Effect of the Ankle–Foot Orthosis Dorsiflexion Angle on Gait Kinematics in Individuals with Hemiparetic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Experimental Setup and Conditions
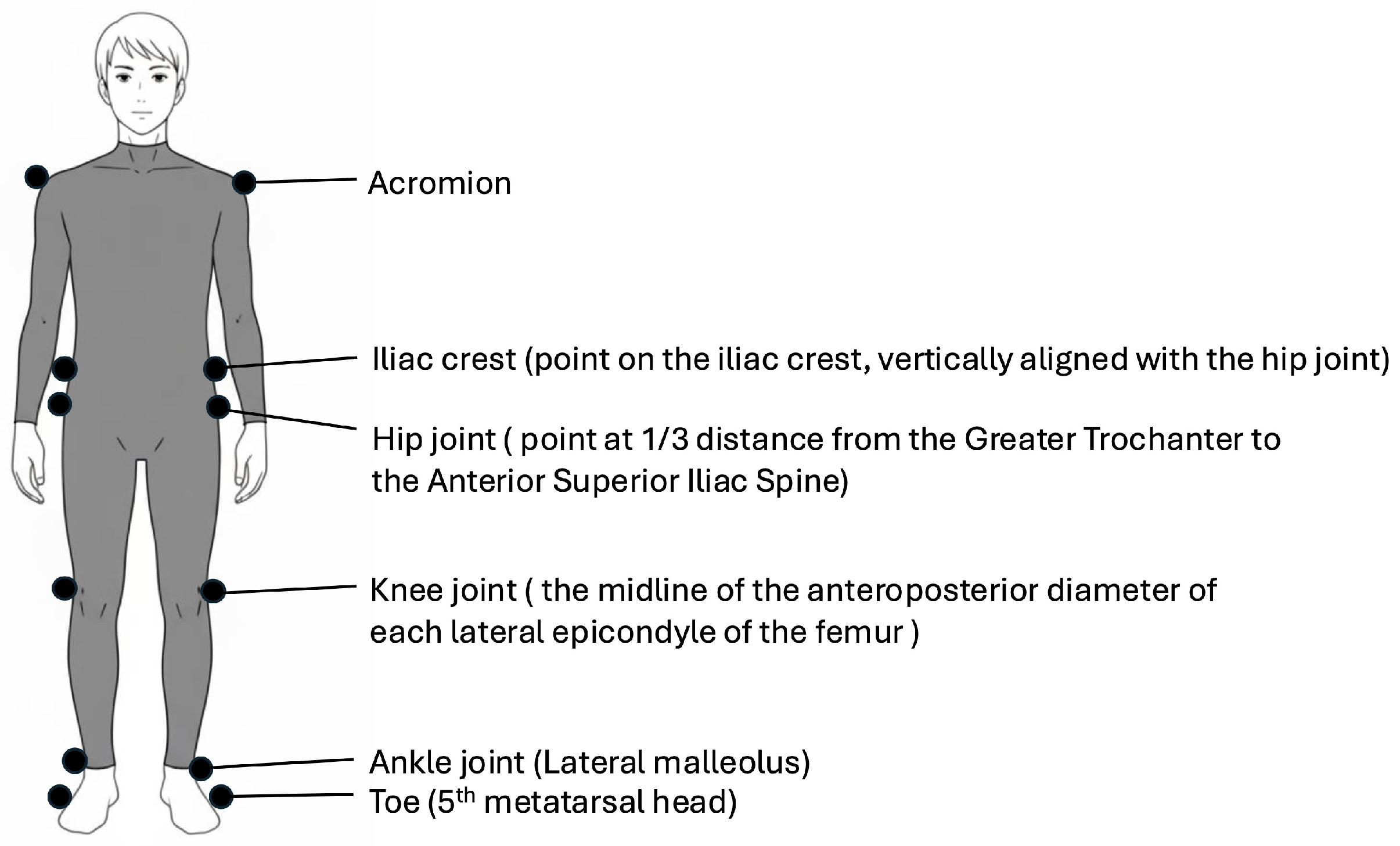
2.4. Kinematic Analysis
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Spatiotemporal Gait Parameters
3.3. Joint Angles
3.4. Toe Clearance and Its Components
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFOs | Ankle-foot orthoses |
| APS-AFO | Adjustable posterior strut AFO |
References
- Pollock, A.; St George, B.; Fenton, M.; Firkins, L. Top ten research priorities relating to life after stroke. Lancet Neurol. 2012, 11, 209. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function, 1st ed.; Slack Incorporated: Thorofare, NJ, USA, 1992. [Google Scholar]
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM R 2014, 6, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- De Quervain, I.A.; Simon, S.R.; Leurgans, S.; Pease, W.S.; McAllister, D. Gait pattern in the early recovery period after stroke. J. Bone Jt. Surg. Am. 1996, 78, 1506–1514. [Google Scholar] [CrossRef]
- Sulzer, J.S.; Gordon, K.E.; Dhaher, Y.Y.; Peshkin, M.A.; Patton, J.L. Preswing knee flexion assistance is coupled with hip abduction in people with stiff-knee gait after stroke. Stroke 2010, 41, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Merlo, A.; Damiano, B. A method to differentiate the causes of stiff-knee gait in stroke patients. Gait Posture 2013, 38, 165–169. [Google Scholar] [CrossRef]
- Cruz, T.H.; Lewek, M.D.; Dhaher, Y.Y. Biomechanical impairments and gait adaptations post-stroke: Multi-factorial associations. J. Biomech. 2009, 42, 1673–1677. [Google Scholar] [CrossRef]
- Stanhope, V.A.; Knarr, B.A.; Reisman, D.S.; Higginson, J.S. Frontal plane compensatory strategies associated with self-selected walking speed in individuals post-stroke. Clin. Biomech. 2014, 29, 518–522. [Google Scholar] [CrossRef]
- Detrembleur, C.; Dierick, F.; Stoquart, G.; Chantraine, F.; Lejeune, T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture 2003, 18, 47–55. [Google Scholar] [CrossRef]
- Danielsson, A.; Sunnerhagen, K.S. Energy expenditure in stroke subjects walking with a carbon composite ankle foot orthosis. J. Rehabil. Med. 2004, 36, 165–168. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Paulus, R.; van Uden, C.J.; Kooloos, J.G.; Hopman, M.T. Decreased energy cost and improved gait pattern using a new orthosis in persons with long-term stroke. Arch. Phys. Med. Rehabil. 2007, 88, 181–186. [Google Scholar] [CrossRef]
