Abstract
The prevalence of wet age-related macular degeneration (AMD) in the US is expected to increase to 82 million by 2050. Addressing the specialized needs for this population will become increasingly challenging as prevalence rises. Frequent anti-vascular endothelial growth factor (anti-VEGF) injections have been the recourse for this population; however, the burden wet AMD places on patients underscores the critical need for durable therapeutic approaches. Gene therapy is a bioengineered treatment that has transformed the management of previously untreatable disorders. Ongoing advancements and refinements in its biomechanism could lead to more sustainable treatment options for wet AMD. In this article, we provide recent updates on gene therapy trials for wet AMD.
1. Introduction
Age-related macular degeneration (AMD) is a progressive and debilitating condition. Its multifaceted pathophysiology is influenced by both genetic predisposition and environmental factors [1,2]. The natural progression of AMD leads to two distinct advanced clinical presentations. One is dry age-related macular degeneration, where vision loss is characterized by progressive retinal atrophy. The other form is neovascular age-related macular degeneration (nAMD or often referred to as wet AMD), which is identified by exudative choroidal neovascularization. When nAMD is left untreated, it can lead to rapid vision loss. It is well known that environmental factors such as smoking and obesity influence the progression of AMD [1], in addition to genetic mutations that confer increased risk [3]. The products of the Complement Factor H (CFH) gene have also been implicated in inflammatory and immune pathways, which further support the role of inflammatory indicators such as the tumor necrosis factor (TNF)-α, complement components and interleukin-1 and 6 (IL-1, IL-6) in the pathogenesis of nAMD [4].
The formation of choroidal neovascularization depends on the release of vascular endothelial growth factor (VEGF) from the retinal pigment epithelium toward the inner choroid, where high levels of VEGF receptors are located [5]. In classic ischemic retinal diseases, VEGF is induced by hypoxia. However, in nAMD, local immune reactivity and inflammatory markers are the predominant triggers. VEGF interacts with the endothelial cell receptors VEGFR receptor 1 and VEGFR receptor 2, which triggers an intracellular signal transduction cascade [6].
The introduction of anti-vascular endothelial growth factor (anti-VEGF) agents has been revolutionary in the field of retinal medicine, particularly for neovascular retinal diseases. For patients affected with wet AMD, the variable frequency and cost of anti-VEGF injections driven by treatment regimens are important economic considerations. Additionally, the economic impact of wet AMD is significant, with anti-VEGF accounting for over $40 billion annually and 12% of the US Medicare part B budget [7,8]. Holistic cost-saving analysis studies across longer-acting anti-VEGF agents found that they differ in acquisition cost and in the frequency of required injections [9]. When considering projected injection frequency, wholesale acquisition cost, time, and distance travelled, the triannual visit cost for ranibizumab was $72,080, $39,946 for aflibercept and $33,265 for farcimab.
Gene therapy is an innovative approach that has been applied to retinal disease, demonstrating promising efficacy in untreatable retinal diseases and other ocular conditions [10]. The first transformative step in ophthalmic gene therapy was the use of a recombinant adeno-associated virus (AAV) carrying RPE65-complementary DNA (cDNA) for patients with RPE65-associated Leber’s congenital amaurosis (LCA) [11]. This subretinal approach laid the foundation for the field. Since then, the sustained clinical improvements and favorable immunological profile of this therapy have inspired advances in vector design and driven clinical trials for Stargardt disease, choroideremia, retinitis pigmentosa, and Usher syndrome [10,12,13,14,15].
Over the past few years, significant progress has been made in innovative treatments for wet AMD that aim to provide long-lasting effects compared with frequent anti-VEGF injections. Gene therapy for wet AMD has shown particularly promising results. Based on cohort size, efficacy, and stage of advancement, Ixo-vec (ADVM-022), ABBV-RGX-314, and 4D-150 have been the most promising candidates (Table 1). Many of these clinical trials have completed phase 2 testing with phase 3 outcomes forthcoming. The following article reviews the current state of the art for gene therapies applied to AMD.

Table 1.
Overview of Wet AMD gene therapy drugs. VA: visual acuity; CST: Central Subfield Thickness.
2. Gene and Cell Therapy
The major gene therapy modalities currently investigated in clinical trials are gene replacement, gene editing, ribonucleic acid (RNA) modulation, cell-based therapy, and oncolytic virotherapy [16]. The mechanism of gene replacement uses a viral vector to deliver a functional copy of a defective gene to restore normal protein function. Similarly, oncolytic virotherapy uses viral vectors to selectively lyse cancer cells. Gene editing and RNA modulation techniques directly modify the genetic material to alter its expression.
The current gene therapies in clinical trials for wet AMD mainly employ gene replacement or RNA modulation to suppress retinal vascular endothelial growth factor (VEGF). The main vectors used in these trials are adeno-associated viral (AAV) vectors, which offer several advantages. AAV vectors minimize immune reactivity and can target specific cell populations via engineered serotypes [17]. For many inherited ocular conditions being approached with gene therapy, the etiology is predominantly monogenic [11,18], which makes AAV vectors more appealing given their small but precise genetic payload capacity. In addition, their episomal DNA is stable without integrating into the host genome, theoretically decreasing oncogenicity [19]. This allows them to persist in postmitotic cells such as retinal cells, but progressively lost in dividing cells [20,21].
