Joint Kinematics and Gait Pattern in Multiple Sclerosis: A 3D Analysis Comparative Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
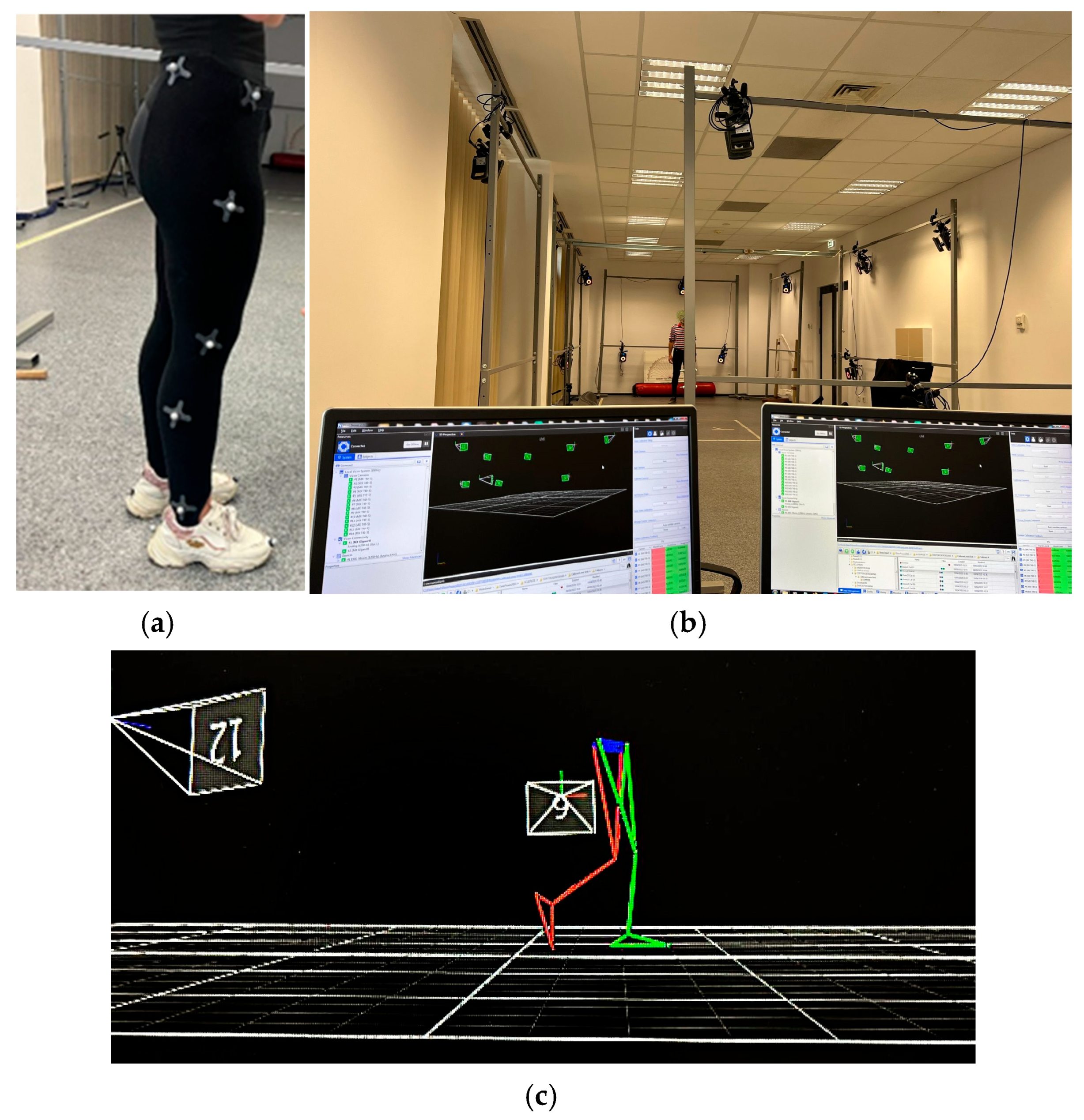