- Franceschini, M.; Massucci, M.; Ferrari, L.; Agosti, M.; Paroli, C. Effects of an ankle-foot orthosis on spatiotemporal parameters and energy cost of hemiparetic gait. Clin. Rehabil. 2003, 17, 368–372. [Google Scholar] [CrossRef]
- Pongpipatpaiboon, K.; Mukaino, M.; Matsuda, F.; Ohtsuka, K.; Tanikawa, H.; Yamada, J.; Tsuchiyama, K.; Saitoh, E. The impact of ankle-foot orthoses on toe clearance strategy in hemiparetic gait: A cross-sectional study. J. Neuroeng. Rehabil. 2018, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Moosabhoy, M.A.; Gard, S.A. Methodology for determining the sensitivity of swing leg toe clearance and leg length to swing leg joint angles during gait. Gait Posture 2006, 24, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, H.L.; Shepherd, M.K.; Lawson, B.E. A passive dorsiflexing ankle prosthesis to increase minimum foot clearance during swing. Wearable Technol. 2023, 4, e15. [Google Scholar] [CrossRef]
- Hosokawa, H.; Tamiya, F.; Fujii, R.; Ishimoto, R.; Mukaino, M.; Otaka, Y. Changes in toe clearance due to adjusting the dorsiflexion angle of ankle–foot orthoses: A study in healthy individuals. Bioengineering 2024, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Mukaino, M.; Ohtsuka, K.; Tsuchiyama, K.; Matsuda, F.; Inagaki, K.; Yamada, J.; Tanikawa, H.; Saitoh, E. Feasibility of a simplified, clinically oriented, three-dimensional gait analysis system for the gait evaluation of stroke patients. Progr Rehabil. Med. 2016, 1, 20160001. [Google Scholar] [CrossRef][Green Version]
- Mizuno, M.; Saitoh, E.; Iwata, E.; Okada, M.; Teranishi, T.; Itoh, M.; Hayashi, M.; Oda, Y. The development of a new posterior strut AFO with an adjustable joint: Its concept and a consideration of basic function. Bull. Jap. Soc. Prosthet. Orthot. 2005, 21, 225–233. [Google Scholar][Green Version]
- Tanino, G.; Tomita, Y.; Mizuno, S.; Maeda, H.; Miyasaka, H.; Orand, A.; Takeda, K.; Sonoda, S. Development of an ankle torque measurement device for measuring ankle torque during walking. J. Phys. Ther. Sci. 2015, 27, 1477–1480. [Google Scholar] [CrossRef]
- Maeshima, S.; Okazaki, H.; Okamoto, S.; Mizuno, S.; Asano, N.; Maeda, H.; Masaki, M.; Matsuo, H.; Tsunoda, T.; Sonoda, S. A comparison of knee-ankle-foot orthoses with either metal struts or an adjustable posterior strut in hemiplegic stroke patients. J. Stroke Cerebrovasc. Dis. 2015, 24, 1312–1316. [Google Scholar] [CrossRef]
