Abstract
Artificial intelligence (AI) is transforming cardiac electrophysiology across the entire care pathway, from arrhythmia detection on 12-lead electrocardiograms (ECGs) and wearables to the guidance of catheter ablation procedures, through to outcome prediction and therapeutic personalization. End-to-end deep learning (DL) models have achieved cardiologist-level performance in rhythm classification and prognostic estimation on standard ECGs, with a reported arrhythmia classification accuracy of ≥95% and an atrial fibrillation detection sensitivity/specificity of ≥96%. The application of AI to wearable devices enables population-scale screening and digital triage pathways. In the electrophysiology (EP) laboratory, AI standardizes the interpretation of intracardiac electrograms (EGMs) and supports target selection, and machine learning (ML)-guided strategies have improved ablation outcomes. In patients with cardiac implantable electronic devices (CIEDs), remote monitoring feeds multiparametric models capable of anticipating heart-failure decompensation and arrhythmic risk. This review outlines the principal modeling paradigms of supervised learning (regression models, support vector machines, neural networks, and random forests) and unsupervised learning (clustering, dimensionality reduction, association rule learning) and examines emerging technologies in electrophysiology (digital twins, physics-informed neural networks, DL for imaging, graph neural networks, and on-device AI). However, major challenges remain for clinical translation, including an external validation rate below 30% and workflow integration below 20%, which represent core obstacles to real-world adoption. A joint clinical engineering roadmap is essential to translate prototypes into reliable, bedside tools.
1. Introduction
In recent years, artificial intelligence (AI) has been redefining cardiology, owing to the availability of large datasets derived from high-frequency physiologic signals, multimodal imaging, longitudinal time series, and heterogeneous clinical variables that align well with advances in machine learning (ML) and deep learning (DL) methods [1].
Specifically, electrophysiology (EP) is the cardiology subspecialty with the broadest availability of structured and semi-structured data, ranging from 12-lead electrocardiograms (ECGs) and photoplethysmograms from wearable devices to intracardiac potentials, three-dimensional (3D) electroanatomical maps (EAMs), and continuous data streams from cardiac implantable electronic devices (CIEDs) [2,3] (Figure 1).
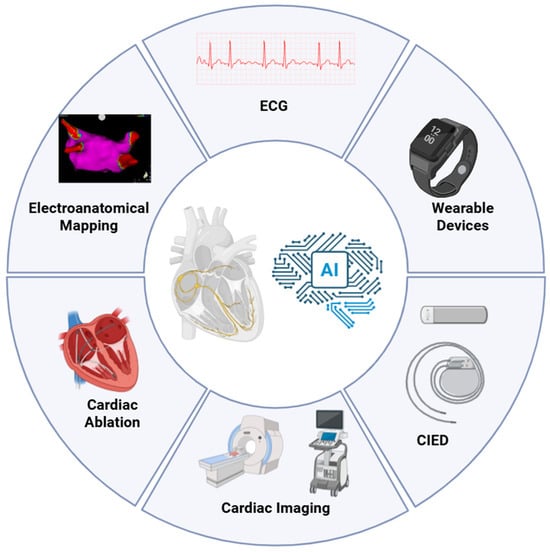
Figure 1.
Domains of AI application across the cardiac electrophysiology care pathway. Schematic overview of the principal data sources and procedural touchpoints leveraged by AI in EP. AI integrates non-invasive signals (12-lead ECG and wearable PPG), intracardiac data from the EP lab (EGMs and 3D EAM systems), cardiac imaging (echocardiography, CT, CMR), and continuous device streams from CIEDs to enable screening, risk stratification, guidance of catheter ablation, and longitudinal monitoring. AI, Artificial Intelligence; CIED, Cardiac Implantable Electronic Device; CMR, Cardiovascular Magnetic Resonance; CT, Computed Tomography; ECG, Electrocardiogram.
Within this context, AI has produced particularly robust evidence: automated arrhythmia classification, support for localizing ablation targets, personalized prognostic estimation, and proactive remote monitoring [4]. The recent literature documents an acceleration both in model performance and in the availability of prospective and real-world studies that begin to quantify clinical impact; nonetheless, challenges remain regarding external validation, integration into operational workflows, and model interpretability [5].
On the surface-signal front, the ECG has become the “test bench” of computational cardiology: end-to-end convolutional neural networks (CNNs) trained on raw traces have achieved, or surpassed, cardiologist-level performance in rhythm classification, demonstrating that clinically useful information is encoded in patterns that escape the human eye [6]. The pioneering work by Hannun and colleagues on more than 90,000 ambulatory recordings is often cited as proof of principle for the scalability and accuracy of these approaches [7]. In parallel, the Artificial-Intelligence Electrocardiography (AI-ECG) line of research has expanded the scope from diagnosis to prognosis: algorithms trained on standard ECGs can identify a reduced ejection fraction or occult systolic dysfunction, with prospective validations in routine clinical populations and evaluations in emergency settings [8]. Research has also been facilitated by the availability of large, well-annotated public datasets, such as Public ECG dataset “PTB-XL” (PTB-XL) and its extension PTB-XL+, which enable benchmarking and generalization studies [9,10]. The convergence of AI and wearable devices has extended screening to the population level, opening avenues for digital triage pathways and targeted follow-up (FU) [11].
In patients with CIEDs, remote monitoring enables multiparametric models capable of anticipating heart-failure decompensation and arrhythmic risk [12]. Growing evidence from both prospective studies and real-world data shows that structured alerts embedded within codified care pathways are associated with appropriate management and improved outcomes, offering windows for intervention before clinical deterioration [13].
Concurrently, applications based on DL are increasingly used to connect anatomy and signals: from U-Net architectures for atrial segmentation on late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) or computed tomography (CT) to aid ablation planning to models combining imaging and clinical data to predict responses to cardiac resynchronization therapy (CRT) or post-ablation outcomes [12,14,15,16].
In the EP laboratory, AI supports the standardized interpretation of EGMs and selection of activation patterns that guide mapping and ablation [17,18]. AI models have demonstrated reproducible identification of critical areas in atrial fibrillation (AF) and the classification of arrhythmogenic potentials in ventricular tachycardias (VTs), with concrete promise to reduce inter-operator variability, harmonize substrate tagging, and expedite intraprocedural decision-making [19,20].
A complementary and innovative thread is represented by emerging technologies that integrate electrical signals, geometry, and physics patient specific digital twins (DTs), physics-informed neural networks (PINNs), DL for imaging, graph-based AI on sparse data, and wearables with on-device AI [21,22,23]. These tools enable truly personalized care, reducing procedural time and risk and standardizing workflows without replacing clinical judgment.
The proper application of AI in electrophysiology requires a clear understanding of how models are built, their assumptions, and their limitations. In other words, clinicians and engineers need a common framework and terminology for understanding how models learn, are evaluated, and are validated and monitored over time. Current guidelines reflect this need; they specify methodological and reporting requirements to ensure that AI-based predictive models are developed, tested, and described transparently, avoiding overfitting and systematic bias [24].
AI in EP has moved beyond the pioneering phase across multiple domains, from AI-ECG to wearable-based screening and from support for intracavitary mapping to remote monitoring with CIEDs [24]. To translate these innovations into clinical benefit, an integrated clinical engineering workflow is essential, articulating model assumptions, quantifying uncertainty, ensuring calibration and external validation, and designing explainable, physiologically coherent tools [25]. From this perspective, tight integration among data, algorithms, and clinical practice is not optional but foundational to the era of precision medicine, enabling AI to inform truly personalized, timely, and measurable decision-making in electrophysiology.
2. Clinical Landscape of Artificial Intelligence in Electrophysiology
Clinical electrophysiology generates large volumes of data ideally suited for AI analysis [24]. Applications range from arrhythmia recognition to the guidance of ablation and from outcome prediction to personalized therapy. A useful synthesis distinguishes three principal domains: non-invasive electrocardiographic signals, EP laboratory data (EGMs, EAM, procedural imaging), and longitudinal information from clinical FU and implantable devices (CIEDs) (Figure 2).
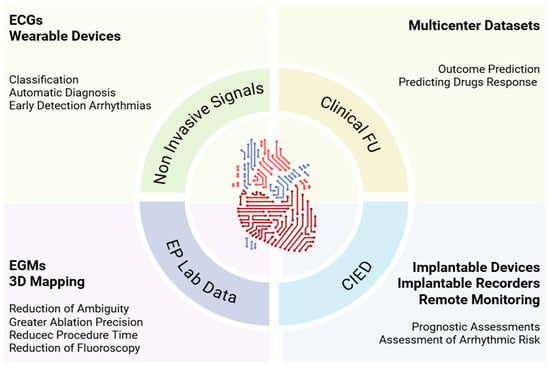
Figure 2.
Clinical landscape of AI in EP. AI supports EP from ECGs and wearables that enable earlier arrhythmia detection, to EP-lab learning from EGMs and 3D maps that sharpen ablation and reduce time and fluoroscopy, to CIED-based remote monitoring that refines prognosis and risk. Combined with clinical FU and multicenter datasets, models predict outcomes and drug response, turning routine data into decision support. 3D, Three Dimensional; AI, Artificial Intelligence; CIED, Cardiac Implantable Electronic Device; EP, Electrophysiology; ECGs, Electrocardiograms; EGMs, Intracardiac Electrograms; FU, Follow-Up; Lab, Laboratory.
Across all three, model performance is rapidly improving, although challenges persist in external validation, workflow integration, and interpretability [5].
2.1. Non-Invasive Electrocardiographic Signals
Non-invasive electrocardiographic signals provide an enormous volume of data. Automated ECG analysis was among the earliest applications of AI in cardiology [7]. Standard ECGs produce simultaneous multilead time series from which algorithms can extract beat-to-beat rhythm and P/QRS/ST-T morphology features [26]. Twelve-lead recordings project the same cardiac electrical vector into space, enabling the encoding of conduction blocks, arrhythmias, hypertrophy, and ischemia through inter-lead correlations. Adding clinical metadata (e.g., sex, age, symptoms) further enhances risk stratification [27]. The availability of large public datasets has accelerated research and enabled standardized benchmarking [28]. PTB-XL provides >21,000 recordings and is now a reference dataset for the training/validation of 12-lead ECG algorithms; extensions such as PTB-XL+ add derived features, facilitating studies on explainability and generalization [9].
In this setting, end-to-end DL models, particularly CNNs that apply learnable filters directly to raw traces to autonomously derive discriminative representations, have outperformed handcrafted-feature approaches for tasks such as arrhythmia classification and the detection of latent information not visible to human readers. Hannun and colleagues developed a CNN capable of classifying 12 rhythms from >90,000 ambulatory recordings on single-lead data [7]. These CNNs achieved a mean AUC of 0.97 and an F1 score of 0.837, with “cardiologist-level” performance for automated rhythm classification. Ribeiro et al., in contrast, operated on 12-lead ECGs and targeted six abnormalities (rhythmic and morphological) trained on >2 million exams; on a cardiologist-annotated test set, it reported F1 > 80% for all classes and a specificity of >99% [28]. AI-ECG by Attia et al. is different again: it does not classify rhythm types but detects a latent AF “signature” in sinus rhythm using 649,931 ECGs, with an AUC of 0.87 and an accuracy of ~79–83% [29].
Beyond diagnosis, AI-ECG has shifted the focus toward prognosis: algorithms trained on standard ECGs can identify reduced left-ventricular ejection fraction in prospectively validated settings, anticipate the need for echocardiography, and flag at-risk individuals even in the absence of evident clinical signs [30]. For arrhythmias, DL models demonstrate sensitivity/specificity comparable to, or exceeding, traditional methods for AF detection, including challenging contexts such as paroxysmal episodes and noisy signals [29].
In parallel, the advent of wearable devices (e.g., smartwatches) has opened the door to continuous, population-level screening, enabling the earlier identification of clinically silent arrhythmias. Clinically approved (FDA/CE) applications include on-device single-lead ECGs (e.g., smartwatch iECG, handheld devices/ECG patches) and large-scale PPG screening modules [31]. For iECG devices, the most informative metrics are sensitivity/specificity and, for notification algorithms, positive predictive value, along with practical indicators such as signal quality and the proportion of inconclusive tracings [23,32,33,34,35,36]. The Apple Heart Study demonstrated the feasibility of AF detection via photoplethysmography (PPG) in very large cohorts, inaugurating a new era of digital cardiology [37]. For clinical ECG patches, studies more often report diagnostic yield relative to the 24 h Holter rather than per-patient sensitivity/specificity, reflecting their role in detecting paroxysmal arrhythmias over prolonged monitoring windows (Table 1) [35,38].

Table 1.
Clinically approved applications. Clinically approved solutions include Apple Watch, AliveCor KardiaMobile, Withings ScanWatch, Fitbit, Samsung and FibriCheck and clinical patches (iRhythm, Rooti Rx). AF, Atrial Fibrillation; AFL, Atrial Flutter; AUC, Area Under the Curve; CE, CE mark (Conformité Européenne); ECG, Electrocardiogram; FDA, U.S. Food and Drug Administration; iECG, Single-Lead Integrated ECG; IHRN, Irregular Heart Rhythm Notification; PPG, Photoplethysmography; PPV, Positive Predictive Value; vs., Versus.