Gene replacement and gene editing are common techniques employed in the therapies for monogenic IRDs. Monogenic inherited retinal diseases (IRDs) are characterized by a single defective gene, which presents a more defined roadmap for therapeutic development. The mendelian inheritance pattern makes it easier to target the root cause and delay disease progression. Bioengineering challenges are possibly less demanding for IRD gene therapy. Selecting a broad promoter and a vector capable of delivering a single gene payload is often sufficient to achieve pan-retinal expression in many conditions.
In contrast, nAMD, requires complex vector engineering that also avoids excessive VEGF inhibition, which can lead to retinal atrophy. Unlike monogenic IRDs, gene therapy for AMD does not aim to correct a specific genetic mutation. Instead, it provides sustained delivery of therapeutic proteins that can alter the disease course.
Another layer of complexity for nAMD gene therapy is the delivery method. Subretinal injection can reduce the immune response risk due to the blood–retina barrier, which normally safeguards the retina from the systemic immune system [22] (Figure 1A). However, in nAMD this barrier may be compromised, and the surgical procedure itself confers risks of infection, hemorrhage, and retinal detachment to name a few.
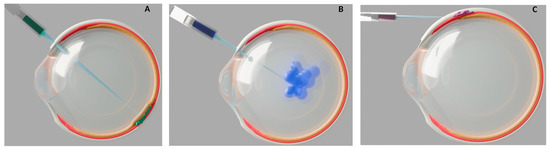
Figure 1.
Schematic representations of ocular injection techniques for nAMD gene therapy. Cross-section illustrations of the human eye show three methods of delivering bioengineered gene therapy for neovascular age-related macular degeneration (nAMD), with relevant anatomical layers color coded: retina (orange), choroid (red), sclera (white). (A) Subretinal injection: The therapeutic agent (green) is delivered via a subretinal approach, using a syringe. (B) Intravitreal injection: The therapeutic agent (blue) is injected into the vitreous cavity through an intravitreal approach. (C) Suprachoroidal injection: The therapeutic agent (purple) is delivered into the suprachoroidal space.
A less invasive delivery approach is intravitreal injection, which can be performed in outpatient settings. However, this may increase the chance of exposure to systemic circulating antibodies [23] (Figure 1B). From a bioengineering aspect, intravitreal delivery also reduces vector efficacy, due to the inner limiting membrane limiting the penetration of vectors to outer retinal layers [24].
Suprachoroidal injections may offer a balanced option by reducing the immune response risks compared to an intravitreal delivery and potentially allowing outpatient administration (Figure 1C). However, this approach may have less pharmacokinetic advantage, as increased drug clearance through choroidal circulation can decrease the duration of treatment in suprachoroidal space [25]. This rapid clearance increases the risk that the vectors do not have sufficient time to transduce target tissues and exert their effects.
3. Phase 3 Clinical Trials
The clinical translation of AAV technology has facilitated a spectrum of trials for the treatment of wet AMD. The leading phase 3 gene therapy trials have been RGX-314, Ixo-vec(ADVM-022) and 4D-150.
RGX-314 is an AAV vector with serotype 8 (AAV8), containing cDNA encoding a monoclonal antibody fragment antigen binding (Fab) protein that mimics ranibizumab, and use a chicken β-actin promoter (CB7) [26]. Serotype 8 provides efficient transduction in murine retinal cells [27], has moderate neutralizing seroprevalence, and superior efficiency when crossing blood vessel barriers compared to other serotypes [28]. The CB7 promoter is effective for sustained gene expression [29]. The expressed Fab fragment serves as an antagonist, neutralizing VEGF-A. While ranibizumab targets all VEGF-A isoforms [30], no biophysical data are available on RGX-314’s anti-VEGF Fab fragment affinity, isoform specificity, or kinetic parameters.
The phase 1/2a dose-escalation (NCT03066258) study of RGX-314 showed sustained RGX-314 protein concentrations in aqueous humor at the two year endpoint [26]. RGX-314 is delivered by a small-gauge vitrectomy, followed by a single subretinal injection without a pre-bleb. In this phase 1/2a dose escalation study of 42 participants with wet AMD, 13 participants experienced 20 serious adverse events (macula pigment mottling, macula-sparing retinal detachment, endophthalmitis, reduced visual acuity, recurrent transitional cell carcinoma, and the remaining were systemic events). In cohort three (6 × 1010 genome copies (GC)/eye) and four (1.6 × 1011 GC/eye), 50% gained ≥15 letters, respectively, at week 106.
RGX-314 phase 2 trials (AAVIATE and ATMOSPHERE) are evaluating a less invasive, office-based suprachoroidal delivery, without vitrectomy. According to Regenexbio, in AAVIATE (n = 116), there was a 60–80% decrease in the number of supplemental intravitreal anti-VEGF injections needed per year across dose cohorts. In cohorts receiving 6 × 1010 GC/eye, the mean BCVA was maintained at ±5 ETDRS letters or improved over two years, with the highest dose (1 × 1012 GC/eye) showing dose dependent efficacy [26]. However, multiple moderate treatment-emergent adverse events were reported, such as episcleritis and an increase in IOP. The ongoing phase 3 trials (ATMOSPHERE and ASCENT) with approximately 765 patients globally are evaluating suprachoroidal and subretinal RGX-314, respectively (NCT05407636).