- Mukaino, M.; Ohtsuka, K.; Tanikawa, H.; Matsuda, F.; Yamada, J.; Itoh, N.; Saitoh, E. Clinical-oriented three-dimensional gait analysis method for evaluating gait disorder. J. Vis. Exp. 2018, 133, 57063. [Google Scholar] [CrossRef]
- Matsuda, F.; Mukaino, M.; Ohtsuka, K.; Tanikawa, H.; Tsuchiyama, K.; Teranishi, T.; Kanada, Y.; Kagaya, H.; Saitoh, E. Biomechanical factors behind toe clearance during the swing phase in hemiparetic patients. Top. Stroke Rehabil. 2017, 24, 177–182. [Google Scholar] [CrossRef]
- Tsuchiyama, K.; Mukaino, M.; Ohtsuka, K.; Matsuda, F.; Tanikawa, H.; Yamada, J.; Pongpipatpaiboon, K.; Kanada, Y.; Saitoh, E.; Otaka, Y. Effects of ankle-foot orthoses on the stability of post-stroke hemiparetic gait. Eur. J. Phys. Rehabil. Med. 2022, 58, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Chino, N.; Sonoda, S.; Domen, K.; Saitoh, E.; Kimura, A. Stroke Impairment Assessment Set(SIAS). A new evaluation instrument for stroke patients. Jpn. J. Rehabil. Med. 1994, 31, 119–125. [Google Scholar] [CrossRef]
- Bouchalová, V.; Houben, E.; Tancsik, D.; Schaekers, L.; Meuws, L.; Feys, P. The influence of an ankle-foot orthosis on the spatiotemporal gait parameters and functional balance in chronic stroke patients. J. Phys. Ther. Sci. 2016, 28, 1621–1628. [Google Scholar] [CrossRef][Green Version]
- Pohl, M.; Mehrholz, J. Immediate effects of an individually designed functional ankle-foot orthosis on stance and gait in hemiparetic patients. Clin. Rehabil. 2006, 20, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Silver-Thorn, B.; Herrmann, A.; Current, T.; McGuire, J. Effect of ankle orientation on heel loading and knee stability for post-stroke individuals wearing ankle-foot orthoses. Prosthet. Orthot. Int. 2011, 35, 150–162. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Ounpuu, S.; Delp, S.L. The importance of swing-phase initial conditions in stiff-knee gait. J. Biomech. 2003, 36, 1111–1116. [Google Scholar] [CrossRef]
- Boehm, W.L.; Gruben, K.G. Post-stroke walking behaviors consistent with altered ground reaction force direction control advise new approaches to research and therapy. Transl. Stroke Res. 2016, 7, 3–11. [Google Scholar] [CrossRef]
- Lee, J.; Lee, R.K.; Seamon, B.A.; Kautz, S.A.; Neptune, R.R.; Sulzer, J. Between-limb difference in peak knee flexion angle can identify persons post-stroke with Stiff-Knee gait. Clin. Biomech. 2024, 120, 106351. [Google Scholar] [CrossRef] [PubMed]
- Mihcin, S.; Sahin, A.M.; Yilmaz, M.; Alpkaya, A.T.; Tuna, M.; Akdeniz, S.; Korkmaz, N.C.; Tosun, A.; Sahin, S. Database covering the prayer movements which were not available previously. Sci. Data 2023, 10, 276. [Google Scholar] [CrossRef]