Beyond marketed devices, a clinical-validation tier includes AI-ECG models that, while not yet cleared for specific diagnostic indications, show prospective and implementation-level performance. Examples are algorithms that infer an “AF signature” in sinus rhythm to flag individuals at risk and extended-wear strategies embedded in care pathways that improve diagnostic efficiency and may accelerate anticoagulation decisions [39].
At the laboratory/early-stage tier, methods remain preclinical but promising. Graph neural networks can prioritize isthmus regions in scar-related atrial tachycardia for targeting, while other deep-learning approaches refine AF risk estimation or mapping from limited inputs [40]. These require multicenter prospective validation and the assessment of clinical impact before translation.
In summary, the ECG is an ideal substrate for supervised end-to-end models owing to the richness of its time series, the relative reliability of labeling, and the availability of large datasets.
2.2. Electrophysiology Laboratory Data
During invasive procedures, the large amount of data collected via multipolar catheters (high-density EGMs and EAM) and 3D imaging systems represent a remarkable resource for AI applications.
Key algorithmic objectives include standardizing and accelerating signal interpretation, identifying informative substrate/ablation targets, and reducing procedural times. Machine learning can standardize electrogram interpretation, reduce ambiguity, and offer a platform potentially usable in real time to support mapping and ablation decisions [41]. For example, to overcome inter-observer variability in EGMs signal analysis during AF, Alhusseini and colleagues showed that a CNN can objectively recognize complex activation patterns on bi-atrial maps and guide AF ablation [42]. Beyond AF, AI has also shown utility in VT for recognizing arrhythmogenic patterns and classifying potential targets. In post-ischemic VT, a supervised classifier trained on EGMs identified arrhythmogenic ventricular potentials, decreasing reliance on purely visual interpretation and standardizing selection of ablation sites [19].
Machine learning is also becoming a practical assistant for determining ablation sites in both AF and VT. In AF, the randomized TAILORED-AF trial showed that an ML-guided strategy—identifying spatiotemporal dispersion areas on EGMs and treating them in addition to pulmonary vein isolation (PVI)—was superior to PVI alone in achieving AF freedom at 12 months (88% vs. 70%), demonstrating that objective, reproducible signal analysis can effectively direct lesion selection [11].
In post-ischemic VT, Baldazzi et al. developed and validated a supervised approach to automatically recognize abnormal ventricular potentials on bipolar electrograms, achieving cross-validation accuracies of ≥93% [19]. Model-flagged sites aligned with en-trances/isthmuses and regions ultimately ablated, suggesting the feasibility of standardizing substrate tagging and expediting target identification, particularly in complex circuits. From an efficiency standpoint, Fox et al. reported that AI-assisted, 12-lead, ECG-informed arrhythmia mapping was associated with shorter procedures (−22.6%) and reduced fluoroscopy time (−43.7%) without adverse effects on outcomes or complications; 6-month arrhythmia-free survival was 73.5% (63.3% in the control group) [43].
2.3. Longitudinal Information from Clinical Follow-Up and Implantable Cardiac Electronic Devices (CIEDs)
Another rapidly expanding area is outcome prediction after pharmacologic therapy or ablation. Supervised algorithms trained on large multicenter datasets have shown the ability to integrate clinical, electrophysiological, and imaging variables to estimate individual risk, an approach aligned with personalized medicine that aims to prospectively identify patients most likely to benefit from a given treatment. In the cardioversion setting, ML models combining clinical features and ECG have accurately predicted short-term recurrence and suggested personalized surveillance schemes [44,45]. In parallel, exploratory in silico studies have demonstrated the feasibility of predicting antiarrhythmic drug responses in virtual populations, which is useful for hypothesis generation and rhythm-control selection [46].
In recent years, supervised models have achieved good accuracy in predicting AF recurrence after ablation by combining clinical, electrocardiographic, and imaging variables [47,48,49,50].
Extending these predictive frameworks from static, episode-based datasets to continuous, real-world physiology, CIEDs and implantable loop recorders provide continuous data streams and a privileged substrate for predictive models, also thanks to remote monitoring. The most robust evidence concerns the prediction of heart failure (HF) decompensation and arrhythmic risk (Table 2). In patients monitored remotely, multiparametric algorithms trained and validated in prospective cohorts are automating HF risk estimation. Prospective and real-world studies are consolidating the association of structured alerts—embedded in codified care pathways—with improved outcomes and reduced rehospitalizations when integrated into organized clinical workflows [42,51,52,53,54]. On the arrhythmic front, daily analysis of remote Implantable Cardioverter-Defibrillator (ICD) data enables models that predict imminent appropriate therapies, electrical storms, or AF recurrence, offering a window to optimize medical therapy, device programming, or the timing of interventions [55,56].

Table 2.
Core parameters of multiparametric CIED models. This table summarizes the main device-derived inputs used by contemporary multiparametric algorithms embedded in CIED. Each combines daily trends from multiple sensors. AF, Atrial Fibrillation; AT, Atrial Tachyarrhythmia; CIED, Cardiac Implantable Electronic Device; HF, Heart Failure; HFRS, Heart Failure Risk Status; HR, Heart Rate; HRV, Heart-Rate Variability; RR, Respiratory Rate; RSBI, Rapid Shallow-Breathing Index; VP, Ventricular Pacing; VT, Ventricular Tachycardia; VF, Ventricular Fibrillation.
The integration of AI into clinical decision support systems (CDSS) for electrophysiology is consolidating. The goal is not to replace clinicians but to standardize high-variability steps and accelerate repetitive decisions from signals and maps.
In summary, the clinical landscape indicates that AI has already produced tools with potentially meaningful impact in everyday practice. To make this impact systematic, mechanisms must be made explicit, training/validation criteria transparent, and the limitations hampering adoption acknowledged. Here, the engineering contribution is decisive: translating algorithms into robust, explainable, interoperable solutions; ensuring calibration, safety, and post-deployment monitoring; and enabling the prospective evaluation of clinically relevant endpoints.
3. Technical Foundations and Engineering Considerations
In cardiac electrophysiology, AI plays a pivotal role in advancing new diagnostic and therapeutic techniques. Machine learning (ML) is an active research area with broad clinical application [57]. Understanding the underlying models and learning principles is essential to assess the utility, reliability, and limitations of AI in electrophysiology. The ML methods most frequently applied in this field are supervised (Table 3) and unsupervised learning (Table 4).

Table 3.
Supervised machine-learning methods in cardiac electrophysiology. Summary of common algorithms, core method, and representative clinical applications in EP. In supervised learning a model is trained on human-labeled inputs and output datasets, in order to identify a set of patterns that can be used for mapping the features of interest on correct outcomes. For instance, this allows for addressing clinical questions. AF, Atrial Fibrillation; CPU, Central Processing Unit; ECG, Electrocardiogram; EGM, Electrogram; GPU, Graphics Processing Unit.

Table 4.
Unsupervised machine-learning methods in cardiac electrophysiology. Summary of common algorithms, core method, and representative clinical applications in EP. In unsupervised learning no predefined labels or ground-truth outputs are available. The algorithm autonomously identifies the set of patterns by analyzing latent structures, relationships, and clusters within the data. CPU, Central Processing Unit; DBSCAN, Density-Based Spatial Clustering of Applications with Noise; ECG, Electrocardiogram; EGM, Electrogram; GPU, Graphics Processing Unit; PCA, Principal Component Analysis; T-SNE, T-Distributed Stochastic Neighbor Embedding; UMAP, Uniform Manifold Approximation and Projection.
3.1. Supervised Learning
Supervised algorithms are widely used and are trained on datasets containing both input data “X” (ECGs, EGMs, clinical data, imaging data, procedural parameters) and the correct outputs “y” (diagnosis, site of origin, risk of recurrence, time-to-event). The model learns the mapping from inputs to the correct outputs (X→y) with the goal of generalizing to unseen data and answering the relevant clinical question. Here, labeled input are needed; the latter are generally the responsibility of a human operator to ensure the truthfulness and correctness of the information.
3.1.1. Least-Squares Regression (LSR)
Least-squares regression (LSR) characterizes the relationship between a continuous variable “y” (ms, mV, Ω, mm, %) and one or more measured inputs. LSR estimates the line that passes “as close as possible” to all points by minimizing the sum of squared residuals (vertical point-to-line distances), yielding a rule for predicting values at new points and coefficients (Δ) that quantify each variable’s contribution to the output. When relationships are nonlinear (e.g., exponential or logistic), LSR can be extended to fit curves rather than straight lines.
In electrophysiology, LSR is applied according to the clinical question, classification, regression, survival/time-to-event, or segmentation. Classification is used to categorize inputs; for example, ML models have classified the site of origin of VT from the ECG [58]. Regression is used when the outcome is continuous, such as estimating an index, burden (%), conduction velocity (CV), local impedance, or lesion depth from procedural parameters like power, duration, contact force, or impedance. Sprenger et al. used regression to predict the Ablation Index (AIx) and Lesion Size Index (LSI) during radiofrequency (RF) ablation for AF from local impedance values and application duration [59]. Giffard-Roisin et al. applied a regression model to predict response to CRT using non invasive data; body-surface potential mapping enabled the preoperative estimation of activation onset and tissue conductivity [60]. For survival outcomes, the objective is time-to-event, widely used to predict time to recurrence after AF ablation [61,62,63]. Segmentation models provide “point-by-point” maps, classifying each site as healthy, border, or fibrotic based on intracardiac signals and enabling identification of target zones for ablation [64].
3.1.2. Support Vector Machines (SVMs)
Support vector machines (SVMs) are fast and efficient models, particularly useful in electrophysiology when datasets are relatively small but high-dimensional. Their primary function is classification by identifying a decision boundary (hyperplane) that separates classes. In simple cases, the boundary is linear; for nonlinearly separable data, a curved hyperplane in a transformed feature space is used.
In electrophysiology, SVMs have been applied to AF screening from ECG, risk stratification, and prognostic prediction from electrophysiological signals [65,66,67,68]. For screening, a major application is arrhythmia detection from short, single-lead ECGs recorded by wearable devices [69,70,71,72]. For sudden cardiac death risk, Rodriguez et al. used an SVM model in dilated cardiomyopathy based on heart-rate variability (HRV) and blood-pressure variability (BPV) [73]. SVMs have also been used to estimate postoperative AF risk from long-term ECG analysis and to predict favorable prognosis (CRT response) or adverse outcomes as VT or ventricular fibrillation (VF) susceptibility in ischemic cardiomyopathy [67,74,75].
3.1.3. Supervised Neural Networks
Supervised neural networks (NNs) comprise “artificial neurons” arranged in layers that receive inputs, process them, and produce outputs. During training, networks are provided both raw inputs and the corresponding correct outputs, learning complex patterns that elude traditional models.
Clinically, NNs are widely used because they can learn the relative importance of features and infer relationships to generate predictions such as AF onset, HF progression, post-MI arrhythmia, or diagnoses of conditions predisposing to sudden cardiac death. Several studies, including the algorithm by Attia and colleagues, trained networks to diagnose AF from ECGs recorded during normal sinus rhythm [76,77]. Another application is the early detection of rare cardiac diseases strongly associated with sudden death; for example, Goto et al. developed a model for ECG-based prescreening of cardiac amyloidosis [78]. Augusto et al. reported the superior, reproducible automated measurement of maximal left-ventricular-wall thickness, with implications for the timely diagnosis and treatment of hypertrophic cardiomyopathy. Prognostic modelling is also feasible: MacGregor et al. built an algorithm to identify patients with dilated cardiomyopathy likely to respond to medical therapy alone [79]. Multiple NN models have been developed to stratify arrhythmic risk in post-MI patients [80,81].
3.1.4. Random Forest
Random forests consist of ensembles of decision trees built via bootstrapping, with each tree trained on heterogeneous samples from the same dataset (clinical features, derived ECG/EGM variables, imaging) and random feature subsets at each split. For classification, predictions are made by majority vote (or averaged probabilities); for regression, they are made by averaging tree estimates.
This approach is widely used to predict responses to CRT using multiple features, including clinical data, ECG variables, echocardiographic measurements, biomarker levels, and left-ventricular lead position [82,83]. Random forests have also been used to estimate recurrence risk after ablation and are commonly applied in screening to identify features associated with AF diagnosis [84,85,86].
3.2. Unsupervised Learning
Unlike supervised learning, where algorithms learn from labelled data (inputs with corresponding correct outputs), unsupervised learning relies on unannotated datasets. The goal is not to “predict” a known outcome but to identify hidden structures, relationships, patterns, or clusters. This is especially promising in electrophysiology, where signals are often complex, noisy, and non-intuitive, and where a diagnostic gold standard or well-defined outcomes may be lacking. Unsupervised algorithms can generate new clinical hypotheses, explore patient/signal subgroups with shared characteristics, and uncover recurrent electrophysiological patterns that guide therapy personalization or reveal new ablation targets.
3.2.1. Clustering
Clustering is the most common technique for automatically grouping data into homogeneous clusters. K-means clustering partitions data into k groups by minimizing distances to cluster centroids [87]. DBSCAN (Density-Based Spatial Clustering of Applications with Noise) is advantageous for irregular distributions, identifying “dense” regions and separating them from sparse, noisy areas [88]. Hierarchical clustering constructs a dendrogram to explore cluster structure at different levels of granularity [87].