Ixo-vec (ADVM-022) is an AAV vector with serotype 2/7m8 peptide (AAV2.7m8) that contains a CD11-optimized cDNA that encodes a complete aflibercept protein with its associated binding domain VEGF-A/B, placental growth factor and the fragment crystallizable region (Fc) portion of human immunoglobulin (IgG1) [31]. The 7m8 peptide insertion disrupts AAV2 binding to heparan sulfate proteoglycans in the inner limiting membrane (ILM) at the vitreoretinal junction [24]. It is believed that this disruption facilitates infusion into the retinal cells, based on its high transduction of retinal tissues [32,33]. It also helps reduce antibody recognition, given AAV2’s low neutralizing seroprevalence [28].
Results from the phase 1 and phase 2 studies (NCT03748784; NCT05536973) revealed that aflibercept levels in aqueous humor were stable and maintained through study completion. The intervention for the Ixo-vec (ADVM-022) trials includes administration of one intravitreal injection dosage in an outpatient setting with oral or topical steroids.
In the phase 1 dose-ranging (6 × 1011 vector genome(vg)/eye or 2 × 1011 vg/eye) study of 30 patients with wet AMD, 20% of patients in the higher dosage (6 × 1011 vg/eye) cohort and 7% in the lower dosage cohort experienced serious adverse events (cataract, uveitis, retinal detachment, or dry AMD progression). In the phase 2 randomized, double-masked, dose-ranging study of 60 patients with enhanced topical steroids, no serious adverse events were observed. There were two discontinuations due to unrelated adverse events (lung malignancy and cardiopulmonary arrest), and no systemic immunological reactions were observed in either study phases.
In the phase 1 study, 80% of patients in the higher dose cohort were injection-free at the two years landmark and 53% of the lower dose cohort remained injection-free at two years. In both cohorts there was ≥80% reduction in annualized anti-VEGF injections. In the phase 2 study, 76% of patients in the 6 × 1010 vg/eye cohort remained injection-free at 26 weeks, whereas 83% of those in the 2 × 1011 vg/eye cohort remained injection-free at 26 weeks [34]. The current phase 3 (ARTEMIS) non-inferiority study evaluating the 6 × 1011 vg/eye dosage vs. aflibercept every eight weeks with prophylactic steroids is actively enrolling participants (NCT06856577) [35].
4D-150 is different from the other AAV vectors, because it is a synthetic AAV capsid (R100) and has a dual transgene payload. Similarly to Ixo-vec, it contains a codon-optimized cDNA encoding aflibercept, but it also encodes a miRNA-30 backbone targeting VEGF-C mRNA. With this additional feature it is able to antagonize four VEGF family members (A, B, C and PIGF) [36,37].
Although R100 is a relatively new engineered AAV variant, it has shown marked transduction efficiency in preclinical studies. In vitro functional assessment in human retinal cells, derived from induced pluripotent stem cells, revealed that R100 had a 2.6- to 3.75-fold higher transduction compared to AAV2 [38]. However, this assay lacks the complexity of human retinal tissue to effectively assess the biological challenges faced by vectors injected intravitreally, such as the ILM, vitreous humor, extracellular matrix, and human immunological response. In Cynomolgus macaques and African green monkeys, bilateral R100 resulted in widespread and stable transduction across all retinal cells, leading to clinical trials in human subjects [38].
The protocols for the 4D-150 clinical trials included the administration of a single intravitreal injection of 4D-150. In the phase 1 open-label dose, exploration (3 × 1010, 1 × 1010, and 6 × 109 vg/eye) study of 15 patients with topical steroid taper post injection, no serious adverse events were observed. The 3 × 1010 vg/eye dosage was the most efficient with a mean central subfield thickness (CST) reduced by 92 µm at 36 weeks, and an 84% reduction in annualized anti-VEGF injections [39]. However, the results were worse for the 1 × 1010 dose cohort with a CST +38 ±37 µm at 24 weeks and stable for the 6 × 109 vg/eye dose cohort with +3 ±25 µm [40].
In the randomized, controlled phase 2 (PRISM; NCT05197270) clinical trial, patients assigned to two treatment arms (1 × 1010 vg/eye and 3 × 1010 vg/eye) demonstrated satisfactory steroid compliance and no serious adverse events [41]. The current phase 3 (4FRONT-1; NCT07064759) trial will cover a more global population with over 800 patients, with a fixed 3 × 1010 vg/eye dosage as the intervention [42].
4. Phase 2 Clinical Trials
In addition to the phase 3 clinical trials discussed, several therapies are currently being investigated in the early stages of clinical development. LX102 and rAAV.sFlt-1 are currently in phase 2 clinical trials.