- Puh, U.; Baer, G.D. A comparison of treadmill walking and overground walking in independently ambulant stroke patients: A pilot study. Disabil. Rehabil. 2009, 31, 202–210. [Google Scholar] [CrossRef]
- Nikamp, C.D.M.; van der Palen, J.; Hermens, H.J.; Rietman, J.S.; Buurke, J.H. The influence of early or delayed provision of ankle-foot orthoses on pelvis, hip and knee kinematics in patients with sub-acute stroke: A randomized controlled trial. Gait Posture 2018, 63, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Cruz, T.H.; Dhaher, Y.Y. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait Posture 2009, 30, 312–316. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | |
|---|---|
| Age, years | 62.3 ± 14.4 |
| Sex, male/female | 19/7 |
| Type of stroke, hemorrhagic/ischemic | 14/12 |
| Affected side, right/left | 14/12 |
| Stroke Impairment Assessment Set total lower-limb score | 9.7 ± 3.3 |
| Days after onset | 55 (37–621) |
| Functional Independence Measure-Walking score, 4/5/6 | 6/9/11 |
| Variable | AFO Dorsiflexion Angles | p Value † | |||
|---|---|---|---|---|---|
| 0° | 5° | 10° | 15° | ||
| Stride length, cm | 59.3 ± 30.5 | 57.8 ± 28.5 | 57.8 ± 28.4 | 58.3 ± 28.2 | 0.956 |
| Paretic step length, cm | 30.1 ± 15.5 | 29.3 ± 14.7 | 29.0 ± 15.0 | 28.6 ± 15.0 | 0.485 |
| Non-paretic step length, cm | 29.2 ± 16.5 | 28.5 ± 15.3 | 28.8 ± 14.8 | 29.7 ± 15.0 | 0.557 |
| Stride time, s | 1.70 ± 0.47 | 1.68 ± 0.44 | 1.67 ± 0.45 | 1.66 ± 0.51 | 0.151 |
| Paretic swing time, s | 0.49 ± 0.14 | 0.49 ± 0.15 | 0.51 ± 0.19 | 0.49 ± 0.13 | 0.406 |
| Paretic single-stance time, s | 1.20 ± 0.41 | 1.19 ± 0.34 | 1.16 ± 0.34 | 1.18 ± 0.43 | 0.105 |
| Double-stance time after paretic swing, s | 0.38 ± 0.21 | 0.38 ± 0.15 | 0.38 ± 0.17 | 0.40 ± 0.24 | 0.172 |
| Double-stance time before paretic swing, s | 0.42 ± 0.20 | 0.40 ± 0.18 | 0.37 ± 0.15 | 0.37 ± 0.18 | <0.001 |
| Angles, Degree | AFO Dorsiflexion Angles | p Value † | ||||
|---|---|---|---|---|---|---|
| 0° | 5° | 10° | 15° | |||
| Hip flexion | Swing | 25.4 ± 9.0 | 23.8 ± 8.5 | 25.4 ± 8.2 | 25.4 ± 9.2 | 0.227 |
| Stance | 20.4 ± 8.3 | 21.8 ± 8.0 | 22.3 ± 7.9 | 21.8 ± 8.8 | 0.485 | |
| Hip extension | Swing | −9.1 ± 9.5 | −9.2 ± 9.8 | −8.5 ± 9.5 | −8.1 ± 9.3 | 0.934 |
| Stance | 1.8 ± 8.1 | 1.2 ± 9.4 | 1.5 ± 8.3 | 2.1 ± 8.4 | 0.875 | |
| Knee flexion | Swing | 33.0 ± 20.0 | 31.4 ± 19.7 | 30.7 ± 19.4 | 28.9 ± 19.9 | 0.023 |