In electrophysiology, clustering is mainly used to phenotype patients or signals into homogeneous groups. Zhang et al. stratified participants in the CABANA trial, identifying AF phenotypes with distinct clinical and prognostic features [89]. Treatment personalization, particularly risk categorization, has become a key objective of contemporary clinical studies. Clustering has also been applied to distinguish normal potentials, fractionated signals, and low-voltage activity during EAM, improving identification of critical zones (e.g., macro-reentry pathways or abnormal conduction) for tachyarrhythmia ablation [90]. Kong et al. used clustering to select the optimal filter to remove loss-of-contact and noisy-electrode artifacts when identifying active sites [91].
3.2.2. Dimensionality Reduction
Electrophysiology datasets often comprise thousands of variables (ECG features, imaging parameters, clinical variables, intracardiac signals), necessitating dimensionality reduction. This is a crucial analytical step: it improves visualization, reduces noise, supports clustering, and facilitates more robust models.
Principal component analysis (PCA) identifies principal components—new variables formed as linear combinations of the originals—that explain most of the dataset variance. Dimensionality reduction is frequently used as a preprocessing step for other algorithms, removing redundancy and enhancing interpretability. Yang et al. highlighted PCA use in ECG processing for dimensionality reduction and feature extraction, confirming associations between specific features and future AF onset [92].
Newer methods such as t-Distributed Stochastic Neighbor Embedding (t-SNE) and Uniform Manifold Approximation and Projection (UMAP) provide intuitive two-dimensional representations that preserve local similarity structure. Sánchez Carballo et al. showed that UMAP can reduce high-dimensional ECG data into a more interpretable 2D “map” [93]. Signals from anatomically adjacent cardiac regions clustered together in this 2D space, suggesting that UMAP can yield a “simplified electrical map” that captures local electrical patterns without the full complexity of the original signals.
3.2.3. Association Rule Learning
Association rule learning (ARL) identifies frequent co-occurrences among clinical events or features.
In electrophysiology, ARL can reveal recurrent patterns linking clinical characteristics and arrhythmia types, or procedural parameters and ablation failures [94]. Direct applications in EP remain sparse but represent a promising area [95].
3.2.4. Unsupervised Deep Learning
DL has transformed signal and image analysis by enabling CNNs to automatically extract salient features from complex data such as 12-lead ECGs or cardiac MRI images. The main limitation remains the opacity of decision processes, the “black box”, raising concerns about trust and interpretability in clinical settings [96].
From an engineering standpoint, there is growing interest in explainable AI (XAI), designed to deliver not only predictions but also insight into the reasoning behind them [97,98]. In electrophysiology, this may include highlighting ECG segments most responsible for a tachycardia classification. Such transparency is essential to foster clinician acceptance.
In summary, the engineering foundations provide clinicians with the vocabulary and conceptual framework needed to appraise the reliability of AI models and to engage meaningfully with developers in translating algorithms into robust, explainable, and interoperable tools for clinical electrophysiology.
4. Emerging Technologies
Alongside established applications, several technologies have recently emerged that, through integration with AI, promise to transform clinical electrophysiology (Table 5).

Table 5.
Emerging AI technologies in cardiac electrophysiology. Compact overview of core principles, data inputs, applications, and clinical impact for key technologies. 3D, Three Dimensional; AF, Atrial Fibrillation; CMR, Cardiovascular Magnetic Resonance; CNN, Convolutional Neural Network; CRT, Cardiac Resynchronization Therapy; CT, Computed Tomography; CV, Conduction Velocity; EAM, Electroanatomical Mapping; EAT, Epicardial Adipose Tissue; ECGI, Electrocardiographic Imaging; EGM, Electrogram; EP, Electrophysiology; GCNN, Graph Convolutional Neural Network; ICE, Intracardiac Echocardiography; LA, Left Atrium; LGE, Late Gadolinium Enhancement; PPG, Photoplethysmography; VT, Ventricular Tachycardia.
In the era of precision medicine, tailoring therapies to the individual patient has become a central objective of care [99].
4.1. Digital Twin
A DT is a patient-specific model that replicates cardiac anatomy and physiology, enabling the simulation of electrical activity and in silico testing of therapeutic strategies before intervention [100,101,102].
In AF, patient-specific “virtual ablation” on reconstructed atrial geometries has allowed for the comparison of lesion strategies and was followed by a multicenter randomized study demonstrating feasibility and the ability to predict the most effective lesion sets in persistent AF [103]. Haïssaguerre and colleagues combined high-density ECG with chest CT to reconstruct bi-atrial activation patterns in real time and identify AF drivers (foci/rotors) [104].
For VT, personalized “virtual-heart” models derived from LGE-MRI/CT can predict minimal ablation targets and guide procedures even without invasive mapping [105]. A complementary approach is the reentry vulnerability index (RVI): simple pacing protocols yield maps comparing local recovery (repolarization) with activation time between neighboring points, highlighting regions most susceptible to reentry [106]. In practice, low-RVI clusters co-localize with exits/isthmuses of scar-related VT and indicate effective ablation sites. Additional tools, such as the integration of CT-derived intramyocardial/epicardial fat marker (inFAT), further refine target selection [107].
In cardiac pacing, a major challenge is predicting responses to CRT, given the proportion of non-responders. Mechanical digital-twin “virtual pacing,” personalized using strain and echocardiography, has predicted left-ventricular reverse remodeling after CRT, suggesting a role for DT in candidate selection and therapy optimization [108]. A patient-specific electromechanical model showed that length–tension dependence (Frank–Starling) can already synchronize stress/strain; in such cases, the incremental benefit of CRT may be limited [109]. To support candidate selection, Lumens et al. proposed the Systolic Stretch Index (SSI), which distinguishes true electromechanical discoordination from hypocontractility/scar and predicts post-CRT outcomes [110].
Operationalizing the twin paradigm requires open tools: platforms such as pyCEPS convert proprietary EAM data into open formats, facilitating calibration, integration, and reproducibility [111]. However, use is currently limited to a few major mapping systems (CARTO 3 and EnSite Precision); conversion is not yet universal, and centers using unsupported systems may experience lower conversion success rates. The openCARP v18.1 ecosystem provides an open, reproducible environment (with Python 3.10.12 pipelines) that promotes standardization, transparency, and shareable simulation workflows—key prerequisites for robust, AI-integrated DT [112]. However, simulations with realistic anatomy and high-resolution meshes can take hours for a single-patient model, which does not meet intraoperative real-time needs; current practice is preoperative use with offline calibration and simulation.
A recent review proposed a unifying framework for electrophysiology DT, emphasizing the transition from static models to continuously updated, highly calibrated, patient-specific twins that seamlessly integrate imaging, signals, and clinical data [113]. Looking ahead, DTs may become operational tools to guide personalized decisions, shorten procedures, and increase ablation safety. In summary, preoperative digital-twin simulations can streamline ablation—reducing the number of lesions/passes and enabling more targeted substrate selection—and limit iterations during mapping. These benefits, however, require upfront investments (engineering time, high-resolution imaging/segmentation, computation) and dedicated infrastructure (servers, storage, quality assurance). To date, peer-reviewed, longitudinal cost-effectiveness evidence specifically for digital twins in electrophysiology remains limited.
4.2. Physics-Informed Neural Networks
PINNs are hybrid learning approaches in which neural networks are trained not only on data but also on the physical equations governing myocardial electrical conduction [114].
In practice, PINNs do not just learn from data; they embed physical laws into training. The loss function combines a data term and a physics term (equation residuals), so the model is penalized both when it deviates from measurements and when it violates physiology. Key laws/constraints in cardiac electrophysiology:
- Eikonal equation (activation times). Guides reconstruction of activation-time maps: where conduction is slow (scar/fibrosis), activation must occur later. The PINN prevents unrealistic “jumps” of the wavefront.
- Monodomain/bidomain models (current propagation). Describe how current spreads in an anisotropic myocardium (easier along fibers, harder across) and how it depends on ionic currents (e.g., Hodgkin–Huxley, ten Tusscher–Panfilov, O’Hara–Rudy). The PINN enforces charge conservation, anisotropy, and consistency with ionic models, reducing non-physiologic solutions.
- Boundary/initial conditions. At tissue borders (e.g., chambers or non-conductive scar) current does not cross the boundary (no-flux/Neumann). The PINN respects these anatomical “walls”.
- Physiological constraints. Clinical guardrails: conduction velocity ≥ 0, diffusivity ≥ 0, APD within plausible ranges, anisotropy aligned with fiber orientation. The PINN penalizes solutions outside these ranges.
In electrophysiology, they enable the reconstruction of activation maps from sparse, noisy measurements; the estimation of tissue parameters (diffusivity/CV, anisotropy, excitability and recovery properties); and solutions to inverse problems such as electrocardiographic imaging (ECGi), which infers cardiac electrical activity from torso-surface signals [22]. Compared with purely data-driven methods, PINNs are more robust and yield results that are physiologically plausible and interpretable.
Sahli-Costabal et al. used a PINN constrained by the eikonal equation to produce realistic activation and conduction-velocity maps from few measurements; an active-learning component that recommends the next sampling points suggests the potential to reduce procedural time and improve mapping accuracy [115]. In parallel, Grandits et al. applied PINNs to identify tissue properties (anisotropy, fiber orientation, conductivities) directly from EAM [116]. The concept was extended with FiberNet, which integrates multiple activation maps to robustly infer local fiber orientation, moving closer to truly patient-specific models [117]. Herrero Martin et al. demonstrated tissue characterization and therapy guidance using PINNs: from sparse, noisy data, they reconstructed the full action potential, estimated electrophysiological parameters (Action Potential Duration or APD, excitability, diffusion), and assessed sensitivity to antiarrhythmic drug effects [22]. Dermul et al. unified mechanical and electrical signals with PINNs, reconstructing activation from tissue motion and opening avenues for non-invasive mapping with potential reductions in procedural time and risk [118].
ECGi is a non-invasive technique that, using a multielectrode vest and chest/heart CT or MRI, reconstructs epicardial potentials and activation times via inverse algorithms [119]. In ECGi, Bacoyannis et al. proposed a generative deep-learning approach (Conditional Variational Autoencoder or CVAE) that integrates anatomy and torso potentials to estimate volumetric activation maps probabilistically, showing that data-driven models can capture the spatiotemporal correlations of the inverse problem [2]. More recently, Zhu et al. introduced a PINN with residual learning and local spatiotemporal support that mitigates typical PINN limitations (overfitting to collocation points, training instability, poor scalability), achieving more accurate, noise-robust ECGi reconstructions [120]. Collectively, these studies advance hybrid models in which data and physics combine to improve non-invasive inversion of cardiac electrical activity [120].
Overall, PINNs offer a promising bridge between biophysical simulation and AI, enabling clinically usable cardiac DTs.
4.3. Deep Learning for Cardiac Imaging
DL is already widely used in cardiovascular imaging, and EP-focused applications are expanding rapidly. The automated segmentation of atrial and ventricular chambers enables the rapid, precise reconstruction of structures for use in mapping systems.
U-Net–type architectures segment the left atrium (LA) and myocardium on LGE-CMR, reducing time and inter-operator variability and enabling standardized workflows for ablation planning and the construction of patient-specific models [15,121,122]. Applying U-Nets to cardiac CT similarly segments the LA/right atrium (RA) and epicardial adipose tissue (EAT) within seconds to quantify remodeling and predict AF recurrence after ablation [14]. On intracardiac echocardiography (ICE), dedicated algorithms guide view selection and are improving the robustness of 3D left-atrial rendering, reducing operator dependence [123,124]. Beyond segmentation, DL is informing clinical decision-making: models that integrate imaging (CT/LGE/echo) with clinical data predict AF ablation outcomes and CRT response [48,116,125]. For electro-functional imaging, DL-based ECGi seeks to translate surface ECGs into 3D activation maps, fusing anatomical images (CT/CMR) with electrical signals to non-invasively reconstruct cardiac activity [2].
In summary, DL applied to LGE-CMR, CT, and echocardiography provides complementary building blocks, segmentation, fibrosis/remodeling quantification, and predictive modeling, which accelerate data-driven EP workflows and the transition to patient-specific models integrable with mapping systems [126].
4.4. Graph-Based AI and Convolutional Models for Sparse Data
A rapidly growing area is the use of graph convolutional neural networks (GCNNs) to extract clinically useful information from EGMs acquired with multipolar catheters. These algorithms handle unstructured, incomplete datasets and can estimate complex electrophysiologic parameters even when mapping points are irregularly distributed, raising the possibility of shorter procedures without loss of accuracy.
In the atria, GCNN trained on EAM have predicted critical isthmuses in scar-related tachycardias, showing that learning on graphs can identify ablation targets from imperfect point sets [40,127]. In the ventricles, a GCNN trained on realistic simulations and integrated with 12-lead ECG and CMR reconstructs 3D activation-time maps from sparse points and can suggest subsequent sampling locations, with the potential to reduce procedural duration [128].
For ECGi, recent studies indicate that GNNs may reduce the number of electrodes while preserving reconstruction quality—a step toward less-burdensome, faster-to-configure systems [129].
This line of work complements other DL approaches operating on intracardiac signals and maps, reinforcing the concept that geometry-aware models can accelerate mapping, decrease dependence on sampling density, and standardize intraprocedural interpretation.