LX102 uses an AAV vector with serotype 2, similar to Ixo-vec, with the gene coding for VEGF-Trap (aflibercept). This allows the specific inhibition of VEGF- A and PIGF, but not VEGF- C or D [43]. The intervention for the LX102 clinical trials includes a single intravitreal injection of aflibercept (2 mg/0.05 mL), followed by subretinal injection of LX102 dosages (2 × 1010 or 1.25 × 1011 vg) two weeks later. Although no LX102-related adverse events were reported, more than half of the nine patients in the study experienced procedure-related adverse events, such as conjunctival hyperemia, post-operative visual acuity reduction, increased intraocular pressure (IOP), and mild cell debris in the anterior vitreous [44]. Similarly to phase 1, the current phase 2 randomized, parallel-group (NCT06196840) trial of LX102 is actively recruiting local participants in China.
rAAV.sFlt-1 is composed of a recombinant adeno-associated virus serotype 2 (rAAV2) vector that contains the sFLT-1 transgene variant. The expression of this gene antagonizes VEGF-A, VEGF-B, and PlGF [45,46]. Preclinical studies of this new engineered vector have revealed sustained retinal preservation in transgenic mice [47].
In the phase 1 randomized controlled trial (NCT01494805) the injection involved a 23-gauge three-port pars plana vitrectomy with core vitreous removal and posterior vitreous detachment induction, followed by a subretinal delivery of 100 μL therapy (1 × 1010 or 1 × 1011 vg) via a 41G cannula. Although no systemic adverse events attributed to rAAV.sFLT-1 were noted, one patient developed a systemic immune response to AAV2 antigens and three of the six patients in the treatment cohorts experienced some adverse events (mild cell debris, subconjunctival and subretinal hemorrhage) [48]. In the combined phase 1/2a trial (NCT01494805) with only the high dose, 33 serious adverse events occurred over three years. Three cancer (breast cancer, colon cancer and lung cancer) diagnoses occurred during the trial, although two patients had a history of malignancy prior to starting the clinical trial. There were two non-lethal cardiac events, one transient choroiditis, one chronic eye inflammation, and the remaining serious adverse events were attributed to age-related infirmities. There were no statistically significant differences between the rAAV.sFlt-1 and control groups in visual acuity, retinal thickness, or sFLT-1 protein at any time point during the trial [49]. Given this lack of meaningful efficacy or safety benefits, the development of rAAV.sFlt-1 for wet AMD has been discontinued.
5. Phase 1 Clinical Trials
Wet AMD gene therapy trials that have started phase 1 include but are not limited to NG101, KH658, FT-003, KH631, and AAV2-sFLT01.
Similarly to RGX-314, NG101 is a recombinant adeno-associated virus serotype 8 (rAAV8) vector, but it encodes aflibercept instead of a Fab fragment similar to ranibizumab. A preclinical evaluation of this engineered vector showed effective transduction in vitro via non-retinal cells (CHO-K1 and HEK293 cells) and in vivo (subretinal injection of NG101 1 × 106 to 1 × 109 vg/eye; intravitreal aflibercept injection as a comparator) in a mouse model [50]. This bioassay of NG101 proved to be effective, with sustained expression of aflibercept from the vector persisting one year post injection in mice, although no additional long-term data are available in this mouse model.
The phase 1 dose escalation study clinical trial (NCT05984927) for NG101 received an FDA fast track designation in November 2024 to enroll US- and Canada-based patients. The result of the safety and effectiveness of this gene therapy is expected in 2025.
KH658 is a recombinant AAV vector of an unspecified serotype that contains the transgene for VEGF receptor. Although there have been no published peer-review preclinical studies on KH658, it has started its phase 1 trial (NCT06458595).
Similarly to KH658, there is paucity of published peer-reviewed data on the therapeutic payload or biomechanism of FT-003. However, according to the global clinical-stage biotechnology company that engineered FT-003, the preliminary outcome of the phase 1 clinical study (NCT05611424) conducted in China is showing promising safety results. This limited clinical study of approximately 15 patients revealed that a single intravitreal injection of FT-003 was well tolerated with no serious adverse effect [51]. As of the last update, no safety or efficacy results have been published on ClinicalTrials.gov.
KH631 is composed of a rAAV8 vector with the transgene for the human VEGFR1/VEGFR2-Fc fusion protein that antagonizes all VEGF-A/B and PlGF with great affinity (inhibitory concentration (IC50) value of 269.2 pM). A pharmacokinetics and biodistribution assessment in rhesus monkey via subretinal injections of KH631 at 1 × 1011 vg/eye revealed that the transgene expression was higher in retinal tissue compared to vitreous [52]. Given the promising outcome of KH631, the phase 1 dose escalation study (NCT05672121) is underway to establish its safety and efficacy in humans.
Although many of these gene therapy trials have shown great potential for the future of wet AMD, AAV2-sFLT01 remained at phase 1 (NCT01024998), with no subsequent phase 2 or 3 trials initiated. AAV2-sFLT01 is composed of an AAV2 vector with a transgene encoding domain 2 of sFlt1 linked to a human Fc domain. Like RGX-314, this therapy uses the chicken β-actin promoter to drive gene expression and enable production of the therapeutic protein. sFlt1 naturally binds VEGF-A, VEGF-B, and PlGF [53]. Studies on its molecular and functional properties have shown that it exhibits a high affinity for this protein and that it binds VEGF-A with high affinity [54].