2.2. Experimental Protocol and Data Collection
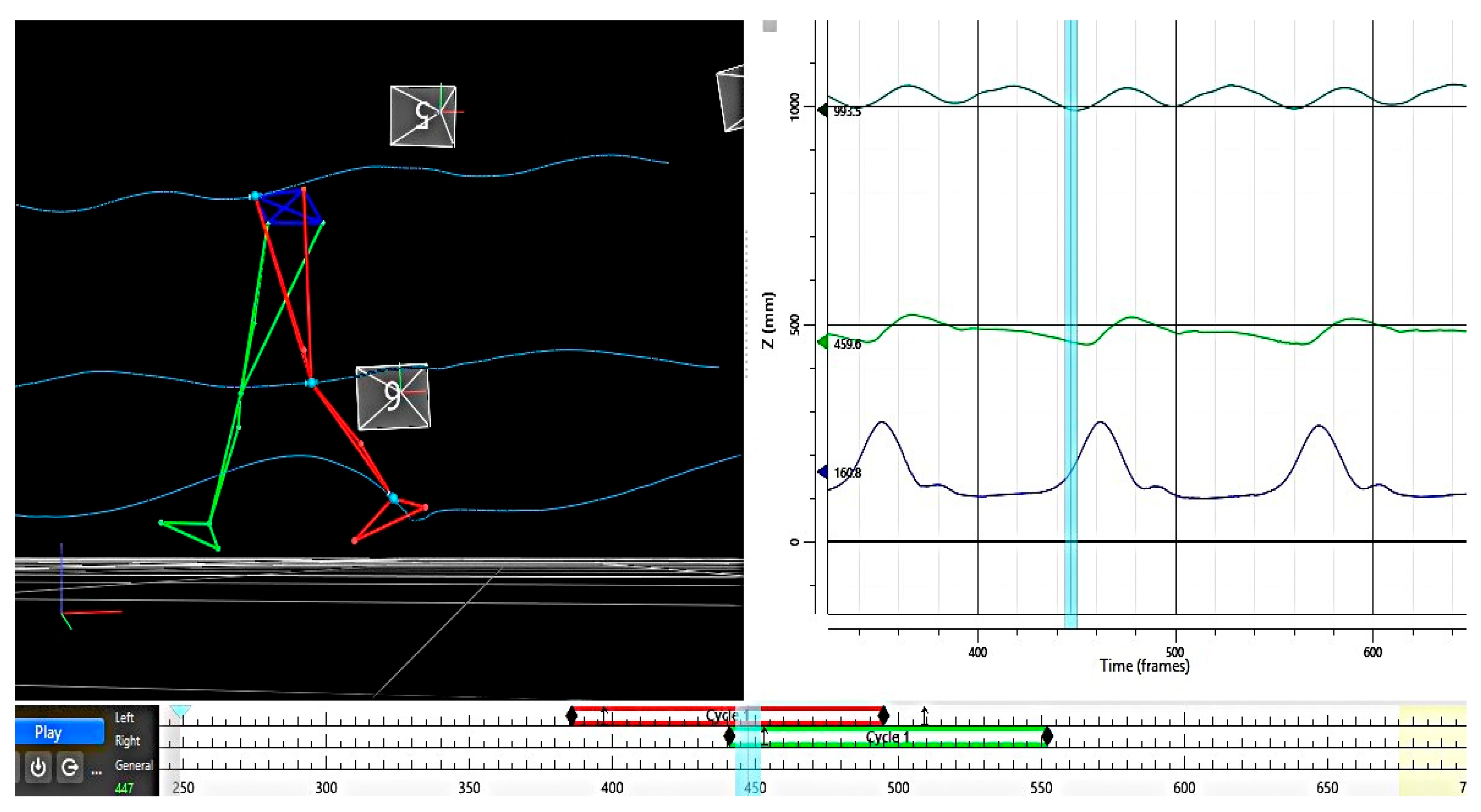
2.3. Data Analysis
- i = the point in the cycle (1 to 100);
- n = the total number of points (100).