| Stance | 29.4 ± 15.6 | 27.3 ± 15.2 | 27.0 ± 15.1 | 24.7 ± 15.5 | 0.022 | |
| Knee extension | Swing | 3.6 ± 9.1 | 4.1 ± 9.0 | 3.9 ± 8.3 | 3.4 ± 9.1 | 0.853 |
| Stance | −3.5 ± 6.6 | −2.8 ± 7.0 | −2.8 ± 5.9 | −3.2 ± 6.8 | 0.731 | |
| Ankle dorsiflexion | Swing | −0.8 ± 4.9 | −0.5 ± 3.8 | 1.6 ± 3.6 | 1.2 ± 4.5 | <0.001 |
| Stance | 4.9 ± 5.3 | 4.3 ± 3.7 | 5.3 ± 3.3 | 5.0 ± 4.6 | 0.145 | |
| Ankle plantarflexion | Swing | 6.8 ± 5.3 | 5.7 ± 3.8 | 4.3 ± 5.0 | 4.0 ± 4.6 | 0.001 |
| Stance | 6.5 ± 4.4 | 5.5 ± 4.0 | 5.0 ± 4.6 | 4.7 ± 4.8 | 0.027 | |
| Variable | AFO Dorsiflexion Angles | p Value † | |||
|---|---|---|---|---|---|
| 0° | 5° | 10° | 15° | ||
| Toe clearance, cm | 3.9 ± 2.0 | 3.7 ± 1.9 | 3.8 ± 2.1 | 3.8 ± 2.1 | 0.557 |
| Toe clearance components | |||||
| Limb shortening secondary to knee flexion, cm | 3.1 ± 3.2 | 2.7 ± 3.2 | 2.9 ± 3.3 | 2.7 ± 3.3 | 0.061 |
| Limb shortening secondary to ankle dorsiflexion, cm | −1.1 ± 1.1 | −0.8 ± 0.7 | −0.7 ± 0.7 | −0.5 ± 0.7 | <0.001 |
| Compensatory hip elevation, cm | 1.8 ± 1.7 | 1.7 ± 1.8 | 1.7 ± 1.8 | 1.6 ± 1.8 | 0.025 |
| Compensatory contralateral vaulting, cm | −0.1 ± 0.9 | −0.0 ± 1.0 | −0.1 ± 1.1 | −0.1 ± 1.0 | 0.831 |
| Compensatory hip abduction, cm | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.163 |
| Compensatory movement ratio | 0.74 ± 0.73 | 0.77 ± 0.75 | 0.70 ± 0.71 | 0.68 ± 0.76 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosokawa, H.; Tamiya, F.; Fujii, R.; Ishimoto, R.; Mukaino, M.; Otaka, Y. Effect of the Ankle–Foot Orthosis Dorsiflexion Angle on Gait Kinematics in Individuals with Hemiparetic Stroke. Bioengineering 2025, 12, 1091. https://doi.org/10.3390/bioengineering12101091
Hosokawa H, Tamiya F, Fujii R, Ishimoto R, Mukaino M, Otaka Y. Effect of the Ankle–Foot Orthosis Dorsiflexion Angle on Gait Kinematics in Individuals with Hemiparetic Stroke. Bioengineering. 2025; 12(10):1091. https://doi.org/10.3390/bioengineering12101091
Chicago/Turabian StyleHosokawa, Hiroshi, Fumiaki Tamiya, Ren Fujii, Ryu Ishimoto, Masahiko Mukaino, and Yohei Otaka. 2025. "Effect of the Ankle–Foot Orthosis Dorsiflexion Angle on Gait Kinematics in Individuals with Hemiparetic Stroke" Bioengineering 12, no. 10: 1091. https://doi.org/10.3390/bioengineering12101091
APA StyleHosokawa, H., Tamiya, F., Fujii, R., Ishimoto, R., Mukaino, M., & Otaka, Y. (2025). Effect of the Ankle–Foot Orthosis Dorsiflexion Angle on Gait Kinematics in Individuals with Hemiparetic Stroke. Bioengineering, 12(10), 1091. https://doi.org/10.3390/bioengineering12101091