Preclinical studies on a mouse oxygen-induced retinopathy model have demonstrated adequate transduction effectivity of AAV2-sFLT01 [55]. However, the phase 1 dose-escalation trial revealed that only five patients in the highest-dosage cohorts (had detectable sFLT01 in their aqueous humor. Given AAV2’s poor neutralizing seroprevalence, only patients with low anti-AAV2 serum antibody titers achieved therapeutic sFLT01 levels in this study. This could also explain why only 4 of the 19 patients in this study had a sustained reduction in CST at 52 weeks [56]. The unreliable transgene expression and inferior outcome compared to anti-VEGF injection has halted the development of this gene therapy.
6. Emerging Molecular Editing Therapy for AMD: CRSPR-CAS13
The CRISPR/Cas gene-editing system is widely considered as one of the most efficient and innovative bioengineered tools in the field of genomics [57]. Its revolutionary status was officially affirmed in 2020, when Doudna and Charpentier were awarded the Nobel Prize in Chemistry for its discovery [58]. In addition, CRISPR-based therapies have been approved for sustained remission in both beta-thalassemia and sickle cell disease [59,60]. Currently, the CRISPR/Cas9 mechanism has not seen the same level of application as the traditional AAV vectors in wet AMD gene therapy research.
HG202 is the only CRISPR-based gene therapy that is currently in clinical trials for wet AMD. Compared to the previously described AAV-based anti-VEGF gene therapies, HG202 is composed of an undisclosed AAV capsid that contains an RNA editing enzyme, Cas13Y, a type VI CRISPR system. This class 2 type VI RNA endonuclease directly degrades VEGF-A mRNA, preventing its expression. This mechanism enables persistent suppression of VEGF-A and its isoforms without relying on continuous transgene expression. Its RNA-targeting capabilities and efficacy have been investigated in transgenic mice [61,62]. However, there are no studies in non-human primates or on long-term durability of Cas13Y currently. HG202’s first-in-human phase 1 trial in China was initiated, and more recently, its phase 1 dose-escalation study (NCT06623279) has started in the US.
7. Weaknesses of Ocular Gene Therapy
One of the main weaknesses of ocular gene therapy has been immunological responses. Many nAMD clinical trials have attempted to mitigate inflammatory reactions by using different injection techniques, enhancing dose-ranging strategies with topical or oral steroids, and by addressing neutralizing seroprevalence of viral vectors. However, significant challenges to both the effectiveness and safety of gene therapy in nAMD remain. Some patients still develop autoantibodies and secondary autoimmune retinopathy after injections.
The current landscape of ongoing clinical trials for neovascular age-related macular degeneration (nAMD) suggests a persistent emphasis on therapies targeting the VEGF pathway. This may have limited potential benefit to patients with a documented history of inadequate responses to anti-VEGF agents. XMVA09 represents an innovative approach by encoding a bispecific antibody designed to simultaneously inhibit both VEGF-A and ANG-2, offering a rationale for broader efficacy, including in VEGF-refractory cases. Nevertheless, several critical challenges remain. The recent investigator-initiated clinical trial assessed XMVA09 in only six nAMD patients, all of whom had a history of multiple anti-VEGF therapy injections in the past, but none had a documented history of resistance to prior anti-VEGF treatments [63]. It is difficult to determine whether therapeutic effects in these subjects were due to VEGF-A inhibition, ANG-2 inhibition, or synergism between both pathways. Future trials would benefit from dedicated cohorts of non-responders and treatment-naive patients to determine response rates in these groups.
In addition, there is uncertainty regarding the long-term results and durability of treatment. For example, the inferiority of AAV2-sFLT01 compared to current anti-VEGF therapies led to discontinuation of its clinical trial. This underscores the critical need to improve both surgical and minimally invasive delivery methods for therapeutic agents. This optimization should focus on injection approaches, vector dosing protocols, and formulation strategies to achieve maximum transduction efficiency and safety.
Although, these vectors are engineered for stable transduction in postmitotic cells; however, the therapeutic benefit may decline over time. Another important point to consider is the accessibility and economic aspect of gene therapy. Established anti-VEGF treatments currently available for nAMD already account for a considerable portion of the Medicare budget. Limited patient access and stringent insurance approval processes are key challenges that may hinder the widespread implementation of gene therapy.
Although these innovative therapies are impactful and important, we must consider the dependability of engineered gene editing therapies. The pathway to FDA approval for bioengineered therapies follows high standards, with strict manufacturing protocols, robust regulatory planning, and comprehensive review of all results before advancing through clinical phases. This approach is not intended to hinder scientific innovation, but rather to safeguard the efficacy and safety of these groundbreaking treatments. There are proven examples demonstrating the durability and effectiveness of gene therapies in clinical medicine. With appropriate regulatory oversight and safety planning, these treatments have already transformed the clinical management of conditions such as RPE65-associated LCA, hemophilia A, sickle cell disease, and beta thalassemia [11,60,64,65]. With ongoing refinements in gene therapy mechanisms, a major breakthrough for wet AMD is within reach. One that promises durable treatment efficacy and lasting sight preservation for an expanding patient population.