3. Results
3.1. Comparative Analysis of ROM for the Hip, Knee, and Ankle Joints (Bilateral)
3.2. Root Mean Square Error (RMSE)
- Throughout the whole gait cycle, only the ankle joint is significantly affected (p = 0.014), whereas the hip and knee do not differ significantly (p > 0.05).
- During the stance phase, no joints are noticeably affected.
- Swing phase: the knee and ankle joints are significantly impacted (p = 0.015 and p = 0.008, respectively), whereas the hip remains unaffected.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.-J.; Chen, W.-W.; Zhang, X. Multiple Sclerosis: Pathology, Diagnosis and Treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Schapiro, R.T. Symptom Management in Multiple Sclerosis. Ann. Neurol. 1994, 36 (Suppl. S1), S123–S129. [Google Scholar] [CrossRef]
- Finlayson, M.L.; Peterson, E.W.; Cho, C.C. Risk Factors for Falling Among People Aged 45 to 90 Years with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2006, 87, 1274–1279. [Google Scholar] [CrossRef]
- Slavkovic, S.; Golubovic, S.; Vojnovic, M.; Nadj, C. Influence of Cognitive and Motor Abilities on the Level of Current Functioning in People with Multiple Sclerosis. Slov. J. Public Health 2019, 58, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yozbatıran, N.; Baskurt, F.; Baskurt, Z.; Ozakbas, S.; Idiman, E. Motor Assessment of Upper Extremity Function and Its Relation with Fatigue, Cognitive Function and Quality of Life in Multiple Sclerosis Patients. J. Neurol. Sci. 2006, 246, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bethoux, F. Gait Disorders in Multiple Sclerosis. Continuum 2013, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Eken, M.M.; Richards, R.; Beckerman, H.; Van Der Krogt, M.; Gerrits, K.; Rietberg, M.; De Groot, V.; Heine, M. Quantifying Muscle Fatigue during Walking in People with Multiple Sclerosis. Clin. Biomech. 2020, 72, 94–101. [Google Scholar] [CrossRef]
- Galea, M.P.; Cofré Lizama, L.E.; Butzkueven, H.; Kilpatrick, T.J. Gait and Balance Deterioration over a 12-Month Period in Multiple Sclerosis Patients with EDSS Scores ≤ 3.0. NeuroRehabilitation 2017, 40, 277–284. [Google Scholar] [CrossRef]
- Bennett, S.E.; Bromley, L.E.; Fisher, N.M.; Tomita, M.R.; Niewczyk, P. Validity and Reliability of Four Clinical Gait Measures in Patients with Multiple Sclerosis. Int. J. MS Care 2017, 19, 247–252. [Google Scholar] [CrossRef]
- Lizrova Preiningerova, J.; Novotna, K.; Rusz, J.; Sucha, L.; Ruzicka, E.; Havrdova, E. Spatial and Temporal Characteristics of Gait as Outcome Measures in Multiple Sclerosis (EDSS 0 to 6.5). J. Neuroeng. Rehabil. 2015, 12, 14. [Google Scholar] [CrossRef]
- Shanahan, C.J.; Boonstra, F.M.C.; Cofré Lizama, L.E.; Strik, M.; Moffat, B.A.; Khan, F.; Kilpatrick, T.J.; Van Der Walt, A.; Galea, M.P.; Kolbe, S.C. Technologies for Advanced Gait and Balance Assessments in People with Multiple Sclerosis. Front. Neurol. 2018, 8, 708. [Google Scholar] [CrossRef]