4.5. Advanced Wearables and On-Device AI
Prevention and monitoring are increasingly centered on wearable devices, enabling large-scale screening and timelier arrhythmia management; recent reviews of AI-enabled wearable ECG confirm the trend and growing clinical impact in electrophysiology [130]. Hardware–software advances are moving computation onto the device (edge-AI/TinyML), allowing for continuous signal analysis and real-time notifications [131]. From a regulatory perspective, on-device features (Apple and Samsung Irregular Rhythm Notification, Apple AFib History, Fitbit AF algorithms) are already marketed as screening support tools [132,133].
Diagnostic performance is high for AF screening with smartwatches, Holters, and ECG patches (sensitivities/specificities ~96–98%), particularly for event detection in post-cryptogenic-stroke screening [134,135]. Novel form factors are emerging: smart rings have early validations for AF and, experimentally, for ventricular arrhythmias [136,137]; in parallel, e-textiles and multichannel wearables show Holter-comparable accuracy in daily practice with greater comfort and adherence [138].
Overall, wearables are evolving from simple sensors to decision-support tools that can be integrated into EP workflows, from remote triage to FU and procedural planning, while generating standardized datasets for patient-specific modeling [139].
In summary, these emerging technologies do not replace traditional clinical approaches; rather, they augment them, providing tools that may reshape the practice of electrophysiology in the coming years.
5. Ethical, Legal, and Regulatory Considerations for AI in Cardiac Electrophysiology
Despite the impressive achievements of artificial intelligence (AI) in cardiac electrophysiology (EP), several limitations and challenges must be critically acknowledged before large-scale clinical implementation.
5.1. Ethical Considerations
AI can broaden access, standardize decisions, and anticipate risk windows at low marginal cost. However, these advantages hinge on the quality and representativeness of the data. Biases in training datasets can produce systematic disparities in diagnostic accuracy across gender, ethnicity, or device type [140,141,142]. Mitigation requires the deliberate inclusion of underrepresented populations, periodic auditing, and the use of explainable AI (XAI) methods [7,143]. Patient trust is fundamental: patients should be aware that AI contributes to, rather than replaces, clinical decision-making [144]. Transparent XAI outputs and traceable algorithmic reasoning are crucial to address “black-box” concerns and to sustain clinician acceptance [143,145]. Finally, the introduction of AI-based decision support should not widen health inequities between well-resourced centers and facilities with limited resources [146].
5.2. Regulatory and Legislative Challenges
In the United States, AI-enabled medical devices are reviewed by the FDA via one of three premarket pathways: 510(k) clearance, De Novo classification, or Premarket Approval (PMA). As most AI software qualifies as Software as a Medical Device (SaMD), development should align with Good Machine Learning Practice (GMLP) principles and, where applicable, a Predetermined Change Control Plan (PCCP). GMLP provides ten lifecycle-wide principles, from design and data governance to clinical validation and post-market monitoring, that function as a practical checklist for submissions and audits. For models that learn or update over time, the PCCP final guidance allows manufacturers to predefine which elements of the algorithm may evolve, how those changes will be controlled, and which guardrails/metrics will be tracked post-market, enabling iterative updates without repeating a full authorization each time.
In the European Union, Regulation (EU) 2017/745 (MDR) strengthens safety, performance, and post-market surveillance requirements for CE marking [147]. The Medical Device Coordination Group (MDCG) 2019-11 Rev.1 remains the key guidance for software qualification/classification. The EU AI Act adds AI-specific, horizontal obligations (risk management, data governance, transparency, logging, human oversight) on top of MDR. In parallel, General Data Protection Regulation (GDPR) for EU and Health Insurance Portability and Accountability Act (HIPAA) for US require robust data protection and cybersecurity [148,149,150].
The spread of AI-enabled wearables for arrhythmia detection has raised data-privacy concerns. PPG/ECG signals are personal health data. Recent analyses indicate that only about one-third (~30–34%) of manufacturers implement encryption and key management in line with protection standards across the entire data pathway, creating a risk of information leakage [151]. On-device AI reduces exposure but does not eliminate security and privacy issues [152]. Accordingly, data minimization, Data Protection Impact Assessments (DPIAs), independent audits, and traceable logging are needed. In addition, there is a lack of specific regulatory guidance for AI algorithms that process cardiac rhythm data in minors (covering consent, parental access, retention, and profiling), warranting pediatric-specific safeguards [153].
Medico-legal liability for algorithmic error remains a legal gray area, underscoring the need for explicit human oversight, clear accountability, and auditable versioning/logging throughout the AI lifecycle [154].
5.3. Clinical Validation and Generalizability
AI has considerable potential in cardiology, yet many models still lack robust external validation on independent cohorts, limiting their reliability beyond the development setting [25]. Fewer than one-third of cardiology AI studies undergo external validation, undermining real-world clinical utility [155]. Real-world heterogeneity, data drift, and differences in acquisition hardware can further erode performance, making in-production monitoring and model maintenance plans essential [156,157]. To ensure both generalizability and clinical benefit, multicenter, prospective studies with clinically meaningful endpoints are needed, ideally designed and reported according to SPIRIT-AI and CONSORT-AI extensions for AI-enabled clinical trials [144,158].
5.4. Integration and Application
For benefits to translate meaningfully to the bedside, healthcare systems need interoperability with electronic health records and mapping platforms, continuous MLOps-based model monitoring, and comprehensive staff training [159]. A responsible AI framework, transparent, explainable, fair, and subject to ongoing validation, is essential for clinical acceptance [24,160]. Multidisciplinary collaboration among clinicians, engineers, bioethicists, and regulators should guide the entire AI lifecycle, ensuring that innovation yields a safer and more equitable electrophysiology practice. Organizational readiness, workflow redesign, role definition, training, and sustainable maintenance costs, often determines success or failure [159,161]. When these conditions are met, evidence points to system-level gains: broader and more sustainable screening, more reproducible in-lab decision-making, earlier interventions, and more personalized therapies.
6. Conclusions
Cardiac electrophysiology is entering a phase in which AI is no longer merely a technical accessory but a structural component of diagnostic, therapeutic, and FU pathways.
Evidence accrued across ECG, wearable devices, intracardiac mapping, imaging, and remote monitoring demonstrates concrete clinical value, standardizing decisions, anticipating windows of risk, and personalizing procedural choices. The most robust signal comes from AI-ECG, where end-to-end NNs have achieved cardiologist-level performance for rhythm classification and, crucially, have shifted the emphasis toward prognosis by identifying latent risk phenotypes from standard traces [7,8,9]. Expansion to wearables has enabled population-scale screening and digital triage pathways driven by algorithmic alerts, with feasibility demonstrated in very large cohorts [37]. In the EP laboratory, supervised models applied to EGMs recognize complex activation patterns, reduce inter-operator variability, and assist target selection in AF and VT; early clinical data indicate shorter procedures and reduced fluoroscopy without compromising outcomes [19]. On the FU side, CIEDs have turned telemetric streams into actionable clinical signals: multiparametric scores anticipate heart-failure decompensation and arrhythmic instability, and prospective/real-world studies suggest that, when embedded within structured pathways with dedicated teams, they can reduce rehospitalizations and enable earlier interventions [13,51,53].
The technological trajectory further suggests that progress will not derive solely from larger or more efficient models but from integrated systems that fuse signals, images, and the physical laws of cardiac conduction. In this context, patient-specific DTs enable pre-procedural simulation, “virtual ablation” in AF, and the identification of minimal VT targets, with feasibility and predictive performance that foreshadow operational use for the planning and quality control of ablation strategies [103,105,108]. In parallel, PINNs, by embedding conduction physics alongside measured data, offer more robust and interpretable solutions to inverse problems and to tissue-property estimation from sparse measurements; prospectively, they may shorten mapping times and intelligently guide acquisition of additional points, with tangible intra-procedural potential [22]. Imaging powered by DL completes the picture: U-Net–type architectures markedly shorten atrial and myocardial segmentation on LGE-CMR/CT, enhance reproducibility of substrate quantification, and integrate with multimodal models that predict CRT response and post-ablation outcomes, laying the groundwork for truly patient-specific workflows [125,126]. Finally, the adoption of GCNN on electro-anatomic graphs shows that clinically useful information can be extracted even from incomplete maps, opening avenues for greater procedural efficiency [40].
Against this backdrop, concrete advantages are emerging. Scalability enables broader screening and risk stratification at low marginal cost per evaluation [7]. Standardization in the EP lab, via algorithmic electrogram analysis, reduces inter-operator variability in activation-pattern interpretation and yields more uniform substrate tagging, directly informing mapping and ablation decisions [11]. Timeliness improves through multiparametric algorithms from remote monitoring, which identify hemodynamic and arrhythmic instability earlier and trigger response pathways before clinical decompensation [162]. Personalization is now tangible through integration of imaging, signals, and simulation [105]. Collectively, these benefits point to potential system-wide impact: more accessible and equitable pathways through scalable screening, more reproducible in-lab decision-making, earlier interventions enabled by telemonitoring, and therapies better tailored to individual patients [11].
Important limitations remain. Generalizability can be undermined by selection bias, distribution shift, and noisy labels; addressing these issues requires multicenter consortia and robustly annotated datasets, together with interoperable tools and formats that promote reproducibility and technology transfer [25,111,112,163,164]. Interpretability must advance toward explanations that are clear, stable, and physiologically coherent [140,141]. Safety demands prospective studies, continuous post-deployment monitoring, and explicit rules for clinician intervention when the model is uncertain or operating out of distribution [143,144,146,165]. Organizational factors ultimately determine bedside value: seamless IT integration, staff training, clearly defined roles, and medico-legal responsibilities across the algorithm’s lifecycle [148,154,156]. Finally, sustainability should be demonstrated in pragmatic trials incorporating rigorous economic analyses and pathway impact, to justify large-scale adoption (Table 6) [159,160,161].

Table 6.
Advantages and limitations of AI in electrophysiology. Summary of key clinical/organizational benefits and methodological/implementation challenges across screening, EP lab, and follow-up. AF, Atrial Fibrillation; AI-ECG, AI Applied To ECG; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; CRT, Cardiac Resynchronization Therapy; EGM, Intracardiac Electrogram; EP, Electrophysiology; EHR, Electronic Health Record; HF, Heart Failure; Mlops, Machine Learning Operations; OOD, Out Of Distribution; VT, Ventricular Tachycardia; XAI, Explainable AI.
A four-stage clinical translation roadmap—from preclinical validation to multicenter trials, regulatory approval, and clinical promotion—can guide real-world adoption (Figure 3).
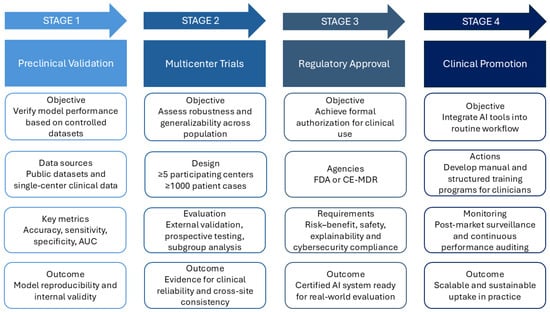
Figure 3.
Clinical translation roadmap for AI in cardiac electrophysiology. Four staged pathways from laboratory prototype to routine care. Stage 1—Preclinical Validation: verify model performance on controlled datasets and single-center data. Stage 2—Multicenter Trials: ≥5 centers and ≥1000 cases to assess robustness and generalizability. Stage 3—Regulatory Approval: submissions to FDA or CE-MDR with documented risk–benefit, safety, explainability, and cybersecurity compliance. Stage 4—Clinical Promotion: integrate into routine workflow with an AI operation manual, structured clinician training and monitor via post-market surveillance and continuous performance auditing. AI, Artificial Intelligence; AUC, Area Under the Curve; CE-MDR, Conformité Européenne—Medical Device Regulation (EU 2017/745); FDA, U.S. Food and Drug Administration.
Looking ahead, AI does not replace clinical judgment; it amplifies precision and timeliness when embedded in co-designed workflows with explicit decision thresholds, utility metrics, and clear governance. If these conditions are met, the convergence of AI-ECG, on-device wearables, ML-guided mapping, multiparametric CIED analytics, DL-enhanced imaging, GNNs, and DT can yield a truly data-driven, patient-specific electrophysiology, where therapeutic choices are simulated before execution, risk windows are identified before clinical destabilization, and uncertainty is surfaced and managed, delivering concrete, measurable benefits for patients.
Author Contributions
Conceptualization, G.C. and D.T.; writing—original draft preparation, G.C. and A.D.C.; writing—review and editing, G.C., A.D.C., N.S., I.L., M.C., P.H.G., P.V., S.S. and S.D.R.; visualization, M.C., P.H.G. and P.V.; funding acquisition, D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Italian Ministry of University and Research: PRIN 2020L45ZWA_005, PRIN-PNRR 2022: P2022NRRB8; PRIN 2020L45ZW4_005, and PNRR—the National Center for Gene Therapy and Drugs based on RNA Technology (CN00000041), and from the Italian Ministry of Health: PSC SALUTE 2014–2020-POS4 “Cal-Hub-Ria” (T4-AN-09) and PNRR MAD-2022–12376814.
