Biomechanical Effects of Cement Augmentation and Prophylactic Vertebroplasty on Adjacent Segment Stability in Multilevel Spinal Fusion: A Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Finite Element Analysis of an Intact Model
2.3. Finite Element Model Verification
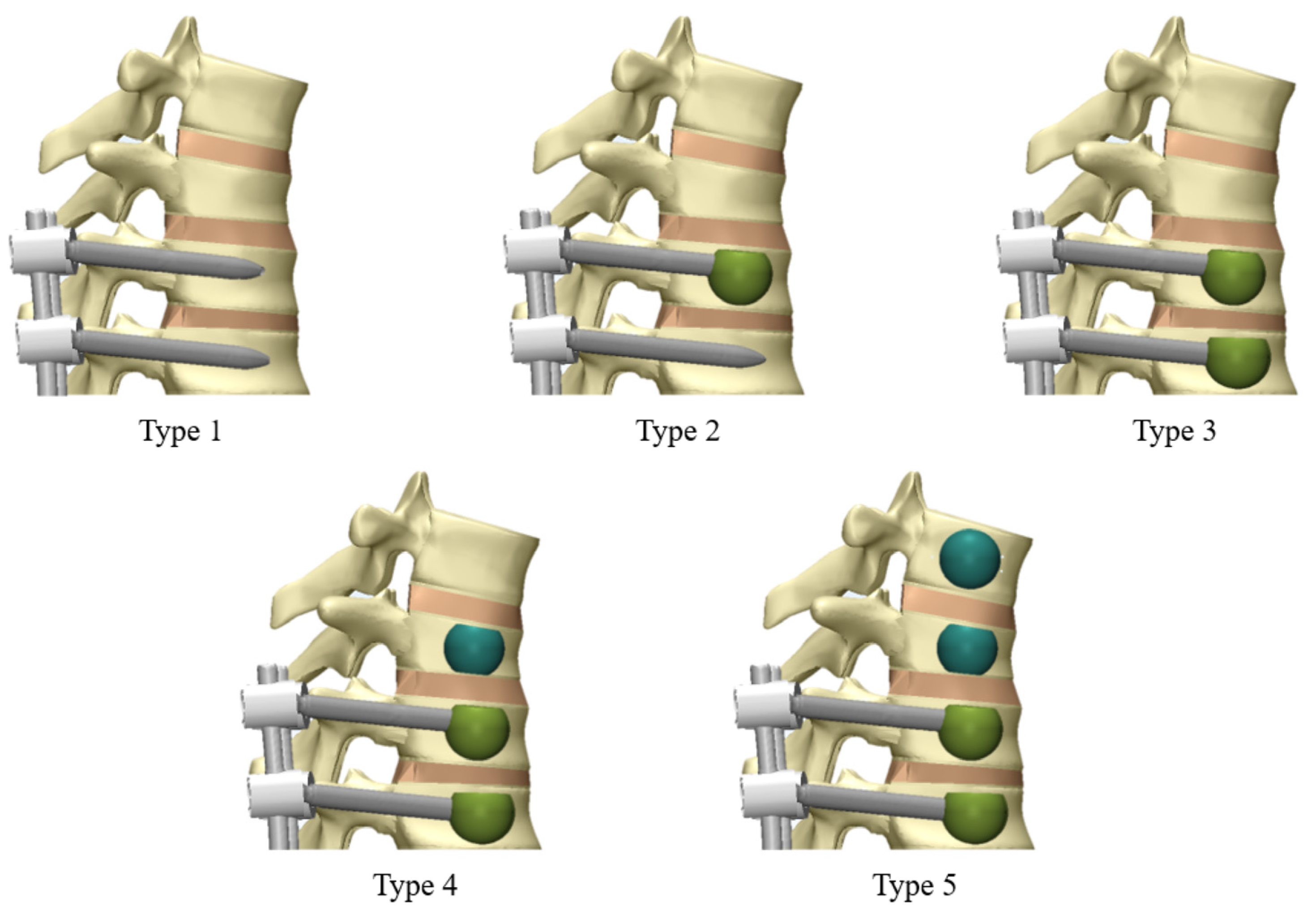
2.4. Finite Element Analysis of Surgical Models
- Type 1: Fusion with pedicle screws only.