- Hobart, J.C.; Riazi, A.; Lamping, D.L.; Fitzpatrick, R.; Thompson, A.J. Measuring the Impact of MS on Walking Ability: The 12-Item MS Walking Scale (MSWS-12). Neurology 2003, 60, 31–36. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Sandroff, B.M.; Motl, R.W. Quantifying Gait Abnormalities in Persons with Multiple Sclerosis with Minimal Disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef]
- Timmermans, S.T.; Van Der Krogt, M.M.; Rietberg, M.B.; Beckerman, H.; De Groot, V. A Delphi Study to Identify Key Gait Patterns and Their Potential Causes in People with Multiple Sclerosis. J. Rehabilitation Med. 2025, 57, jrm42556. [Google Scholar] [CrossRef]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Galvin, R.; Coote, S. Gait Deficits in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait Abnormalities in Minimally Impaired Multiple Sclerosis Patients. Mult. Scler. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Martin, C.L.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, E.; Galea, M.P. Gait and Balance Impairment in Early Multiple Sclerosis in the Absence of Clinical Disability. Mult. Scler. 2006, 12, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Achiron, A.; Dvir, Z. Muscular and Gait Abnormalities in Persons with Early Onset Multiple Sclerosis. J. Neurol. Phys. Ther. 2011, 35, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Bacon, T.E.; Herbert, J. Disability in Multiple Sclerosis: A Reference for Patients and Clinicians. Neurology 2013, 80, 1018–1024. [Google Scholar] [CrossRef]
- Créange, A.; Serre, I.; Levasseur, M.; Audry, D.; Nineb, A.; Boërio, D.; Moreau, T.; Maison, P.; Sindefi-Sep, R. Walking Capacities in Multiple Sclerosis Measured by Global Positioning System Odometer. Mult. Scler. 2007, 13, 220–223. [Google Scholar] [CrossRef]
- Paltamaa, J.; Sarasoja, T.; Leskinen, E.; Wikström, J.; Mälkiä, E. Measuring Deterioration in International Classification of Functioning Domains of People with Multiple Sclerosis Who Are Ambulatory. Phys. Ther. 2008, 88, 176–190. [Google Scholar] [CrossRef]
- Spain, R.I.; Mancini, M.; Horak, F.B.; Bourdette, D. Body-Worn Sensors Capture Variability, but Not Decline, of Gait and Balance Measures in Multiple Sclerosis over 18 Months. Gait Posture 2014, 39, 958–964. [Google Scholar] [CrossRef]
- Zörner, B.; Hostettler, P.; Meyer, C.; Killeen, T.; Gut, P.; Linnebank, M.; Weller, M.; Straumann, D.; Filli, L. Prognosis of Walking Function in Multiple Sclerosis Supported by Gait Pattern Analysis. Mult. Scler. Relat. Disord. 2022, 63, 103802. [Google Scholar] [CrossRef]
- Vienne-Jumeau, A.; Quijoux, F.; Vidal, P.P.; Ricard, D. Value of Gait Analysis for Measuring Disease Severity Using Inertial Sensors in Patients with Multiple Sclerosis: Protocol for a Systematic Review and Meta-Analysis. Syst. Rev. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Abasıyanık, Z.; Kahraman, T.; Veldkamp, R.; Ertekin, Ö.; Kalron, A.; Feys, P. Changes in Gait Characteristics During and Immediately After the 6-Minute Walk Test in Persons with Multiple Sclerosis: A Systematic Review. Phys. Ther. 2022, 102, pzac036. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.D.; Marrie, R.A.; Cohen, J.A. Evaluation of the Six-Minute Walk in Multiple Sclerosis Subjects and Healthy Controls. Mult. Scler. 2008, 14, 383–390. [Google Scholar] [CrossRef]