Data Availability Statement
No new data were created for this review article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D | Three Dimensional |
| AF | Atrial Fibrillation |
| AFib | Atrial Fibrillation |
| AIx | Ablation Index |
| AI | Artificial Intelligence |
| AI-ECG | Artificial-Intelligence Electrocardiography |
| APD | Action Potential Duration |
| ARL | Association Rule Learning |
| BPV | Blood-Pressure Variability |
| CABANA | Catheter ABlation vs. ANtiarrhythmic Drug Therapy for Atrial Fibrillation (trial) |
| CDSS | Clinical Decision Support Systems |
| CE | Conformité Européenne |
| CHEERS | Consolidated Health Economic Evaluation Reporting Standards |
| CIED | Cardiac Implantable Electronic Device |
| CMR | Cardiovascular Magnetic Resonance |
| CNN | Convolutional Neural Network |
| CRT | Cardiac Resynchronization Therapy |
| CT | Computed Tomography |
| CV | Conduction Velocity |
| CVAE | Conditional Variational Autoencoder |
| DBSCAN | Density-Based Spatial Clustering of Applications with Noise |
| DL | Deep Learning |
| DPIA | Data Protection Impact Assessment |
| DT | Digital Twin |
| EAM | Electroanatomical Mapping |
| EAT | Epicardial Adipose Tissue |
| ECG | Electrocardiogram |
| ECGi | Electrocardiographic Imaging |
| EGM | Intracardiac Electrogram |
| edge-AI | On-device/edge Artificial Intelligence |
| EHR | Electronic Health Record |
| EU | European Union |
| FDA | Food and Drug Administration |
| FU | Follow Up |
| GCNN | Graph Convolutional Neural Network |
| GDPR | General Data Protection Regulation |
| GMLP | Good Machine Learning Practice |
| HF | Heart Failure |
| HIPAA | Health Insurance Portability and Accountability Act |
| HRV | Heart-Rate Variability |
| ICD | Implantable Cardioverter-Defibrillator |
| ICE | Intracardiac Echocardiography |
| inFAT | CT-derived intramyocardial/epicardial fat marker |
| LA | Left Atrium |
| LGE-CMR | Late Gadolinium Enhancement Cardiac Magnetic Resonance |
| LSI | Lesion Size Index |
| LSR | Least-Squares Regression |
| MDR | Medical Device Regulation |
| MLOps | Machine Learning Operations |
| NN | Neural Network(s) |
| OOD | Out-Of-Distribution |
| PCA | Principal Component Analysis |
| PCCP | Predetermined Change Control Plan |
| PINN | Physics-Informed Neural Network |
| PMA | Premarket Approval |
| PPG | Photoplethysmography |
| PTB-XL | Public ECG dataset “PTB-XL” |
| PTB-XL+ | Extended release of PTB-XL |
| PVI | Pulmonary Vein Isolation |
| RA | Right Atrium |
| RF | Radiofrequency |
| RVI | Reentry Vulnerability Index |
| SaMD | Software as a Medical Device |
| SSI | Systolic Stretch Index |
| t-SNE | t-Distributed Stochastic Neighbor Embedding |
| TinyML | Tiny/embedded Machine Learning |
| U-Net | U-Net convolutional architecture |
| UMAP | Uniform Manifold Approximation and Projection |
| VF | Ventricular Fibrillation |
| VT | Ventricular Tachycardia |
| XAI | Explainable Artificial Intelligence |
References
- Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention. Biomedicines 2025, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bacoyannis, T.; Ly, B.; Cedilnik, N.; Cochet, H.; Sermesant, M. Deep learning formulation of electrocardiographic imaging integrating image and signal information with data-driven regularization. EP Eur. 2021, 23, i55–i62. [Google Scholar] [CrossRef]
- Kabra, R.; Israni, S.; Vijay, B.; Baru, C.; Mendu, R.; Fellman, M.; Sridhar, A.; Mason, P.; Cheung, J.W.; DiBiase, L.; et al. Emerging role of artificial intelligence in cardiac electrophysiology. Cardiovasc. Digit. Health J. 2022, 3, 263–275. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Singh, J.P.; Ghanbari, H.; McManus, D.D.; Deering, T.F.; Silva, A.J.N.; Mittal, S.; Krahn, A.; Hurwitz, J.L. The potential of artificial intelligence to revolutionize health care delivery, research, and education in cardiac electrophysiology. Heart Rhythm 2024, 21, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.H.-V.; Thu, A.; Twayana, A.R.; Fuertes, A.; Gonzalez, M.; Harrison, J.L.; Mehta, K.A.; James, M.; Basta, M.; Frishman, W.H.; et al. Artificial Intelligence in Cardiac Electrophysiology: Enhancing Mapping and Ablation Precision. Cardiol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.-Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Yao, X.; Lopez-Jimenez, F.; Mohan, T.L.; Pellikka, P.A.; Carter, R.E.; Shah, N.D.; Friedman, P.A.; Noseworthy, P.A. Prospective validation of a deep learning electrocardiogram algorithm for the detection of left ventricular systolic dysfunction. J. Cardiovasc. Electrophysiol. 2019, 30, 668–674. [Google Scholar] [CrossRef]
- Wagner, P.; Strodthoff, N.; Bousseljot, R.-D.; Kreiseler, D.; Lunze, F.I.; Samek, W.; Schaeffter, T. PTB-XL, a large publicly available electrocardiography dataset. Sci. Data 2020, 7, 154. [Google Scholar] [CrossRef]
- Strodthoff, N.; Mehari, T.; Nagel, C.; Aston, P.J.; Sundar, A.; Graff, C.; Kanters, J.K.; Haverkamp, W.; Dössel, O.; Loewe, A.; et al. PTB-XL+, a comprehensive electrocardiographic feature dataset. Sci. Data 2023, 10, 279. [Google Scholar] [CrossRef]
- Deisenhofer, I.; Albenque, J.-P.; Busch, S.; Gitenay, E.; Mountantonakis, S.E.; Roux, A.; Horvilleur, J.; Bakouboula, B.; Oza, S.; Abbey, S.; et al. Artificial intelligence for individualized treatment of persistent atrial fibrillation: A randomized controlled trial. Nat. Med. 2025, 31, 1286–1293. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Sammut-Powell, C.; Martin, G.P.; Callan, P.; Cunnington, C.; Kale, M.; Gerritse, B.; Lanctin, D.; Soken, N.; Campbell, N.G.; et al. Use of a device-based remote management heart failure care pathway is associated with reduced hospitalization and improved patient outcomes: TriageHF Plus real-world evaluation. Eur. Heart J.—Digit. Health 2022, 3, ztac076.2814. [Google Scholar] [CrossRef]
- Boehmer, J.; Sauer, A.J.; Gardner, R.; Stolen, C.M.; Kwan, B.; Wariar, R.; Ruble, S. PRecision Event Monitoring for PatienTs with Heart Failure using HeartLogic (PREEMPT-HF) study design and enrolment. ESC Heart Fail. 2023, 10, 3690–3699. [Google Scholar] [CrossRef]
- Kuo, L.; Wang, G.-J.; Su, P.-H.; Chang, S.-L.; Lin, Y.-J.; Chung, F.-P.; Lo, L.-W.; Hu, Y.-F.; Lin, C.-Y.; Chang, T.-Y.; et al. Deep learning-based workflow for automatic extraction of atria and epicardial adipose tissue on cardiac computed tomography in atrial fibrillation. J. Chin. Med. Assoc. 2024, 87, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jamart, K.; Xiong, Z.; Maso Talou, G.D.; Stiles, M.K.; Zhao, J. Mini Review: Deep Learning for Atrial Segmentation from Late Gadolinium-Enhanced MRIs. Front. Cardiovasc. Med. 2020, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Maurer, M.H.; Thomas, R.P. Explainable Artificial Intelligence in Radiological Cardiovascular Imaging—A Systematic Review. Diagnostics 2025, 15, 1399. [Google Scholar] [CrossRef]
- Zolotarev, A.M.; Hansen, B.J.; Ivanova, E.A.; Helfrich, K.M.; Li, N.; Janssen, P.M.L.; Mohler, P.J.; Mokadam, N.A.; Whitson, B.A.; Fedorov, M.V.; et al. Optical Mapping-Validated Machine Learning Improves Atrial Fibrillation Driver Detection by Multi-Electrode Mapping. Circ. Arrhythmia Electrophysiol. 2020, 13, 1199–1212. [Google Scholar] [CrossRef]
- Whitaker, J.; Baum, T.E.; Qian, P.; Prassl, A.J.; Plank, G.; Blankstein, R.; Cochet, H.; Sauer, W.H.; Bishop, M.J.; Tedrow, U. Frequency Domain Analysis of Endocardial Electrograms for Detection of Nontransmural Myocardial Fibrosis in Nonischemic Cardiomyopathy. JACC Clin. Electrophysiol. 2023, 9, 923–935. [Google Scholar] [CrossRef]
- Baldazzi, G.; Orrù, M.; Viola, G.; Pani, D. Computer-aided detection of arrhythmogenic sites in post-ischemic ventricular tachycardia. Sci. Rep. 2023, 13, 6906. [Google Scholar] [CrossRef]
- Seitz, J.; Durdez, T.M.; Albenque, J.P.; Pisapia, A.; Gitenay, E.; Durand, C.; Monteau, J.; Moubarak, G.; Théodore, G.; Lepillier, A.; et al. Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2022, 33, 2250–2260. [Google Scholar] [CrossRef]
- Sakata, K.; Bradley, R.P.; Prakosa, A.; Yamamoto, C.A.P.; Ali, S.Y.; Loeffler, S.; Tice, B.M.; Boyle, P.M.; Kholmovski, E.G.; Yadav, R.; et al. Assessing the arrhythmogenic propensity of fibrotic substrate using digital twins to inform a mechanisms-based atrial fibrillation ablation strategy. Nat. Cardiovasc. Res. 2024, 3, 857–868. [Google Scholar] [CrossRef]
- Martin, C.H.; Oved, A.; Chowdhury, R.A.; Ullmann, E.; Peters, N.S.; Bharath, A.A.; Varela, M. EP-PINNs: Cardiac Electrophysiology Characterisation Using Physics-Informed Neural Networks. Front. Cardiovasc. Med. 2022, 8, 768419. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Faranesh, A.Z.; Selvaggi, C.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar] [CrossRef]
- Svennberg, E.; Han, J.K.; Caiani, E.G.; Engelhardt, S.; Ernst, S.; Friedman, P.; Garcia, R.; Ghanbari, H.; Hindricks, G.; Man, S.H.; et al. State of the Art of Artificial Intelligence in Clinical Electrophysiology in 2025: A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC Working Group on E-Cardiology. EP Eur. 2025, 27, euaf071. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spaccarotella, C.A.M.; Esposito, G.; Indolfi, C. An Artificial Intelligence Analysis of Electrocardiograms for the Clinical Diagnosis of Cardiovascular Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Scientific Data Curation Team. Metadata Record for: PTB-XL, a Large Publicly Available Electrocardiography Dataset. Figshare. 2020, p. 3235 Bytes. Available online: https://springernature.figshare.com/articles/dataset/Metadata_record_for_PTB-XL_a_large_publicly_available_Electrocardiography_Dataset/12098055 (accessed on 10 October 2025).