- Type 2: Fusion with pedicle screws and cement augmentation at T10.
- Type 3: Fusion with pedicle screws and cement augmentation at T10 and T11.
- Type 4: Fusion with pedicle screws, cement augmentation at T10 and T11, and vertebroplasty at T9.
- Type 5: Fusion with pedicle screws, cement augmentation at T10 and T11, and vertebroplasty at T8 and T9.
2.5. Boundary and Loading Conditions
2.6. Outcome Measures
3. Results
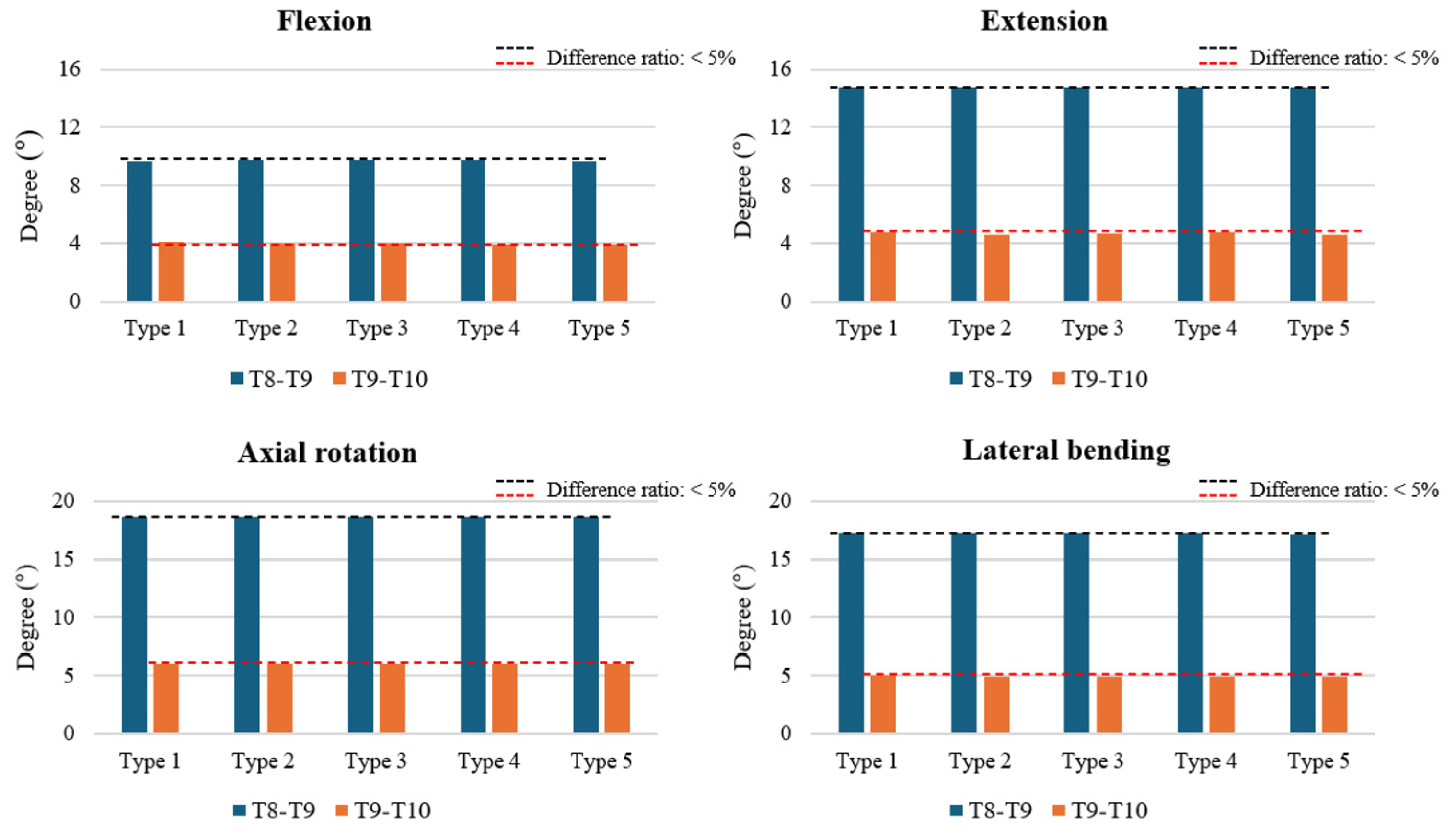
3.1. ROM at T8–T9 and T9–T10
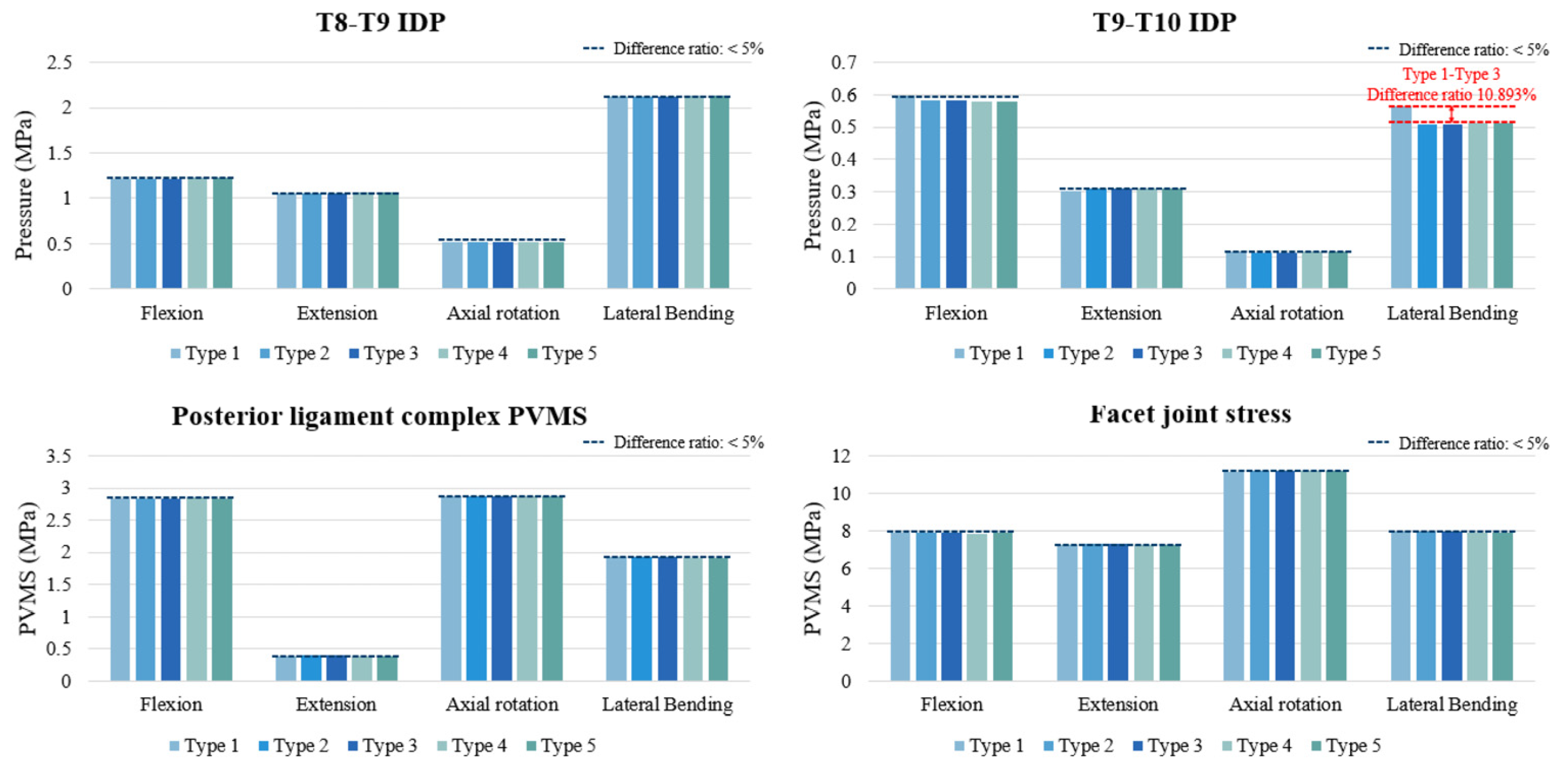
3.2. IDP at T8–T9 and T9–T10
3.3. PVMS in the PLC and Facet Joint Stress (MPa) at T8–T9
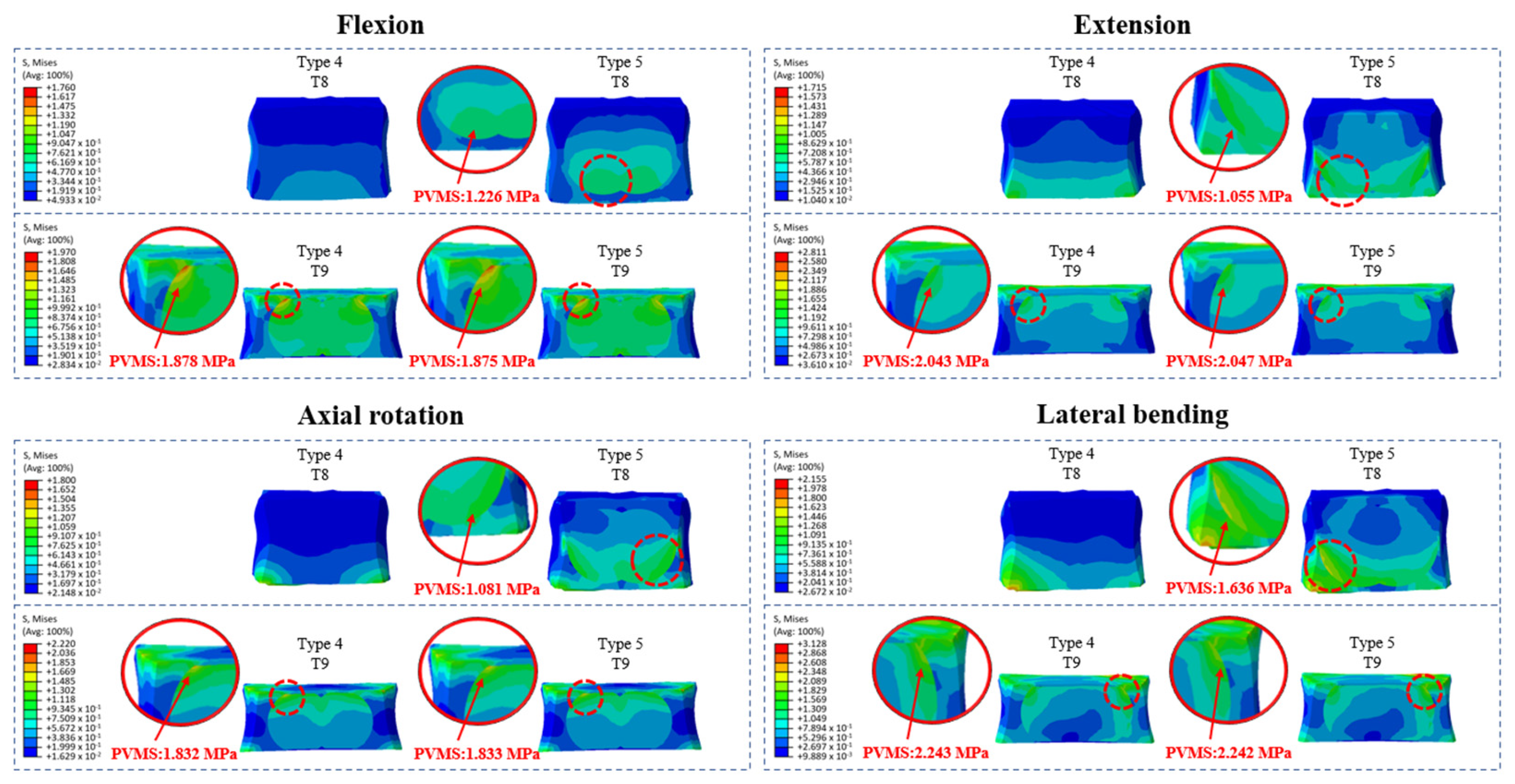