8. Summary Statement
In the above article we review the current state of the art for gene therapies applied to wet-AMD. The leading phase 3 gene therapy trials are RGX-314, Ixo-vec (ADVM-022), and 4D-150. LX102 and rAAV.sFlt-1 are at an earlier stage but may hold promising results. There are multiple wet AMD gene therapy trials that have just started their phase 1 clinical trials, including KH658, FT-003, NG101, KH631, and AAV2-sFLT01. However, HG202 is the only CRISPR-based gene therapy that is currently in clinical trials for wet AMD.
Author Contributions
Conceptualization, N.B., J.S. and J.D.S.; Methodology, N.B., J.S. and J.D.S.; Software, N.B., J.S. and J.D.S.; Validation, N.B., J.S. and J.D.S.; Formal analysis, N.B., J.S. and J.D.S.; Investigation, N.B., J.S. and J.D.S.; Resources, N.B., J.S. and J.D.S.; Data curation, N.B., J.S. and J.D.S.; Writing—original draft, N.B., J.S. and J.D.S.; Writing—review & editing, N.B., J.S. and J.D.S.; Visualization, N.B., J.S. and J.D.S.; Supervision, N.B., J.S. and J.D.S.; Project administration, N.B., J.S. and J.D.S.; Funding acquisition, N.B., J.S. and J.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This article received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seddon, J.M.; Cote, J.; Page, W.F.; Aggen, S.H.; Neale, M.C. The US Twin Study of Age-Related Macular Degeneration: Relative Roles of Genetic and Environmental Influences. Arch. Ophthalmol. 2005, 123, 321–327. [Google Scholar] [CrossRef]
- Orozco, L.D.; Owen, L.A.; Hofmann, J.; Stockwell, A.D.; Tao, J.; Haller, S.; Mukundan, V.T.; Clarke, C.; Lund, J.; Sridhar, A. A systems biology approach uncovers novel disease mechanisms in age-related macular degeneration. Cell Genom. 2023, 3, 100302. [Google Scholar] [CrossRef]
- Spilsbury, K.; Garrett, K.L.; Shen, W.-Y.; Constable, I.J.; Rakoczy, P.E. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef]
- Heloterä, H.; Kaarniranta, K. A linkage between angiogenesis and inflammation in neovascular age-related macular degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Seddon, J.M.; Francis, P.J.; George, S.; Schultz, D.W.; Rosner, B.; Klein, M.L. Association of CFH Y402H and LOC387715 A69S with Progression of Age-Related Macular Degeneration. JAMA 2007, 297, 1793–1800. [Google Scholar] [CrossRef]
- de Córdoba, S.R.g.; Esparza-Gordillo, J.; de Jorge, E.G.; Lopez-Trascasa, M.; Sánchez-Corral, P. The human complement factor H: Functional roles, genetic variations and disease associations. Mol. Immunol. 2004, 41, 355–367. [Google Scholar] [CrossRef]
- Paudel, N.; Brady, L.; Stratieva, P.; Galvin, O.; Lui, B.; Van den Brande, I.; Malkowski, J.-P.; Rebeira, M.; MacAllister, S.; O’Riordan, T.; et al. Economic Burden of Late-Stage Age-Related Macular Degeneration in Bulgaria, Germany, and the US. JAMA Ophthalmol. 2024, 142, 1123–1130. [Google Scholar] [CrossRef]
- Patel, S. Medicare spending on anti–vascular endothelial growth factor medications. Ophthalmol. Retin. 2018, 2, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Meer, E.A.; Oh, D.H.; Brodie, F.L. Time and distance cost of longer acting anti-VEGF therapies for macular degeneration: Contributions to drug cost comparisons. Clin. Ophthalmol. 2022, 16, 4273. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N., Jr.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Trapani, I.; Colella, P.; Sommella, A.; Iodice, C.; Cesi, G.; De Simone, S.; Marrocco, E.; Rossi, S.; Giunti, M.; Palfi, A. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 2014, 6, 194–211. [Google Scholar] [CrossRef]
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D. Triple vectors expand AAV transfer capacity in the retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current state of human gene therapy: Approved products and vectors. Pharmaceuticals 2023, 16, 1416. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry III, W.L.; Strohl, W.R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Hanany, M.; Rivolta, C.; Sharon, D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc. Natl. Acad. Sci. USA 2020, 117, 2710–2716. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Davidsson, M.; Negrini, M.; Hauser, S.; Svanbergsson, A.; Lockowandt, M.; Tomasello, G.; Manfredsson, F.P.; Heuer, A. A comparison of AAV-vector production methods for gene therapy and preclinical assessment. Sci. Rep. 2020, 10, 21532. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, A.; Xu, H.; Kay, M.A. Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J. Virol. 2003, 77, 7689–7695. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef]