- McLoughlin, J.V.; Barr, C.J.; Patritti, B.; Crotty, M.; Lord, S.R.; Sturnieks, D.L. Fatigue Induced Changes to Kinematic and Kinetic Gait Parameters Following Six Minutes of Walking in People with Multiple Sclerosis. Disabil. Rehabil. 2016, 38, 535–543. [Google Scholar] [CrossRef]
- Ramari, C.; Moraes, A.G.; Tauil, C.B.; Von Glehn, F.; Motl, R.; De David, A.C. Knee Flexor Strength and Balance Control Impairment May Explain Declines during Prolonged Walking in Women with Mild Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Drebinger, D.; Rasche, L.; Kroneberg, D.; Althoff, P.; Bellmann-Strobl, J.; Weygandt, M.; Paul, F.; Brandt, A.U.; Schmitz-Hübsch, T. Association Between Fatigue and Motor Exertion in Patients with Multiple Sclerosis—A Prospective Study. Front. Neurol. 2020, 11, 208. [Google Scholar] [CrossRef]
- Motti Ader, L.G.; Greene, B.R.; McManus, K.; Tubridy, N.; Caulfield, B. Short Bouts of Gait Data and Body-Worn Inertial Sensors Can Provide Reliable Measures of Spatiotemporal Gait Parameters from Bilateral Gait Data for Persons with Multiple Sclerosis. Biosensors 2020, 10, 128. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Rochester, L. Moving Forward on Gait Measurement: Toward a More Refined Approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef]
- Vienne, A.; Barrois, R.P.; Buffat, S.; Ricard, D.; Vidal, P.-P. Inertial Sensors to Assess Gait Quality in Patients with Neurological Disorders: A Systematic Review of Technical and Analytical Challenges. Front. Psychol. 2017, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Frechette, M.L.; Meyer, B.M.; Tulipani, L.J.; Gurchiek, R.D.; McGinnis, R.S.; Sosnoff, J.J. Next Steps in Wearable Technology and Community Ambulation in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable Sensor Use for Assessing Standing Balance and Walking Stability in People with Parkinson’s Disease: A Systematic Review. PLoS ONE 2015, 10, e0123705. [Google Scholar] [CrossRef]
- Simon, S.R. Quantification of Human Motion: Gait Analysis—Benefits and Limitations to Its Application to Clinical Problems. J. Biomech. 2004, 37, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Gor-García-Fogeda, M.D.; Cano De La Cuerda, R.; Carratalá Tejada, M.; Alguacil-Diego, I.M.; Molina-Rueda, F. Observational Gait Assessments in People with Neurological Disorders: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 131–140. [Google Scholar] [CrossRef]
- Crenshaw, S.J.; Royer, T.D.; Richards, J.G.; Hudson, D.J. Gait Variability in People with Multiple Sclerosis. Mult. Scler. 2006, 12, 613–619. [Google Scholar] [CrossRef]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling Walking Dysfunction in Multiple Sclerosis: Characterisation, Classification and Progression over Time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef]