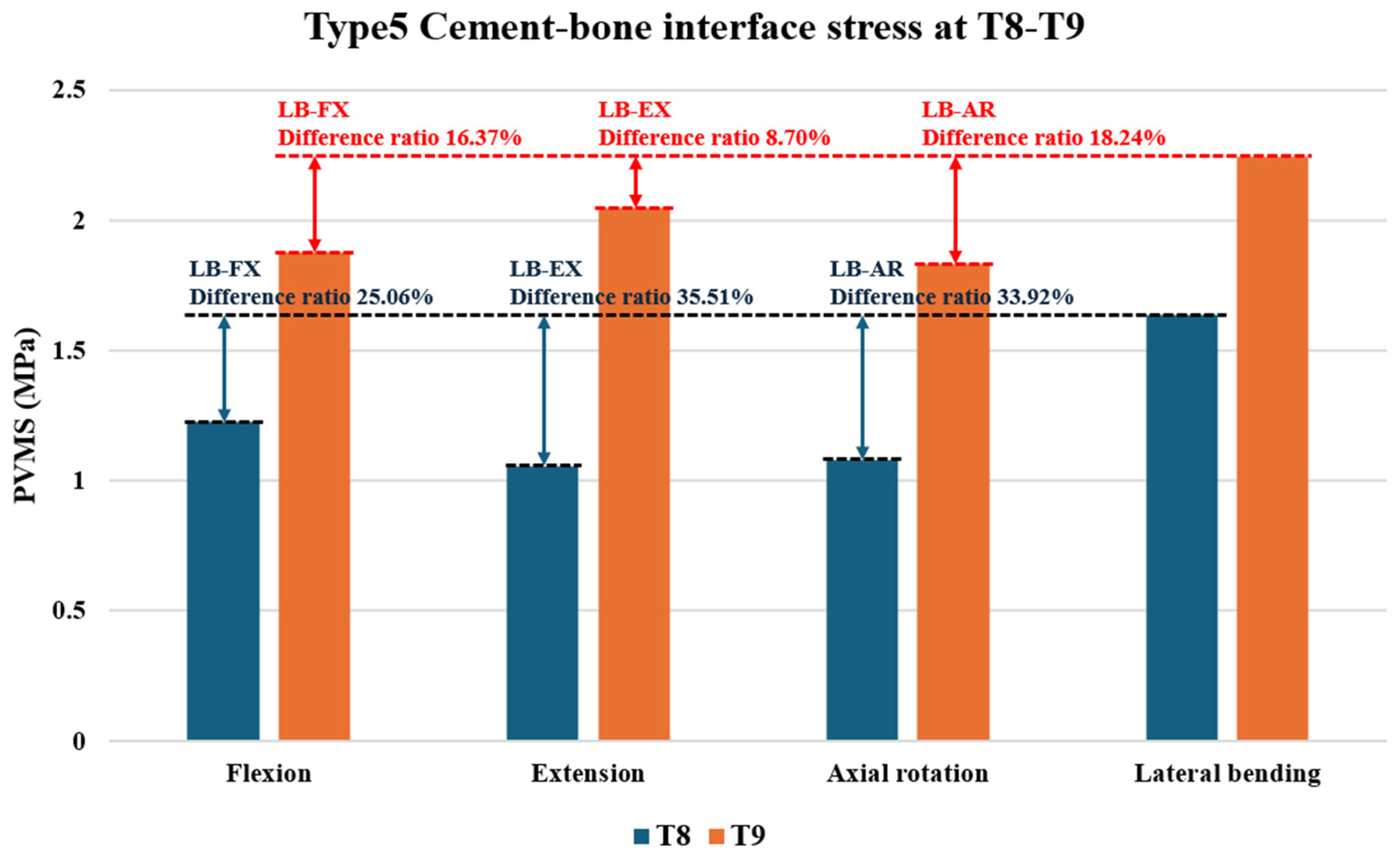
3.4. Cement–Bone Interface Stress Distribution at Adjacent Levels (T8 and T9) During Flexion, Extension, Axial Rotation, and Lateral Bending
4. Discussion
5. Conclusions
- Cement augmentation at the UIV did not significantly affect ROM, IDP, PLC, or facet joint PVMS, indicating preserved stability.
- UIV cement augmentation alone did not increase stress distribution at adjacent levels.
- Prophylactic vertebroplasty at adjacent levels produced uneven cement–cancellous bone interface stresses, particularly at T8 and T9.
- These abnormal stress concentrations may predispose patients to adjacent-level fractures and contribute to PJK or PJF.