- Cui, M.; Su, Q.; Yip, M.; McGowan, J.; Punzo, C.; Gao, G.; Tai, P.W. The AAV2. 7m8 capsid packages a higher degree of heterogeneous vector genomes than AAV2. Gene Ther. 2024, 31, 489–498. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J. Control. Release 2015, 203, 109–117. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Avery, R.; Brown, D.M.; Heier, J.S.; Ho, A.C.; Huddleston, S.M.; Jaffe, G.J.; Khanani, A.M.; Pakola, S.; Pieramici, D.J. Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: A phase 1/2a dose-escalation study. Lancet 2024, 403, 1563–1573. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV serotypes and their applications in gene therapy: An overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.J.; Foti, S.B.; Schwartz, J.W.; Bachaboina, L.; Taylor-Blake, B.; Coleman, J.; Ehlers, M.D.; Zylka, M.J.; McCown, T.J.; Samulski, R.J. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum. Gene Ther. 2011, 22, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef]
- Khabou, H.; Desrosiers, M.; Winckler, C.; Fouquet, S.; Auregan, G.; Bemelmans, A.P.; Sahel, J.A.; Dalkara, D. Insight into the mechanisms of enhanced retinal transduction by the engineered AAV2 capsid variant-7m8. Biotechnol. Bioeng. 2016, 113, 2712–2724. [Google Scholar] [CrossRef]
- Bennett, A.; Keravala, A.; Makal, V.; Kurian, J.; Belbellaa, B.; Aeran, R.; Tseng, Y.-S.; Sousa, D.; Spear, J.; Gasmi, M. Structure comparison of the chimeric AAV2. 7m8 vector with parental AAV2. J. Struct. Biol. 2020, 209, 107433. [Google Scholar] [CrossRef]
- Khanani, A.M.; Boyer, D.S.; Wykoff, C.C.; Regillo, C.D.; Busbee, B.G.; Pieramici, D.; Danzig, C.J.; Joondeph, B.C.; Major, J.C.; Turpcu, A. Safety and efficacy of ixoberogene soroparvovec in neovascular age-related macular degeneration in the United States (OPTIC): A prospective, two-year, multicentre phase 1 study. eClinicalMedicine 2024, 67, 102394. [Google Scholar] [CrossRef] [PubMed]
- Biotechnologies, A. Adverum Biotechnologies Initiates ARTEMIS Phase 3 Study Evaluating Ixo-vec for Wet AMD. Available online: https://investors.adverum.com/press_releases/news-details/2025/Adverum-Biotechnologies-Initiates-ARTEMIS-Phase-3-Study-Evaluating-Ixo-vecfor-Wet-AMD/default.aspx (accessed on 26 May 2025).
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The role of the VEGF family in coronary heart disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Li, M.Z.; Chang, K.; Ge, W.; Golding, M.C.; Rickles, R.J.; Siolas, D.; Hu, G.; Paddison, P.J.; Schlabach, M.R. Second-generation shRNA libraries covering the mouse and human genomes. Nat. Genet. 2005, 37, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Calton, M.A.; Croze, R.H.; Burns, C.; Beliakoff, G.; Vazin, T.; Szymanski, P.; Schmitt, C.; Klein, A.; Leong, M.; Quezada, M. Design and Characterization of a Novel Intravitreal Dual-Transgene Genetic Medicine for Neovascular Retinopathies. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1. [Google Scholar] [CrossRef]
- Therapeutics, D.M. 4D Molecular Therapeutics Announces Interim Clinical Data from On-Going Phase 1/2 Clinical Trial of Intravitreal 4D-150 for Wet Age-Related Macular Degeneration (Wet AMD). Available online: https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4d-molecular-therapeutics-announces-interim-clinical-data-going (accessed on 1 June 2025).
- Khanani, A.M.; Hershberger, V.S.; Kay, C.N.; Hu, A.; Eichenbaum, D.A.; Jaffe, G.J.; Chung, C.; Honarmand, S.; Nien, C.; Lee, S. Interim results for the Phase 1/2 PRISM Trial evaluating 4D-150, a dual-transgene intravitreal genetic medicine in individuals with neovascular (wet) age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5055. [Google Scholar]
- Therapeutics, D.M. 4DMT Presents Positive Interim Data from Randomized Phase 2 PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD. Available online: https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-presents-positive-interim-data-randomized-phase-2-prism (accessed on 27 May 2025).
- Therapeutics, D.M. 4DMT Highlights Robust and Durable Clinical Activity for 4D-150 and Design of 4FRONT Phase 3 Program at 4D-150 Wet AMD Development Day. Available online: https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-highlights-robust-and-durable-clinical-activity-4d-150-and (accessed on 1 June 2025).
