data Requirements | Computational Resources |
|---|---|---|---|---|
| Clustering (k-means, DBSCAN) | Automatically groups data into homogeneous clusters based on similarity. |
|
| CPU only |
| Dimensionality reduction (PCA, t-SNE, UMAP) | Reduces input dimensionality and derives principal components or low-dimensional embeddings that preserve relationships among data points. |
|
| CPU for PCA; GPU optional for t-SNE/UMAP |
| Association Rule Learning | Identifies frequent co-occurrences/correlations among events or features in clinical data |
|
| CPU only |
| Unsupervised deep learning | Automatically extracts salient features from complex data without labels. |
|
| GPU required |
| Technology | Principle | Inputs | Applications in EP | Potential Clinical Impact |
|---|---|---|---|---|
| Digital twin | Patient-specific electromechanical models |
|
|
|
| Physics-informed neural networks | Neural networks constrained by physical laws |
|
|
|
| Deep learning for imaging | CNN/U-Net for segmentation, tissue quantification, and multimodal prediction |
|
|
|
| Graph convolutional neural network | Graph learning over irregular point sets with graph convolutions. |
|
|
|
| Advanced wearables and on-device AI | Real-time, on-device signal analysis |
|
|
|
| Dimension | Key Benefits | Key Concerns |
|---|---|---|
| Advantages | ||
| Scalability | Population-level screening and risk stratification with low marginal cost per evaluation | Risk of under-representation; Excessive alerts without established escalation protocols. |
| Standardization | More consistent EGM interpretation and substrate tagging; reduced inter-operator variability; reproducible mapping/lesion selection | Over-reliance on algorithmic labels; need for external validation and periodic recalibration across centers/devices. |
| Timeliness | Earlier detection of HF decompensation and arrhythmic instability via remote multiparametric analytics; faster, safer workflows in-lab. | False positives/negatives can consume resources or delay care; requires monitored thresholds and clinician oversight. |
| Personalization | Patient-specific planning via digital twins; image- and signal-informed models tailor ablation/CRT and follow-up. | Data quality and model calibration are critical; individual simulations require robust workflow and governance. |
| System-wide impact | Equitable screening access, reproducible lab decisions, proactive remote care, and improved resource allocation | Benefits are conditional on operational readiness, resourcing, and pathway integration. |
| Limitations | ||
| Generalizability | Multicenter datasets and external testing improve portability of models across sites and hardware. | Dataset shift, selection bias, and noisy labels degrade performance; demands continuous performance monitoring. |
| Interpretability | Task-relevant, physiology-aligned explanations increase clinician trust and actionability. | Superficial saliency maps can mislead; require stability testing and uncertainty quantification. |
| Safety | Prospective evaluation, post-market monitoring (MLOps), clear human-in-the-loop controls for uncertainty/OOD. | Governance overhead with the need for audit trails, rigorous versioning, and controls to suspend or revert models. |
| Organizational readiness | Seamless EHR/mapping integration; defined roles; trained teams; medico-legal clarity. | Integration costs/time; unclear accountability can stall or negate value. |
| Economic value | Potential cost offsets via fewer rehospitalizations, shorter procedures/fluoroscopy, targeted follow-up. | Must be proven in pragmatic trials with CHEERS-quality economic reporting; hidden costs of maintenance and updates. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canino, G.; Di Costanzo, A.; Salerno, N.; Leo, I.; Cannataro, M.; Guzzi, P.H.; Veltri, P.; Sorrentino, S.; De Rosa, S.; Torella, D. Artificial Intelligence in Cardiac Electrophysiology: A Clinically Oriented Review with Engineering Primers. Bioengineering 2025, 12, 1102. https://doi.org/10.3390/bioengineering12101102
Canino G, Di Costanzo A, Salerno N, Leo I, Cannataro M, Guzzi PH, Veltri P, Sorrentino S, De Rosa S, Torella D. Artificial Intelligence in Cardiac Electrophysiology: A Clinically Oriented Review with Engineering Primers. Bioengineering. 2025; 12(10):1102. https://doi.org/10.3390/bioengineering12101102
Chicago/Turabian StyleCanino, Giovanni, Assunta Di Costanzo, Nadia Salerno, Isabella Leo, Mario Cannataro, Pietro Hiram Guzzi, Pierangelo Veltri, Sabato Sorrentino, Salvatore De Rosa, and Daniele Torella. 2025. "Artificial Intelligence in Cardiac Electrophysiology: A Clinically Oriented Review with Engineering Primers" Bioengineering 12, no. 10: 1102. https://doi.org/10.3390/bioengineering12101102
APA StyleCanino, G., Di Costanzo, A., Salerno, N., Leo, I., Cannataro, M., Guzzi, P. H., Veltri, P., Sorrentino, S., De Rosa, S., & Torella, D. (2025). Artificial Intelligence in Cardiac Electrophysiology: A Clinically Oriented Review with Engineering Primers. Bioengineering, 12(10), 1102. https://doi.org/10.3390/bioengineering12101102