- The results emphasize the trade-off between immediate fixation strength and long-term fracture risk, especially in osteoporotic or kyphotic patients.
- This study provides biomechanical evidence to guide surgical strategies aimed at minimizing complications and improving outcomes in multilevel spinal fusion.
- Future work should focus on osteoporotic, patient-specific finite element models and validation with clinical or experimental data to further refine surgical strategies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FEM | finite element modeling |
| IDP | intradiscal pressure |
| PJF | proximal junctional failure |
| PJK | proximal junctional kyphosis |
| ROM | range of motion |
| UIV | uppermost instrumented vertebra |
References
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.; Delamarter, R.B. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine 2012, 37, 67–76. [Google Scholar] [CrossRef]
- Martin, B.I.; Mirza, S.K.; Spina, N.; Spiker, W.R.; Lawrence, B.; Brodke, D.S. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine 2019, 44, 369–376. [Google Scholar] [CrossRef]
- Glattes, R.C.; Bridwell, K.H.; Lenke, L.G.; Kim, Y.J.; Rinella, A.; Edwards, C. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: Incidence, outcomes, and risk factor analysis. Spine 2005, 30, 1643–1649. [Google Scholar] [CrossRef]
- Hyun, S.J.; Kim, Y.J.; Kim, Y.B.; Rhim, S.C. Proximal junctional kyphosis: Diagnosis, pathogenesis, and treatment. Neurospine 2015, 12, 86–94. [Google Scholar]
- Kebaish, K.M.; Martin, C.T.; O’Brien, J.R.; LaMotta, I.E.; Voros, G.D.; Belkoff, S.M. Use of vertebroplasty to prevent proximal junctional fractures in adult deformity surgery: A biomechanical cadaveric study. Spine J. 2013, 13, 1897–1903. [Google Scholar] [CrossRef]
- Hart, R.A.; Prendergast, M.A.; Roberts, W.G.; Nesbit, G.M.; Barnwell, S.L. Proximal junctional acute collapse cranial to multi-level lumbar fusion: A cost analysis of prophylactic vertebral augmentation. Spine J. 2008, 8, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Kayanja, M.M.; Togawa, D.; Lieberman, I.H. Prophylactic vertebroplasty of adjacent vertebrae in osteoporotic patients undergoing kyphoplasty: A biomechanical and clinical study. Spine J. 2006, 6, 575–582. [Google Scholar] [CrossRef]
- Dreischarf, M.; Zander, T.; Shirazi-Adl, A.; Puttlitz, C.M.; Adam, C.J.; Chen, C.S.; Goel, V.K.; Kiapour, A.; Kim, Y.H.; Labus, K.M.; et al. Comparison of eight published static finite element models of the intact lumbar spine: Predictive power of models improves when combined together. J. Biomech. 2014, 47, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Choma, T.J.; Pfeiffer, F.M.; Swope, R.W.; Hirner, J.P. Pedicle screw design and cement augmentation in osteoporotic vertebrae: Effects of fenestrations and cement viscosity on fixation and extraction. Spine 2012, 37, E1628–E1632. [Google Scholar] [CrossRef]
- Belkoff, S.M.; Mathis, J.M.; Jasper, L.E.; Deramond, H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine 2001, 26, 1537–1541. [Google Scholar] [CrossRef]
- Polikeit, A.; Nolte, L.P.; Ferguson, S.J. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: Finite-element analysis. Spine 2003, 28, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Chen, W.P.; Wang, H. Treatment of thoracolumbar burst fractures by short-segment pedicle screw fixation using a combination of two additional pedicle screws and vertebroplasty at the level of the fracture: A finite element analysis. BMC Musculoskelet. Disord. 2017, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Jiang, J.M.; Sun, P.D.; Zhang, Z.F.; Ren, H.L. How the clinical dosage of bone cement biomechanically affects adjacent vertebrae. J. Orthop. Surg. Res. 2020, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Doodkorte, R.J.; Vercoulen, T.F.; Roth, A.K.; de Bie, R.A.; Willems, P.C. Instrumentation techniques to prevent proximal junctional kyphosis and proximal junctional failure in adult spinal deformity correction: A systematic review of biomechanical studies. Spine J. 2021, 21, 842–854. [Google Scholar] [CrossRef]