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Feng, J.; Gong, Y.; Wang, H.; Li, L.; Wang, F. Subretinal LX102 gene therapy for neovascular age-related macular degeneration (nAMD): 9-month follow-up of a phase 1 clinical trial. Investig. Ophthalmol. Vis. Sci. 2024, 65, 6107. [Google Scholar]
- Ahmad, S.; Hewett, P.W.; Al-Ani, B.; Sissaoui, S.; Fujisawa, T.; Cudmore, M.J.; Ahmed, A. Autocrine activity of soluble Flt-1 controls endothelial cell function and angiogenesis. Vasc. Cell 2011, 3, 15. [Google Scholar] [CrossRef]
- Hornig, C.; Barleon, B.; Ahmad, S.; Vuorela, P.; Ahmed, A.; Weich, H.A. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab. Investig. 2000, 80, 443–454. [Google Scholar] [CrossRef]
- Luo, L.; Uehara, H.; Zhang, X.; Das, S.K.; Olsen, T.; Holt, D.; Simonis, J.M.; Jackman, K.; Singh, N.; Miya, T.R. Photoreceptor avascular privilege is shielded by soluble VEGF receptor-1. eLife 2013, 2, e00324. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Lai, C.-M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet 2015, 386, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Magno, A.L.; Lai, C.-M.; Pierce, C.M.; Degli-Esposti, M.A.; Blumenkranz, M.S.; Constable, I.J. Three-year follow-up of phase 1 and 2a rAAV. sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am. J. Ophthalmol. 2019, 204, 113–123. [Google Scholar] [CrossRef]
- Shim, J.; Kim, Y.; Bak, J.; Shin, S.; Lee, K.; Hwang, Y.H.; Kong, H.Y.; Han, J.S. Preclinical evaluation of NG101, a potential AAV gene therapy for wet age-related macular degeneration. Mol. Ther. Methods Clin. Dev. 2024, 32, 101366. [Google Scholar] [CrossRef] [PubMed]
- Therapeutics, F. Frontera Therapeutics Receives FDA Clearance for Phase 2 Clinical Trial of FT-003 for Diabetic Macular Edema (DME). Available online: https://fronteratherapeutics.com/news/211.html (accessed on 4 June 2025).
- Ke, X.; Jiang, H.; Li, Q.; Luo, S.; Qin, Y.; Li, J.; Xie, Q.; Zheng, Q. Preclinical evaluation of KH631, a novel rAAV8 gene therapy product for neovascular age-related macular degeneration. Mol. Ther. 2023, 31, 3308–3321. [Google Scholar] [CrossRef]
- Barleon, B.; Totzke, F.; Herzog, C.; Blanke, S.; Kremmer, E.; Siemeister, G.; Marmé, D.; Martiny-Baron, G. Mapping of the sites for ligand binding and receptor dimerization at the extracellular domain of the vascular endothelial growth factor receptor FLT-1. J. Biol. Chem. 1997, 272, 10382–10388. [Google Scholar] [CrossRef]
- Bagley, R.G.; Kurtzberg, L.; Weber, W.; Nguyen, T.-H.; Roth, S.; Krumbholz, R.; Yao, M.; Richards, B.; Zhang, M.; Pechan, P. sFLT01: A novel fusion protein with antiangiogenic activity. Mol. Cancer Ther. 2011, 10, 404–415. [Google Scholar] [CrossRef]
- Pechan, P.; Rubin, H.; Lukason, M.; Ardinger, J.; DuFresne, E.; Hauswirth, W.; Wadsworth, S.; Scaria, A. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009, 16, 10–16. [Google Scholar] [CrossRef]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-f.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Sciences TRSAo. The Nobel Prize in Chemistry. 2020. Available online: https://www.nobelprize.org/prizes/chemistry/2020/press-release/ (accessed on 12 June 2025).
- Leonard, A.; Tisdale, J.F. A new frontier: FDA approvals for gene therapy in sickle cell disease. Mol. Ther. 2024, 32, 264–267. [Google Scholar] [CrossRef]
- Frangoul, H.; Locatelli, F.; Sharma, A.; Bhatia, M.; Mapara, M.; Molinari, L.; Wall, D.; Liem, R.I.; Telfer, P.; Shah, A.J. Exagamglogene autotemcel for severe sickle cell disease. N. Engl. J. Med. 2024, 390, 1649–1662. [Google Scholar] [CrossRef]
- Tong, H.; Huang, J.; Xiao, Q.; He, B.; Dong, X.; Liu, Y.; Yang, X.; Han, D.; Wang, Z.; Wang, X. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat. Biotechnol. 2023, 41, 108–119. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, Y.; Xiao, Q.; He, B.; Geng, G.; Wang, Z.; Cao, B.; Dong, X.; Bai, W.; Wang, Y. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 2021, 18, 499–506. [Google Scholar] [CrossRef]
- Cai, Y.; Gu, Y.; Zhang, J.; Zhu, Y.; Ma, Z.; He, Q.; Sun, Y.; Yuan, M.; Li, X.; Zhu, K. An Engineered Intravitreal Injection Retinal-Pigment-Epithelium-Tropic Adeno-Associated Virus Vector Expressing a Bispecific Antibody Binding VEGF-A and ANG-2 Rescues Neovascular Age-Related Macular Degeneration in Animal Models and Patients. Research 2025, 8, 0717. [Google Scholar] [CrossRef]
- Mahlangu, J.; Kaczmarek, R.; Von Drygalski, A.; Shapiro, S.; Chou, S.-C.; Ozelo, M.C.; Kenet, G.; Peyvandi, F.; Wang, M.; Madan, B. Two-year outcomes of valoctocogene roxaparvovec therapy for hemophilia A. N. Engl. J. Med. 2023, 388, 694–705. [Google Scholar] [CrossRef]
- Pipe, S.W.; Leebeek, F.W.; Recht, M.; Key, N.S.; Castaman, G.; Miesbach, W.; Lattimore, S.; Peerlinck, K.; Van der Valk, P.; Coppens, M. Gene therapy with etranacogene dezaparvovec for hemophilia B. N. Engl. J. Med. 2023, 388, 706–718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).