- Ribeiro, A.H.; Ribeiro, M.H.; Paixão, G.M.M.; Oliveira, D.M.; Gomes, P.R.; Canazart, J.A.; Ferreira, M.P.S.; Andersson, C.R.; Macfarlane, P.W.; Meira, W.; et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat. Commun. 2020, 11, 1760. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Bjerkén, L.V.; Rønborg, S.N.; Jensen, M.T.; Ørting, S.N.; Nielsen, O.W. Artificial intelligence enabled ECG screening for left ventricular systolic dysfunction: A systematic review. Heart Fail. Rev. 2022, 28, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mannhart, D.; Lischer, M.; Knecht, S.; Lavallaz, J.d.F.d.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 232–242. [Google Scholar] [CrossRef]
- Shahid, S.; Iqbal, M.; Saeed, H.; Hira, S.; Batool, A.; Khalid, S.; Tahirkheli, N.K. Diagnostic Accuracy of Apple Watch Electrocardiogram for Atrial Fibrillation. JACC Adv. 2025, 4, 101538. [Google Scholar] [CrossRef]
- Scholten, J.; Jansen, W.P.J.; Horsthuis, T.; Mahes, A.D.; Winter, M.M.; Zwinderman, A.H.; Keijer, J.T.; Minneboo, M.; De Groot, J.R.; Bokma, J.P. Six-lead device superior to single-lead smartwatch ECG in atrial fibrillation detection. Am. Heart J. 2022, 253, 53–58. [Google Scholar] [CrossRef]
- Pengel, L.K.D.; Robbers-Visser, D.; Groenink, M.; Winter, M.M.; Schuuring, M.J.; Bouma, B.J.; Bokma, J.P. A comparison of ECG-based home monitoring devices in adults with CHD. Cardiol. Young 2023, 33, 1129–1135. [Google Scholar] [CrossRef]
- Wijesurendra, R.; Pessoa-Amorim, G.; Buck, G.; Harper, C.; Bulbulia, R.; Jones, N.R.; A’Court, C.; Kurien, R.; Taylor, K.; Casadei, B.; et al. Active Monitoring for AtriaL FIbrillation (AMALFI): Rationale, protocol, and pilot for a pragmatic, randomized, controlled trial of remote screening for asymptomatic atrial fibrillation. Am. Heart J. 2025, 290, 310–324. [Google Scholar] [CrossRef]
- Proesmans, T.; Mortelmans, C.; Van Haelst, R.; Verbrugge, F.; Vandervoort, P.; Vaes, B. Mobile Phone–Based Use of the Photoplethysmography Technique to Detect Atrial Fibrillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR mHealth uHealth 2019, 7, e12284. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Chang, S.-L.; Yeh, Y.-H.; Chung, F.-P.; Hu, Y.-F.; Chou, C.-C.; Hung, K.-C.; Chang, P.-C.; Liao, J.-N.; Chan, Y.-H.; et al. Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int. J. Cardiol. 2021, 332, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.Y.; Cho, M.S.; Kim, M.; Kim, J.; Oh, I.-Y.; Cho, Y.; Lee, J.H. Artificial intelligence predicts undiagnosed atrial fibrillation in patients with embolic stroke of undetermined source using sinus rhythm electrocardiograms. Heart Rhythm 2024, 21, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Saluja, D.; Huang, Z.; Majumder, J.; Zeldin, L.; Yarmohammadi, H.; Biviano, A.; Wan, E.Y.; Ciaccio, E.J.; Hendon, C.P.; Garan, H. Automated prediction of isthmus areas in scar-related atrial tachycardias using artificial intelligence. J. Cardiovasc. Electrophysiol. 2024, 35, 1401–1411. [Google Scholar] [CrossRef]
- Rodrigo, M.; Alhusseini, M.I.; Rogers, A.J.; Krittanawong, C.; Thakur, S.; Feng, R.; Ganesan, P.; Narayan, S.M. Atrial fibrillation signatures on intracardiac electrograms identified by deep learning. Comput. Biol. Med. 2022, 145, 105451. [Google Scholar] [CrossRef]
- Alhusseini, M.I.; Abuzaid, F.; Rogers, A.J.; Zaman, J.A.B.; Baykaner, T.; Clopton, P.; Bailis, P.; Zaharia, M.; Wang, P.J.; Rappel, W.-J.; et al. Machine Learning to Classify Intracardiac Electrical Patterns During Atrial Fibrillation: Machine Learning of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e008160. [Google Scholar] [CrossRef]
- Fox, S.R.; Toomu, A.; Gu, K.; Kang, J.; Sung, K.; Han, F.T.; Hoffmayer, K.S.; Hsu, J.C.; Raissi, F.; Feld, G.K.; et al. Impact of artificial intelligence arrhythmia mapping on time to first ablation, procedure duration, and fluoroscopy use. J. Cardiovasc. Electrophysiol. 2024, 35, 916–928. [Google Scholar] [CrossRef]
- Park, J. Machine Learning for Predicting Atrial Fibrillation Recurrence After Cardioversion: A Modest Leap Forward. Korean Circ. J. 2023, 53, 690–692. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, E.; Ju, H.; Ahn, H.-J.; Lee, S.-R.; Choi, E.-K.; Suh, J.; Oh, S.; Rhee, W. Machine Learning Prediction for the Recurrence After Electrical Cardioversion of Patients with Persistent Atrial Fibrillation. Korean Circ. J. 2023, 53, 677–689. [Google Scholar] [CrossRef]
- De La Nava, A.M.S.; Arenal, Á.; Fernández-Avilés, F.; Atienza, F. Artificial Intelligence-Driven Algorithm for Drug Effect Prediction on Atrial Fibrillation: An in silico Population of Models Approach. Front. Physiol. 2021, 12, 768468. [Google Scholar] [CrossRef] [PubMed]
- Gruwez, H.; Barthels, M.; Dhont, S.; Meekers, E.; Wouters, F.; Pierlet, N.; Nuyens, D.; Rivero-Ayerza, M.; Van Herendael, H.; Pison, L.; et al. Predicting atrial fibrillation recurrence after catheter ablation using an artificial intelligence-enabled electrocardiogram algorithm. EP Eur. 2024, 26, euae102.167. [Google Scholar] [CrossRef]
- Razeghi, O.; Kapoor, R.; Alhusseini, M.I.; Fazal, M.; Tang, S.; Roney, C.H.; Rogers, A.J.; Lee, A.; Wang, P.J.; Clopton, P.; et al. Atrial fibrillation ablation outcome prediction with a machine learning fusion framework incorporating cardiac computed tomography. J. Cardiovasc. Electrophysiol. 2023, 34, 1164–1174. [Google Scholar] [CrossRef]
- Saglietto, A.; Gaita, F.; Blomstrom-Lundqvist, C.; Arbelo, E.; Dagres, N.; Brugada, J.; Maggioni, A.P.; Tavazzi, L.; Kautzner, J.; De Ferrari, G.M.; et al. AFA-Recur: An ESC EORP AFA-LT registry machine-learning web calculator predicting atrial fibrillation recurrence after ablation. EP Eur. 2023, 25, 92–100. [Google Scholar] [CrossRef]
- Brahier, M.S.; Zou, F.; Abdulkareem, M.; Kochi, S.; Migliarese, F.; Thomaides, A.; Ma, X.; Wu, C.; Sandfort, V.; Bergquist, P.J.; et al. Using machine learning to enhance prediction of atrial fibrillation recurrence after catheter ablation. J. Arrhythmia 2023, 39, 868–875. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Albert, N.M.; Allen, L.A.; Ahmed, R.; Averina, V.; Boehmer, J.P.; Cowie, M.R.; Chien, C.V.; Galvao, M.; Klein, L.; et al. Multiple cArdiac seNsors for mAnaGEment of Heart Failure (MANAGE-HF)—Phase I Evaluation of the Integration and Safety of the HeartLogic Multisensor Algorithm in Patients with Heart Failure. J. Card. Fail. 2022, 28, 1245–1254. [Google Scholar] [CrossRef]
- Virani, S.A.; Sharma, V.; McCann, M.; Koehler, J.; Tsang, B.; Zieroth, S. Prospective evaluation of integrated device diagnostics for heart failure management: Results of the TRIAGE-HF study. ESC Heart Fail. 2018, 5, 809–817. [Google Scholar] [CrossRef]
- Zanotto, G.; Capucci, A. HeartInsight: From SELENE HF to implementation in clinical practice. Eur. Heart J. Suppl. 2023, 25, C337–C343. [Google Scholar] [CrossRef]
- Ginder, C.; Li, J.; Halperin, J.L.; Akar, J.G.; Martin, D.T.; Chattopadhyay, I.; Upadhyay, G.A. Predicting Malignant Ventricular Arrhythmias Using Real-Time Remote Monitoring. J. Am. Coll. Cardiol. 2023, 81, 949–961. [Google Scholar] [CrossRef]
- Varma, N.; Braunschweig, F.; Burri, H.; Hindricks, G.; Linz, D.; Michowitz, Y.; Ricci, R.P.; Nielsen, J.C. Remote monitoring of cardiac implantable electronic devices and disease management. EP Eur. 2023, 25, euad233. [Google Scholar] [CrossRef]
- Loeffler, S.E.; Trayanova, N. Primer on Machine Learning in Electrophysiology. Arrhythmia Electrophysiol. Rev. 2023, 12, e06. [Google Scholar] [CrossRef]
- Missel, R.; Gyawali, P.K.; Murkute, J.V.; Li, Z.; Zhou, S.; AbdelWahab, A.; Davis, J.; Warren, J.; Sapp, J.L.; Wang, L. A hybrid machine learning approach to localizing the origin of ventricular tachycardia using 12-lead electrocardiograms. Comput. Biol. Med. 2020, 126, 104013. [Google Scholar] [CrossRef]
- Sprenger, L.; Moser, F.; Maslova, V.; Zaman, A.; Nonnenmacher, M.; Willert, S.; Frank, D.; Lian, E. Prediction of Ablation Index and Lesion Size Index for Local Impedance Drop-Guided Ablation. J. Clin. Med. 2025, 14, 832. [Google Scholar] [CrossRef]
- Giffard-Roisin, S.; Jackson, T.; Fovargue, L.; Lee, J.; Delingette, H.; Razavi, R.; Ayache, N.; Sermesant, M. Noninvasive Personalization of a Cardiac Electrophysiology Model from Body Surface Potential Mapping. IEEE Trans. Biomed. Eng. 2017, 64, 2206–2218. [Google Scholar] [CrossRef]
- Budzianowski, J.; Kaczmarek-Majer, K.; Rzeźniczak, J.; Słomczyński, M.; Wichrowski, F.; Hiczkiewicz, D.; Musielak, B.; Grydz, Ł.; Hiczkiewicz, J.; Burchardt, P. Machine learning model for predicting late recurrence of atrial fibrillation after catheter ablation. Sci. Rep. 2023, 13, 15213. [Google Scholar] [CrossRef]
- Nie, L.; Zhang, T.; Wang, W.; Han, X.; Liu, M.; Zhang, S.; Feng, W.; Wang, Y.; Chen, Y. Machine learning-based prediction model for recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Front. Cardiovasc. Med. 2025, 12, 1642409. [Google Scholar] [CrossRef]
- Jia, S.; Mou, H.; Wu, Y.; Lin, W.; Zeng, Y.; Chen, Y.; Chen, Y.; Zhang, Q.; Wang, W.; Feng, C.; et al. A Simple Logistic Regression Model for Predicting the Likelihood of Recurrence of Atrial Fibrillation Within 1 Year After Initial Radio-Frequency Catheter Ablation Therapy. Front. Cardiovasc. Med. 2022, 8, 819341. [Google Scholar] [CrossRef]
- Sánchez, J.; Luongo, G.; Nothstein, M.; Unger, L.A.; Saiz, J.; Trenor, B.; Luik, A.; Dössel, O.; Loewe, A. Using Machine Learning to Characterize Atrial Fibrotic Substrate from Intracardiac Signals with a Hybrid in silico and in vivo Dataset. Front. Physiol. 2021, 12, 699291. [Google Scholar] [CrossRef]
- Corrado, C.; Williams, S.; Roney, C.; Plank, G.; O’nEill, M.; Niederer, S. Using machine learning to identify local cellular properties that support re-entrant activation in patient-specific models of atrial fibrillation. EP Eur. 2021, 23 (Suppl. S1), i12–i20. [Google Scholar] [CrossRef]
- Pander, T. An Improved Approach for Atrial Fibrillation Detection in Long-Term ECG Using Decomposition Transforms and Least-Squares Support Vector Machine. Appl. Sci. 2023, 13, 12187. [Google Scholar] [CrossRef]
- Rogers, A.J.; Selvalingam, A.; Alhusseini, M.I.; Krummen, D.E.; Corrado, C.; Abuzaid, F.; Baykaner, T.; Meyer, C.; Clopton, P.; Giles, W.; et al. Machine Learned Cellular Phenotypes in Cardiomyopathy Predict Sudden Death. Circ. Res. 2021, 128, 172–184. [Google Scholar] [CrossRef]
- Melgani, F.; Bazi, Y. Classification of Electrocardiogram Signals with Support Vector Machines and Particle Swarm Optimization. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 667–677. [Google Scholar] [CrossRef]
- Smisek, R.; Hejc, J.; Ronzhina, M.; Nemcova, A.; Marsanova, L.; Kolarova, J.; Smital, L.; Vitek, M. Multi-stage SVM approach for cardiac arrhythmias detection in short single-lead ECG recorded by a wearable device. Physiol. Meas. 2018, 39, 094003. [Google Scholar] [CrossRef]
- Rad, A.B.; Galloway, C.; Treiman, D.; Xue, J.; Li, Q.; Sameni, R.; Albert, D.; Clifford, G.D. Atrial fibrillation detection in outpatient electrocardiogram monitoring: An algorithmic crowdsourcing approach. PLoS ONE 2021, 16, e0259916. [Google Scholar] [CrossRef]
- Mäkynen, M.; Ng, G.A.; Li, X.; Schlindwein, F.S. Wearable Devices Combined with Artificial Intelligence—A Future Technology for Atrial Fibrillation Detection? Sensors 2022, 22, 8588. [Google Scholar] [CrossRef]
- Martinez-Alanis, M.; Bojorges-Valdez, E.; Wessel, N.; Lerma, C. Prediction of Sudden Cardiac Death Risk with a Support Vector Machine Based on Heart Rate Variability and Heartprint Indices. Sensors 2020, 20, 5483. [Google Scholar] [CrossRef]