- Sawada, Y.; Takahashi, S.; Terai, H.; Kato, M.; Toyoda, H.; Suzuki, A.; Tamai, K.; Yabu, A.; Iwamae, M.; Nakamura, H. Short-term risk factors for distal junctional kyphosis after spinal reconstruction surgery in patients with osteoporotic vertebrae. Asian Spine J. 2024, 18, 101–109. [Google Scholar] [CrossRef]
- Murata, K.; Otsuki, B.; Shimizu, T.; Sono, T.; Fujibayashi, S.; Matsuda, S. Sagittal section Hounsfield units of the upper instrumented vertebrae as a predictor of proximal junctional vertebral fractures following adult spinal deformity surgery. Asian Spine J. 2024, 18, 209–217. [Google Scholar] [CrossRef]
- Meng, H.; Li, Q.; Lin, J.; Yang, Y.; Fei, Q. Intradiscal cement leakage (ICL) increases the stress on adjacent vertebrae after kyphoplasty for osteoporotic vertebra compression fracture (OVCF): A finite-element study. Sci. Rep. 2023, 13, 15984. [Google Scholar] [CrossRef]
- Son, D.M.; Lee, S.B.; Lee, S.J.; Park, T.H.; Jang, J.E.; Jeong, S.J.; Kang, Y.M.; Lee, B.H. Biomechanical comparison of multilevel lumbar instrumented fusions in adult spinal deformity according to the upper and lower fusion levels: A finite element analysis. BioMed Res. Int. 2022, 2022, 2534350. [Google Scholar] [CrossRef]
- Sohn, S.; Park, T.H.; Chung, C.K.; Kim, Y.J.; Jang, J.W.; Han, I.B.; Lee, S.J. Biomechanical characterization of three iliac screw fixation techniques: A finite element study. J. Clin. Neurosci. 2018, 52, 109–114. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, Y.; Gong, Z.; Jiang, C.; Jin, L.; Li, Z.; Chen, Z.; Jiang, C.; Jiang, X. A finite element analysis on comparing the stability of different posterior fixation methods for thoracic total en bloc spondylectomy. J. Orthop. Surg. Res. 2020, 15, 314. [Google Scholar] [CrossRef]
- Yamamoto, I.; Panjabi, M.M.; Crisco, T.; Oxland, T. Three-dimensional movements of the whole lumbar spine and lumbosacral joint. Spine 1989, 14, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Schultz, A.B.; Andersson, G.B. Load-displacement behavior of sacroiliac joints. J. Orthop. Res. 1987, 5, 92–101. [Google Scholar] [CrossRef]
- Burns, C.B.; Dua, K.; Trasolini, N.A.; Komatsu, D.E.; Barsi, J.M. Biomechanical comparison of spinopelvic fixation constructs: Iliac screw versus S2-alar-iliac screw. Spine Deform. 2016, 4, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shang, J.; Zhou, Y.; Li, C.; Liu, H. Finite element analysis of a new pedicle screw-plate system for minimally invasive transforaminal lumbar interbody fusion. PLoS ONE 2015, 10, e0144637. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, J.W.; Park, T.H.; Lee, S.B.; Moon, S.H.; Lee, B.H. Biomechanical comparison and three-dimensional analysis of cement distribution patterns for different pedicle screw designs. BioMed Res. Int. 2022, 2022, 8293524. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Havey, R.M.; Meade, K.P.; Lee, B.; Dunlap, B. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine 1999, 24, 1003–1009. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, H.J.; Park, S.J.; Kang, D.H.; Lee, C.S. A comprehensive review of risk factors and prevention strategies: How to minimize mechanical complications in corrective surgery for adult spinal deformity. Asian Spine J. 2025, 19, 463–475. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bridwell, K.H.; Lenke, L.G.; Kim, J.; Cho, S.K. Proximal junctional kyphosis in adolescent idiopathic scoliosis following segmental posterior spinal instrumentation and fusion: Minimum 5-year follow-up. Spine 2005, 30, 2045–2050. [Google Scholar] [CrossRef]