- Givon, U.; Zeilig, G.; Achiron, A. Gait Analysis in Multiple Sclerosis: Characterization of Temporal–Spatial Parameters Using GAITRite Functional Ambulation System. Gait Posture 2009, 29, 138–142. [Google Scholar] [CrossRef]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait Variability and Disability in Multiple Sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Effect of Spasticity on Kinematics of Gait and Muscular Activation in People with Multiple Sclerosis. J. Neurol. Sci. 2015, 358, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis Parameters in Patients Affected by Multiple Sclerosis: Analysis of Kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Broom, L.; Ellison, B.A.; Worley, A.; Wagenaar, L.; Sörberg, E.; Ashton, C.; Bennett, D.A.; Buchman, A.S.; Saper, C.B.; Shih, L.C.; et al. A Translational Approach to Capture Gait Signatures of Neurological Disorders in Mice and Humans. Sci. Rep. 2017, 7, 3225. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.L.; Scott, S.M.; Hooper, J.E.; Cowan, P.; Mercer, T.H. Gait Kinematics of People with Multiple Sclerosis and the Acute Application of Functional Electrical Stimulation. Gait Posture 2014, 39, 1092–1096. [Google Scholar] [CrossRef]
- Cutter, G.R. Development of a Multiple Sclerosis Functional Composite as a Clinical Trial Outcome Measure. Brain 1999, 122, 871–882. [Google Scholar] [CrossRef]
- Fritz, N.E.; Newsome, S.D.; Eloyan, A.; Marasigan, R.E.R.; Calabresi, P.A.; Zackowski, K.M. Longitudinal Relationships among Posturography and Gait Measures in Multiple Sclerosis. Neurology 2015, 84, 2048–2056. [Google Scholar] [CrossRef]
- Cadavid, D.; Jurgensen, S.; Lee, S. Impact of Natalizumab on Ambulatory Improvement in Secondary Progressive and Disabled Relapsing-Remitting Multiple Sclerosis. PLoS ONE 2013, 8, e53297. [Google Scholar] [CrossRef]
- Huisinga, J.M.; Schmid, K.K.; Filipi, M.L.; Stergiou, N. Gait Mechanics Are Different Between Healthy Controls and Patients with Multiple Sclerosis. J. Appl. Biomech. 2013, 29, 303–311. [Google Scholar] [CrossRef]
- Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; Miangolarra-Page, J.C.; Carratalá-Tejada, M. The Timing of Kinematic and Kinetic Parameters during Gait Cycle as a Marker of Early Gait Deterioration in Multiple Sclerosis Subjects with Mild Disability. J. Clin. Med. 2022, 11, 1892. [Google Scholar] [CrossRef]
- Kasser, S.L.; Jacobs, J.V.; Foley, J.T.; Cardinal, B.J.; Maddalozzo, G.F. A Prospective Evaluation of Balance, Gait, and Strength to Predict Falling in Women with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2011, 92, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Bethoux, F.; Bennett, S. Evaluating Walking in Patients with Multiple Sclerosis. Int. J. MS Care 2011, 13, 4–14. [Google Scholar] [CrossRef]
- Fernández-Vázquez, D.; Calvo-Malón, G.; Molina-Rueda, F.; López-González, R.; Carratalá-Tejada, M.; Navarro-López, V.; Miangolarra-Page, J.C. Kinematic Gait Analysis in People with Mild-Disability Multiple Sclerosis Using Statistical Parametric Mapping: A Cross-Sectional Study. Sensors 2023, 23, 7671. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kalron, A.; Dvir, Z.; Givon, U.; Baransi, H.; Achiron, A. Gait and Jogging Parameters in People with Minimally Impaired Multiple Sclerosis. Gait Posture 2014, 39, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Massot, C.; Bègue, J.; Simoneau-Buessinger, E.; Donze, C.; Caderby, T.; Leteneur, S. Patients with Multiple Sclerosis and Low Disability Display Cautious Rotational Behavior during Gait Initiation. Clin. Biomech. 2025, 122, 106431. [Google Scholar] [CrossRef]