- Rodriguez, J.; Schulz, S.; Giraldo, B.F.; Voss, A. Risk Stratification in Idiopathic Dilated Cardiomyopathy Patients Using Cardiovascular Coupling Analysis. Front. Physiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- He, K.; Liang, W.; Liu, S.; Bian, L.; Xu, Y.; Luo, C.; Li, Y.; Yue, H.; Yang, C.; Wu, Z. Long-term single-lead electrocardiogram monitoring to detect new-onset postoperative atrial fibrillation in patients after cardiac surgery. Front. Cardiovasc. Med. 2022, 9, 1001883. [Google Scholar] [CrossRef]
- Manohar, A.; Colvert, G.M.; Yang, J.; Chen, Z.; Ledesma-Carbayo, M.J.; Kronborg, M.B.; Sommer, A.; Nørgaard, B.L.; Nielsen, J.C.; McVeigh, E.R. Prediction of Cardiac Resynchronization Therapy Response Using a Lead Placement Score Derived from 4-Dimensional Computed Tomography. Circ. Cardiovasc. Imaging 2022, 15, 603–613. [Google Scholar] [CrossRef]
- Raghunath, S.; Pfeifer, J.M.; Ulloa-Cerna, A.E.; Nemani, A.; Carbonati, T.; Jing, L.; vanMaanen, D.P.; Hartzel, D.N.; Ruhl, J.A.; Lagerman, B.F.; et al. Deep Neural Networks Can Predict New-Onset Atrial Fibrillation from the 12-Lead ECG and Help Identify Those at Risk of Atrial Fibrillation–Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Mahara, K.; Beussink-Nelson, L.; Ikura, H.; Katsumata, Y.; Endo, J.; Gaggin, H.K.; Shah, S.J.; Itabashi, Y.; MacRae, C.A.; et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat. Commun. 2021, 12, 2726. [Google Scholar] [CrossRef]
- Agliari, E.; Barra, A.; Barra, O.A.; Fachechi, A.; Franceschi Vento, L.; Moretti, L. Detecting cardiac pathologies via machine learning on heart-rate variability time series and related markers. Sci. Rep. 2020, 10, 8845. [Google Scholar] [CrossRef]
- O’Brien, H.; Whitaker, J.; Singh Sidhu, B.; Gould, J.; Kurzendorfer, T.; O’Neill, M.D.; Rajani, R.; Grigoryan, K.; Rinaldi, C.A.; Taylor, J.; et al. Automated Left Ventricle Ischemic Scar Detection in CT Using Deep Neural Networks. Front. Cardiovasc. Med. 2021, 8, 655252. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Sun, L.; Cai, J.; Wang, S.; Zeng, L.; Sun, S. Application of machine learning to predict the occurrence of arrhythmia after acute myocardial infarction. BMC Med. Inform. Decis. Mak. 2021, 21, 301. [Google Scholar] [CrossRef]
- Howell, S.J.; Stivland, T.; Stein, K.; Ellenbogen, K.A.; Tereshchenko, L.G. Using Machine-Learning for Prediction of the Response to Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2021, 7, 1505–1515. [Google Scholar] [CrossRef]
- Gallard, A.; Hubert, A.; Smiseth, O.; Voigt, J.-U.; Le Rolle, V.; Leclercq, C.; Bidaut, A.; Galli, E.; Donal, E.; Hernandez, A.I. Prediction of response to cardiac resynchronization therapy using a multi-feature learning method. Int. J. Cardiovasc. Imaging 2021, 37, 989–998. [Google Scholar] [CrossRef]
- Komlósi, F.; Tóth, P.; Bohus, G.; Vámosi, P.; Tokodi, M.; Szegedi, N.; Salló, Z.; Piros, K.; Perge, P.; Osztheimer, I.; et al. Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease. Bioengineering 2023, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D.; De Deus, L.F.; Sehgal, N. Evaluation of Atrial Fibrillation Detection in Short-Term Photoplethysmography (PPG) Signals Using Artificial Intelligence. Cureus 2023, 15, e45111. [Google Scholar] [CrossRef]
- Rad, A.B.; Kirsch, M.; Li, Q.; Xue, J.; Sameni, R.; Albert, D.; Clifford, G.D. A Crowdsourced AI Framework for Atrial Fibrillation Detection in Apple Watch and Kardia Mobile ECGs. Sensors 2024, 24, 5708. [Google Scholar] [CrossRef]
- Donoso, F.I.; Figueroa, R.L.; Lecannelier, E.A.; Pino, E.J.; Rojas, A.J. Clustering of atrial fibrillation based on surface ECG measurements. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: New York, NY, USA, 2013; pp. 4203–4206. [Google Scholar]
- Li, X.; Almeida, T.P.; Dastagir, N.; Guillem, M.S.; Salinet, J.; Chu, G.S.; Stafford, P.J.; Schlindwein, F.S.; Ng, G.A. Standardizing Single-Frame Phase Singularity Identification Algorithms and Parameters in Phase Mapping During Human Atrial Fibrillation. Front. Physiol. 2020, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Li, Y.; Li, Q.; Liu, X.; Weng, S.; Yin, Y.; Liang, Z.; Zhang, T.; Wang, Y. Atrial fibrillation phenotypes identified through cluster analysis in the CABANA study. Int. J. Cardiol. 2025, 438, 133606. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.P.; Soriano, D.C.; Mase, M.; Ravelli, F.; Bezerra, A.S.; Li, X.; Chu, G.S.; Salinet, J.; Stafford, P.J.; Andre Ng, G.; et al. Unsupervised Classification of Atrial Electrograms for Electroanatomic Mapping of Human Persistent Atrial Fibrillation. IEEE Trans. Biomed. Eng. 2021, 68, 1131–1141. [Google Scholar] [CrossRef]
- Kong, X.; Ravikumar, V.; Mulpuru, S.K.; Roukoz, H.; Tolkacheva, E.G. A Data-Driven Preprocessing Framework for Atrial Fibrillation Intracardiac Electrocardiogram Analysis. Entropy 2023, 25, 332. [Google Scholar] [CrossRef]
- Yang, W.; Deo, R.; Guo, W. Functional feature extraction and validation from twelve-lead electrocardiograms to identify atrial fibrillation. Commun. Med. 2025, 5, 32. [Google Scholar] [CrossRef]
- Sánchez-Carballo, E.; Melgarejo-Meseguer, F.M.; Vijayakumar, R.; Sánchez-Muñoz, J.J.; García-Alberola, A.; Rudy, Y.; Rojo-Álvarez, J.L. Interpretable manifold learning for T-wave alternans assessment with electrocardiographic imaging. Eng. Appl. Artif. Intell. 2025, 143, 109996. [Google Scholar] [CrossRef]
- Jung, S.-J.; Son, C.-S.; Kim, M.-S.; Kim, D.-J.; Park, H.-S.; Kim, Y.-N. Association Rules to Identify Complications of Cerebral Infarction in Patients with Atrial Fibrillation. Healthc. Inform. Res. 2013, 19, 25–32. [Google Scholar] [CrossRef]
- Loring, Z.; Holmes, D.N.; Matsouaka, R.A.; Curtis, A.B.; Day, J.D.; Desai, N.; Ellenbogen, K.A.; Feld, G.K.; Fonarow, G.C.; Frankel, D.S.; et al. Procedural Patterns and Safety of Atrial Fibrillation Ablation: Findings from Get with The Guidelines-Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e007944. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, C. Deep learning and electrocardiography: Systematic review of current techniques in cardiovascular disease diagnosis and management. Biomed. Eng. OnLine 2025, 24, 23. [Google Scholar] [CrossRef]
- Goettling, M.; Hammer, A.; Malberg, H.; Schmidt, M. xECGArch: A trustworthy deep learning architecture for interpretable ECG analysis considering short-term and long-term features. Sci. Rep. 2024, 14, 13122. [Google Scholar] [CrossRef]
- Salih, A.; Boscolo Galazzo, I.; Gkontra, P.; Lee, A.M.; Lekadir, K.; Raisi-Estabragh, Z.; Petersen, S.E. Explainable Artificial Intelligence and Cardiac Imaging: Toward More Interpretable Models. Circ. Cardiovasc. Imaging 2023, 16, e014519. [Google Scholar] [CrossRef]
- Antman, E.M.; Loscalzo, J. Precision medicine in cardiology. Nat. Rev. Cardiol. 2016, 13, 591–602. [Google Scholar] [CrossRef]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, Y.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef]
- Seemann, G.; Höper, C.; Sachse, F.B.; Dössel, O.; Holden, A.V.; Zhang, H. Heterogeneous three-dimensional anatomical and electrophysiological model of human atria. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2006, 364, 1465–1481. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Lyon, A.; Shade, J.; Heijman, J. Computational modeling of cardiac electrophysiology and arrhythmogenesis: Toward clinical translation. Physiol. Rev. 2024, 104, 1265–1333. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Kwon, S.-S.; Wi, J.; Park, M.; Lee, H.-S.; Park, J.-S.; Lee, Y.-S.; Shim, E.B.; Pak, H.-N. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: Comparison with clinical catheter ablation. Prog. Biophys. Mol. Biol. 2014, 116, 40–47. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Hocini, M.; Shah, A.J.; Derval, N.; Sacher, F.; Jais, P.; Dubois, R. Noninvasive Panoramic Mapping of Human Atrial Fibrillation Mechanisms: A Feasibility Report. J. Cardiovasc. Electrophysiol. 2013, 24, 711–717. [Google Scholar] [CrossRef]
- Prakosa, A.; Arevalo, H.J.; Deng, D.; Boyle, P.M.; Nikolov, P.P.; Ashikaga, H.; Blauer, J.J.E.; Ghafoori, E.; Park, C.J.; Blake, R.C.; et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat. Biomed. Eng. 2018, 2, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Hill, Y.R.; Child, N.; Hanson, B.; Wallman, M.; Coronel, R.; Plank, G.; Rinaldi, C.A.; Gill, J.; Smith, N.P.; Taggart, P.; et al. Investigating a Novel Activation-Repolarisation Time Metric to Predict Localised Vulnerability to Reentry Using Computational Modelling. PLoS ONE 2016, 11, e0149342. [Google Scholar] [CrossRef] [PubMed]
- Sung, E.; Prakosa, A.; Aronis, K.N.; Zhou, S.; Zimmerman, S.L.; Tandri, H.; Nazarian, S.; Berger, R.D.; Chrispin, J.; Trayanova, N.A. Personalized Digital-Heart Technology for Ventricular Tachycardia Ablation Targeting in Hearts with Infiltrating Adiposity. Circ. Arrhythmia Electrophysiol. 2020, 13, e008912. [Google Scholar] [CrossRef]
- Koopsen, T.; Gerrits, W.; Van Osta, N.; Van Loon, T.; Wouters, P.; Prinzen, F.W.; Vernooy, K.; Delhaas, T.; Teske, A.J.; Meine, M.; et al. Virtual pacing of a patient’s digital twin to predict left ventricular reverse remodelling after cardiac resynchronization therapy. EP Eur. 2023, 26, euae009. [Google Scholar] [CrossRef]
- Niederer, S.A.; Plank, G.; Chinchapatnam, P.; Ginks, M.; Lamata, P.; Rhode, K.S.; Rinaldi, C.A.; Razavi, R.; Smith, N.P. Length-dependent tension in the failing heart and the efficacy of cardiac resynchronization therapy. Cardiovasc. Res. 2011, 89, 336–343. [Google Scholar] [CrossRef]
- Lumens, J.; Tayal, B.; Walmsley, J.; Delgado-Montero, A.; Huntjens, P.R.; Schwartzman, D.; Althouse, A.D.; Delhaas, T.; Prinzen, F.W.; Gorcsan, J. Differentiating Electromechanical from Non–Electrical Substrates of Mechanical Discoordination to Identify Responders to Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2015, 8, e003744. [Google Scholar] [CrossRef]
- Arnold, R.; Prassl, A.J.; Neic, A.; Thaler, F.; Augustin, C.M.; Gsell, M.A.F.; Gillette, K.; Manninger, M.; Scherr, D.; Plank, G. pyCEPS: A cross-platform electroanatomic mapping data to computational model conversion platform for the calibration of digital twin models of cardiac electrophysiology. Comput. Methods Programs Biomed. 2024, 254, 108299. [Google Scholar] [CrossRef]
- Plank, G.; Loewe, A.; Neic, A.; Augustin, C.; Huang, Y.-L.; Gsell, M.A.F.; Karabelas, E.; Nothstein, M.; Prassl, A.J.; Sánchez, J.; et al. The openCARP simulation environment for cardiac electrophysiology. Comput. Methods Programs Biomed. 2021, 208, 106223. [Google Scholar] [CrossRef] [PubMed]
- Bhagirath, P.; Strocchi, M.; Bishop, M.J.; Boyle, P.M.; Plank, G. From bits to bedside: Entering the age of digital twins in cardiac electrophysiology. EP Eur. 2024, 26, euae295. [Google Scholar] [CrossRef]
- Karniadakis, G.E.; Kevrekidis, I.G.; Lu, L.; Perdikaris, P.; Wang, S.; Yang, L. Physics-informed machine learning. Nat. Rev. Phys. 2021, 3, 422–440. [Google Scholar] [CrossRef]
- Sahli Costabal, F.; Yang, Y.; Perdikaris, P.; Hurtado, D.E.; Kuhl, E. Physics-Informed Neural Networks for Cardiac Activation Mapping. Front. Phys. 2020, 8, 42. [Google Scholar] [CrossRef]
- Grandits, T.; Pezzuto, S.; Costabal, F.S.; Perdikaris, P.; Pock, T.; Plank, G.; Krause, R. Learning Atrial Fiber Orientations and Conductivity Tensors from Intracardiac Maps Using Physics-Informed Neural Networks. In Functional Imaging and Modeling of the Heart; Ennis, D.B., Perotti, L.E., Wang, V.Y., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12738, pp. 650–658. [Google Scholar] [CrossRef]