- Lange, T.; Schmoelz, W.; Gosheger, G.; Eichinger, M.; Heinrichs, C.H.; Boevingloh, A.S.; Schulte, T.L. Is a gradual reduction of stiffness on top of posterior instrumentation possible with a suitable proximal implant? A biomechanical study. Spine J. 2017, 17, 1148–1155. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Nguyen, T.T.; Kuo, Y.J.; Chen, Y.P. Impact of sarcopenia on outcomes following vertebral augmentation for osteoporotic vertebral compression fracture: A systematic review and meta-analysis. Asian Spine J. 2025, 19, 476–489. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, J.Y.; Park, J.H.; Ham, D.W.; Park, S.M.; Yeom, J.S.; Kim, H.J. Transpedicular injection of rhBMP-2 with β-tricalcium phosphate to reduce the proximal junctional kyphosis after adult spinal deformity correction: Preliminary study: Preliminary study. Sci. Rep. 2024, 14, 6660. [Google Scholar] [CrossRef]
- Kim, W.J.; Ma, S.B.; Shin, H.M.; Song, D.G.; Lee, J.W.; Chang, S.H.; Park, K.Y.; Choy, W.S.; Oh, T.H. Correlation of sagittal imbalance and recollapse after percutaneous vertebroplasty for thoracolumbar osteoporotic vertebral compression fracture: A multivariate study of risk factors. Asian Spine J. 2022, 16, 231–240. [Google Scholar] [CrossRef]
- Borkowski, S.L.; Tamrazian, E.; Bowen, R.E.; Scaduto, A.A.; Ebramzadeh, E.; Sangiorgio, S.N. Challenging the Conventional Standard for Thoracic Spine Range of Motion: A Systematic Review. JBJS Rev. 2016, 4, e5. [Google Scholar] [CrossRef]
- Wilke, H.J.; Herkommer, A.; Werner, K.; Liebsch, C. In vitro analysis of the segmental flexibility of the thoracic spine. PLoS ONE 2017, 12, e0177823. [Google Scholar] [CrossRef]
- Mannen, E.M.; Friis, E.A.; Sis, H.L.; Wong, B.M.; Cadel, E.S.; Anderson, D.E. The Rib Cage Stiffens the Thoracic Spine in a Cadaveric Model with Body Weight Load Under Dynamic Moments. J. Mech. Behav. Biomed. Mater. 2018, 84, 258–264. [Google Scholar] [CrossRef]
- Liebsch, C.; Wilke, H.J. The rib cage stabilizes the human thoracic spine: An in vitro study using stepwise reduction of rib cage structures. PLoS ONE 2017, 12, e0184699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.W.; Kim, D.H.; Kang, K.M.; Park, T.H.; Oh, Y.R.; Lee, S.J.; Lee, B.H. Biomechanical Effects of Cement Augmentation and Prophylactic Vertebroplasty on Adjacent Segment Stability in Multilevel Spinal Fusion: A Finite Element Analysis. Bioengineering 2025, 12, 1071. https://doi.org/10.3390/bioengineering12101071
Shin JW, Kim DH, Kang KM, Park TH, Oh YR, Lee SJ, Lee BH. Biomechanical Effects of Cement Augmentation and Prophylactic Vertebroplasty on Adjacent Segment Stability in Multilevel Spinal Fusion: A Finite Element Analysis. Bioengineering. 2025; 12(10):1071. https://doi.org/10.3390/bioengineering12101071
Chicago/Turabian StyleShin, Jae Won, Dae Hyeon Kim, Ki Mun Kang, Tae Hyun Park, Yu Rim Oh, Sung Jae Lee, and Byung Ho Lee. 2025. "Biomechanical Effects of Cement Augmentation and Prophylactic Vertebroplasty on Adjacent Segment Stability in Multilevel Spinal Fusion: A Finite Element Analysis" Bioengineering 12, no. 10: 1071. https://doi.org/10.3390/bioengineering12101071
APA StyleShin, J. W., Kim, D. H., Kang, K. M., Park, T. H., Oh, Y. R., Lee, S. J., & Lee, B. H. (2025). Biomechanical Effects of Cement Augmentation and Prophylactic Vertebroplasty on Adjacent Segment Stability in Multilevel Spinal Fusion: A Finite Element Analysis. Bioengineering, 12(10), 1071. https://doi.org/10.3390/bioengineering12101071