- Cofré Lizama, L.E.; Khan, F.; Lee, P.V.; Galea, M.P. The Use of Laboratory Gait Analysis for Understanding Gait Deterioration in People with Multiple Sclerosis. Mult. Scler. 2016, 22, 1768–1776. [Google Scholar] [CrossRef]
- Massot, C.; Simoneau-Buessinger, E.; Agnani, O.; Donze, C.; Leteneur, S. Anticipatory Postural Adjustment during Gait Initiation in Multiple Sclerosis Patients: A Systematic Review. Gait Posture 2019, 73, 180–188. [Google Scholar] [CrossRef]
- Ahdab, R.; Shatila, M.M.; Shatila, A.R.; Khazen, G.; Freiha, J.; Salem, M.; Makhoul, K.; El Nawar, R.; El Nemr, S.; Ayache, S.S.; et al. Cortical Excitability Measures May Predict Clinical Response to Fampridine in Patients with Multiple Sclerosis and Gait Impairment. Brain Sci. 2019, 9, 357. [Google Scholar] [CrossRef] [PubMed]
| Joint | Side | Control (Mean ± SD) | MS (Mean ± SD) | Control Minimum/ Maximum | MS Minimum/ Maximum | Difference | p-Value |
|---|---|---|---|---|---|---|---|
| Hip | Right | 37.05 ± 10.13 | 29.20 ± 9.33 | 28.39/59.92 | 18.95/44.09 | −7.85° | 0.034 * |
| Left | 32.67 ± 3.06 | 27.84 ± 6.69 | 26.64/39.01 | 22.97/39.01 | −4.83° | 0.013 * | |
| Knee | Right | 72.79 ± 29.57 | 56.20 ± 7.33 | 50.81/139.41 | 47.74/66.70 | −16.59° | 0.048 * |
| Left | 61.06 ± 5.55 | 51.27 ± 10.87 | 50.36/67.303 | 39.02/67.98 | −9.79° | 0.010 * | |
| Ankle | Right | 34.59 ± 22.35 | 28.33 ± 4.58 | 23.64/111.95 | 24.04/36.95 | −6.26° | 0.265 |
| Left | 27.39 ± 4.17 | 27.14 ± 1.52 | 20.53/36.37 | 24.23/28.63 | −0.25° | 0.851 |
| Control Subject No. | RMSE—Hip | RMSE—Knee | RMSE—Foot |
|---|---|---|---|
| S1 | 2.09 | 2.92 | 5.93 |
| S2 | 4.09 | 14.53 | 7.15 |
| S3 | 3.50 | 10.14 | 3.53 |
| S4 | 4.32 | 5.84 | 4.58 |
| S5 | 3.07 | 8.41 | 4.24 |
| S6 | 4.48 | 16.30 | 12.06 |
| S7 | 1.36 | 3.69 | 3.29 |
| S8 | 0.70 | 3.31 | 2.60 |
| S9 | 1.37 | 5.29 | 3.45 |
| S10 | 0.91 | 2.68 | 3.84 |
| S11 | 0.82 | 2.58 | 4.21 |
| S12 | 3.70 | 5.00 | 3.32 |
| S13 | 1.76 | 2.59 | 2.35 |
| S14 | 2.34 | 3.76 | 6.53 |
| S15 | 2.04 | 3.15 | 7.18 |
| 1st Quartile | 1.36 | 3.03 | 3.38 |
| Median | 2.09 | 3.76 | 4.21 |
| 3rd Quartile | 3.60 | 7.13 | 6.23 |
| Mean | 2.44 | 6.01 | 4.95 |
| Standard deviation | 1.32 | 4.41 | 2.51 |
| CV | 54.38 | 73.41 | 50.70 |
| MS Patient No. | RMSE—Hip | RMSE—Knee | RMSE—Foot |
|---|---|---|---|
| P1 | 4.23 | 9.11 | 15.44 |
| P2 | 3.45 | 6.12 | 5.25 |
| P3 | 1.60 | 6.12 | 5.71 |
| P4 | 1.60 | 10.83 | 5.71 |
| P5 | 4.07 | 7.91 | 5.51 |
| P6 | 4.21 | 9.85 | 16.44 |
| P7 | 3.94 | 7.12 | 6.25 |
| P8 | 1.96 | 5.12 | 6.71 |
| P9 | 2.60 | 10.02 | 5.67 |
| P10 | 4.97 | 8.58 | 6.51 |
| P11 | 4.06 | 8.11 | 14.44 |
| P12 | 2.45 | 7.12 | 4.25 |
| P13 | 0.60 | 5.12 | 4.71 |
| P14 | 1.99 | 11.83 | 5.99 |
| P15 | 3.07 | 9.11 | 4.51 |
| 1st Quartile | 1.98 | 6.62 | 5.38 |
| Median | 3.07 | 7.91 | 5.71 |
| 3rd Quartile | 4.07 | 9.48 | 6.61 |