- Herrera, C.R.; Grandits, T.; Plank, G.; Perdikaris, P.; Costabal, F.S.; Pezzuto, S. Physics-informed neural networks to learn cardiac fiber orientation from multiple electroanatomical maps. arXiv 2022, arXiv:2201.12362. [Google Scholar] [CrossRef]
- Dermul, N.; Dierckx, H. Reconstruction of excitation waves from mechanical deformation using physics-informed neural networks. Sci. Rep. 2024, 14, 16975. [Google Scholar] [CrossRef]
- Ghanem, R.N.; Jia, P.; Ramanathan, C.; Ryu, K.; Markowitz, A.; Rudy, Y. Noninvasive Electrocardiographic Imaging (ECGI): Comparison to intraoperative mapping in patients. Heart Rhythm 2005, 2, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Bilchick, K.; Xie, J. Physics-informed residual learning with spatiotemporal local support for inverse ECG reconstruction. Sci. Rep. 2025, 15, 31747. [Google Scholar] [CrossRef]
- Xiong, Z.; Fedorov, V.V.; Fu, X.; Cheng, E.; Macleod, R.; Zhao, J. Fully Automatic Left Atrium Segmentation from Late Gadolinium Enhanced Magnetic Resonance Imaging Using a Dual Fully Convolutional Neural Network. IEEE Trans. Med. Imaging 2019, 38, 515–524. [Google Scholar] [CrossRef]
- Borra, D.; Andalò, A.; Paci, M.; Fabbri, C.; Corsi, C. A fully automated left atrium segmentation approach from late gadolinium enhanced magnetic resonance imaging based on a convolutional neural network. Quant. Imaging Med. Surg. 2020, 10, 1894–1907. [Google Scholar] [CrossRef]
- Di Biase, L.; Zou, F.; Lin, A.N.; Grupposo, V.; Marazzato, J.; Tarantino, N.; Della Rocca, D.; Mohanty, S.; Natale, A.; Alhuarrat, M.A.D.; et al. Feasibility of three-dimensional artificial intelligence algorithm integration with intracardiac echocardiography for left atrial imaging during atrial fibrillation catheter ablation. EP Eur. 2023, 25, euad211. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, S.Y.; Gala, D.; Makaryus, A.N. Integration of artificial intelligence into cardiac ultrasonography practice. Expert Rev. Med. Devices 2025, 22, 869–879. [Google Scholar] [CrossRef]
- Puyol-Antón, E.; Sidhu, B.S.; Gould, J.; Porter, B.; Elliott, M.K.; Mehta, V.; Rinaldi, C.A.; King, A.P. A multimodal deep learning model for cardiac resynchronisation therapy response prediction. Med. Image Anal. 2022, 79, 102465. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Zhao, C.; Ling, X.; Malhotra, S.; Qian, Z.; Wang, Y.; Hou, X.; Zou, J.; Zhou, W. A method using deep learning to discover new predictors from left-ventricular mechanical dyssynchrony for CRT response. J. Nucl. Cardiol. 2023, 30, 201–213. [Google Scholar] [CrossRef]
- Ogbomo-Harmitt, S.; Muffoletto, M.; Zeidan, A.; Qureshi, A.; King, A.P.; Aslanidi, O. Exploring interpretability in deep learning prediction of successful ablation therapy for atrial fibrillation. Front. Physiol. 2023, 14, 1054401. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.; Passerini, T.; Audigier, C.; Lluch, È.; Mihalef, V.; Ashikaga, H.; Maier, A.; Halperin, H.; Mansi, T. Extrapolation of Ventricular Activation Times from Sparse Electroanatomical Data Using Graph Convolutional Neural Networks. Front. Physiol. 2021, 12, 694869. [Google Scholar] [CrossRef] [PubMed]
- Verardo, G.; Perez-Ramirez, D.F.; Bruchfeld, S.; Boman, M.; Chiesa, M.; Koch, S.; Maguire, G.Q.; Kostic, D. Reducing the Number of Leads for ECG Imaging with Graph Neural Networks and Meaningful Latent Space. In Statistical Atlases and Computational Models of the Heart. Workshop, CMRxRecon and MBAS Challenge Papers; Camara, O., Puyol-Antón, E., Sermesant, M., Suinesiaputra, A., Zhao, J., Wang, C., Tao, Q., Young, A., Eds.; Lecture Notes in Computer Science; Springer Nature: Cham, Switzerland, 2025; Volume 15448, pp. 301–312. ISBN 978-3-031-87755-1. [Google Scholar] [CrossRef]
- Neri, L.; Oberdier, M.T.; Van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef]
- Heydari, S.; Mahmoud, Q.H. Tiny Machine Learning and On-Device Inference: A Survey of Applications, Challenges, and Future Directions. Sensors 2025, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Wasserlauf, J.; Vogel, K.; Whisler, C.; Benjamin, E.; Helm, R.; Steinhaus, D.A.; Yousuf, O.; Passman, R.S. Accuracy of the Apple watch for detection of AF: A multicenter experience. J. Cardiovasc. Electrophysiol. 2023, 34, 1103–1107. [Google Scholar] [CrossRef]
- Isenegger, C.; Mannhart, D.; Arnet, R.; Jordan, F.; Du Fay De Lavallaz, J.; Krisai, P.; Knecht, S.; Kühne, M.; Sticherling, C.; Badertscher, P. Accuracy of Smartwatches for Atrial Fibrillation Detection Over Time. JACC Clin. Electrophysiol. 2024, 10, 2735–2737. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.-I.; Oh, I.-Y.; Jeong, J.H.; Lee, H.S.; Choi, Y.Y.; Lee, J.H.; Cho, Y.; Shim, J.; Son, H.S.; et al. A Patch-Type Electrocardiography Is Superior to Holter Monitoring for Detecting Paroxysmal Cardiac Arrhythmias. J. Korean Med. Sci. 2025, 40, e168. [Google Scholar] [CrossRef]
- Sibomana, O.; Hakayuwa, C.M.; Obianke, A.; Gahire, H.; Munyantore, J.; Chilala, M.M. Diagnostic accuracy of ECG smart chest patches versus PPG smartwatches for atrial fibrillation detection: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2025, 25, 132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Choi, E.K.; Lee, S.R.; Ahn, H.J.; Lee, B.; Oh, S.; Lip, G.Y.H. Atrial fibrillation detection in ambulatory patients using a smart ring powered by deep learning analysis of continuous photoplethysmography monitoring. Eur. Heart J. 2022, 43, ehac544.415. [Google Scholar] [CrossRef]
- Van Steijn, N.J.; Pepplinkhuizen, S.; Postema, P.G.; Knops, R.E.; Winter, M.M. Ventricular arrhythmia detection with a wearable ring-type photoplethysmography sensor: A feasibility study. Heart Rhythm 2025, S1547527125025548. [Google Scholar] [CrossRef] [PubMed]
- Avanu, A.E.; Dodi, G. Wear Your Heart on Your Sleeve: Smart Textile ECG Wearables for Comfort, Integration, Signal Quality and Continuous Monitoring in Paroxysmal Atrial Fibrillation. Sensors 2025, 25, 676. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Eldesouky, M.; Antoun, I.; Lau, E.Y.M.; Koya, A.; Vali, Z.; Suleman, S.A.; Donaldson, J.; Ng, G.A. Wearable Devices for Arrhythmia Detection: Advancements and Clinical Implications. Sensors 2025, 25, 2848. [Google Scholar] [CrossRef]
- Ghassemi, M.; Oakden-Rayner, L.; Beam, A.L. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit. Health 2021, 3, e745–e750. [Google Scholar] [CrossRef]
- Rudin, C. Stop Explaining Black Box Machine Learning Models for High Stakes Decisions and Use Interpretable Models Instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef]
- Liu, X.; Cruz Rivera, S.; Moher, D.; Calvert, M.J.; Denniston, A.K.; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: The CONSORT-AI extension. Nat. Med. 2020, 26, 1364–1374. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Liu, X.; Chan, A.-W.; Denniston, A.K.; Calvert, M.J.; SPIRIT-AI and CONSORT-AI Working Group; SPIRIT-AI and CONSORT-AI Steering Group; SPIRIT-AI and CONSORT-AI Consensus Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: The SPIRIT-AI extension. Nat. Med. 2020, 26, 1351–1363. [Google Scholar] [CrossRef]
- Tjoa, E.; Guan, C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4793–4813. [Google Scholar] [CrossRef] [PubMed]
- Vasey, B.; Nagendran, M.; Campbell, B.; Clifton, D.A.; Collins, G.S.; Denaxas, S.; Denniston, A.K.; Faes, L.; Geerts, B.; Ibrahim, M.; et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat. Med. 2022, 28, 924–933. [Google Scholar] [CrossRef]
- Melvin, T. The European Medical Device Regulation-What Biomedical Engineers Need to Know. IEEE J. Transl. Eng. Health Med. 2022, 10, 4800105. [Google Scholar] [CrossRef]
- Brady, A.P.; Allen, B.; Chong, J.; Kotter, E.; Kottler, N.; Mongan, J.; Oakden-Rayner, L.; Dos Santos, D.P.; Tang, A.; Wald, C.; et al. Developing, purchasing, implementing and monitoring AI tools in radiology: Practical considerations. A multi-society statement from the ACR, CAR, ESR, RANZCR & RSNA. Insights Into Imaging 2024, 15, 16. [Google Scholar] [CrossRef]
- Conduah, A.K.; Ofoe, S.; Siaw-Marfo, D. Data privacy in healthcare: Global challenges and solutions. Digit. Health 2025, 11, 20552076251343959. [Google Scholar] [CrossRef]
- Williams, P.; Woodward, A. Cybersecurity vulnerabilities in medical devices: A complex environment and multifaceted problem. Med. Devices: Evid. Res. 2015, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Tangari, G.; Ikram, M.; Ijaz, K.; Kaafar, M.A.; Berkovsky, S. Mobile health and privacy: Cross sectional study. BMJ 2021, 373, n1248. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, C.; Lee, I.; Lee, K.; Ong, K.-L. A survey on security and privacy issues in wearable health monitoring devices. Comput. Secur. 2025, 155, 104453. [Google Scholar] [CrossRef]
- Leone, D.M.; O’sullivan, D.; Bravo-Jaimes, K. Artificial Intelligence in Pediatric Electrocardiography: A Comprehensive Review. Children 2024, 12, 25. [Google Scholar] [CrossRef]
- Apfelbacher, T.; Koçman, S.E.; Prokosch, H.-U.; Christoph, J. A Governance Framework for the Implementation and Operation of AI Applications in a University Hospital. Stud. Health Technol. Inform. 2024, 316, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Wessler, B.S.; Nelson, J.; Park, J.G.; McGinnes, H.; Gulati, G.; Brazil, R.; Van Calster, B.; van Klaveren, D.; Venema, E.; Steyerberg, E.; et al. External Validations of Cardiovascular Clinical Prediction Models: A Large-Scale Review of the Literature. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007858. [Google Scholar] [CrossRef]
- Okada, N.; Kubota, A.; Imamura, T.; Suwa, H.; Kawaguchi, Y.; Ohshio, G.; Seino, Y.; Imamura, M. Evaluation of cholecystokinin, gastrin, CCK-A receptor, and CCK-B/gastrin receptor gene expressions in gastric cancer. Cancer Lett. 1996, 106, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Sinha, S.; Zhai, B.; Fudulu, D.; Chan, J.; Narayan, P.; Judge, A.; Caputo, M.; Dimagli, A.; Benedetto, U.; et al. Performance Drift in Machine Learning Models for Cardiac Surgery Risk Prediction: Retrospective Analysis. JMIRx Med. 2024, 5, e45973. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022, 25, 3–9. [Google Scholar] [CrossRef]
- Voets, M.M.; Veltman, J.; Slump, C.H.; Siesling, S.; Koffijberg, H. Systematic Review of Health Economic Evaluations Focused on Artificial Intelligence in Healthcare: The Tortoise and the Cheetah. Value Health 2022, 25, 340–349. [Google Scholar] [CrossRef]
- El Arab, R.A.; Al Moosa, O.A. Systematic review of cost effectiveness and budget impact of artificial intelligence in healthcare. NPJ Digit. Med. 2025, 8, 548. [Google Scholar] [CrossRef]
- Wu, H.; Jin, K.; Yip, C.C.; Koh, V.; Ye, J. A systematic review of economic evaluation of artificial intelligence-based screening for eye diseases: From possibility to reality. Surv. Ophthalmol. 2024, 69, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, N.; Laviola, D.; Mariani, M.V.; Nardini, A.; Adamo, F.; Mahfouz, K.; Colaiaco, C.; Ammirati, F.; Santini, L.; Lavalle, C. Remote monitoring and heart failure. Eur. Heart J. Suppl. 2025, 27, i126–i131. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Sahiner, B.; Chen, W.; Samala, R.K.; Petrick, N. Data drift in medical machine learning: Implications and potential remedies. Br. J. Radiol. 2023, 96, 20220878. [Google Scholar] [CrossRef]
- Andersen, E.S.; Birk-Korch, J.B.; Hansen, R.S.; Fly, L.H.; Röttger, R.; Arcani, D.M.C.; Brasen, C.L.; Brandslund, I.; Madsen, J.S. Monitoring performance of clinical artificial intelligence in health care: A scoping review. JBI Evid. Synth. 2024, 22, 2423–2446. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).