| Mean | 2.99 | 8.04 | 7.54 |
| Standard deviation | 1.27 | 2.02 | 4.16 |
| Group | RMSE Metric | F | p | Conclusion |
|---|---|---|---|---|
| Control | RMSE for the entire gait cycle | 0.695 | 0.411 | No significant differences |
| RMSE for stance phase | 0.060 | 0.808 | No significant differences | |
| RMSE for swing phase | 1.32 | 0.260 | No significant differences | |
| MS | RMSE for the entire gait cycle | 0.172 | 0.682 | No significant differences |
| RMSE for stance phase | 0.190 | 0.666 | No significant differences | |
| RMSE for swing phase | 0.012 | 0.915 | No significant differences |
| Phase | Parameter | MS Group | Control Group | Difference/ Cohen’s d’ Test | p-Value | Interpretation/Significance | |
|---|---|---|---|---|---|---|---|
| Complete cycle | Mean ± SD | Hip | 2.98 ± 1.26 | 2.44 ± 1.31 | +0.54/0.58 | 0.296 | Not significant |
| Knee | 8.04 ± 2.02 | 6.02 ± 4.41 | +2.02/0.65 | 0.130 | Not significant | ||
| Ankle | 7.54 ± 4.16 | 4.93 ± 2.52 | +2.61/1.46 | 0.014 * | Significant | ||
| F-value | 0.172 | 0.695 | Not significant | ||||
| p-value | 0.682 | 0.411 | Not significant | ||||
| R2 | 0.006 | 0.024 | Not significant | ||||
| Stance phase | Mean ± SD | Hip | 2.60 ± 1.61 | 2.08 ± 1.52 | +0.52/0.48 | 0.227 | Not significant |
| Knee | 6.55 ± 1.00 | 5.28 ± 5.90 | +1.27/0.3 | 0.400 | Not significant | ||
| Ankle | 6.18 ± 3.15 | 4.86 ± 3.26 | +1.32/0.57 | 0.170 | Not significant | ||
| F-value | 0.190 | 0.060 | Not significant | ||||
| p-value | 0.666 | 0.808 | Not significant | ||||
| R2 | 0.007 | 0.002 | Not significant | ||||
| Swing phase | Mean ± SD | Hip | 3.21 ± 0.90 | 2.60 ± 1.64 | +0.61/0.53 | 0.237 | Not significant |
| Knee | 9.40 ± 3.76 | 5.81 ± 3.53 | +3.59/1.44 | 0.015 * | Significant | ||
| Ankle | 9.21 ± 5.51 | 4.59 ± 2.11 | +4.62/3.10 | 0.008 * | Significant | ||
| F-value | 0.012 | 1.320 | Not significant | ||||
| p-value | 0.915 | 0.260 | Not significant | ||||
| R2 | 0.000 | 0.045 | Not significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosulescu, R.; Marin, M.I.; Albu, E.; Albu, B.C.; Neamtu, M.C.; Rosulescu, E. Joint Kinematics and Gait Pattern in Multiple Sclerosis: A 3D Analysis Comparative Approach. Bioengineering 2025, 12, 1067. https://doi.org/10.3390/bioengineering12101067
Rosulescu R, Marin MI, Albu E, Albu BC, Neamtu MC, Rosulescu E. Joint Kinematics and Gait Pattern in Multiple Sclerosis: A 3D Analysis Comparative Approach. Bioengineering. 2025; 12(10):1067. https://doi.org/10.3390/bioengineering12101067
Chicago/Turabian StyleRosulescu, Radu, Mihnea Ion Marin, Elena Albu, Bogdan Cristian Albu, Marius Cristian Neamtu, and Eugenia Rosulescu. 2025. "Joint Kinematics and Gait Pattern in Multiple Sclerosis: A 3D Analysis Comparative Approach" Bioengineering 12, no. 10: 1067. https://doi.org/10.3390/bioengineering12101067
APA StyleRosulescu, R., Marin, M. I., Albu, E., Albu, B. C., Neamtu, M. C., & Rosulescu, E. (2025). Joint Kinematics and Gait Pattern in Multiple Sclerosis: A 3D Analysis Comparative Approach. Bioengineering, 12(10), 1067. https://doi.org/10.3390/bioengineering12101067







