Promoting Re-Epithelialization in Diabetic Foot Wounds Using Integrative Therapeutic Approaches
Abstract
1. Introduction
1.1. Epidemiology of Diabetes Mellitus
1.2. Diabetic Foot Complications
1.3. Pathophysiological Mechanisms of Impaired Healing
1.4. Current Therapies and Limitations
- Metabolic control: strict regulation of blood glucose level and associated metabolic risk factors.
- Wound care procedures: cleaning, antisepsis, debridement, and appropriate dressings.
- Surgical interventions: ranging from minor drainage to amputations in severe, non-salvageable cases.
- Patient education: focusing on hygiene, protective footwear, and early recognition and reporting of lesions.
- Adjunctive therapies: such as hyperbaric oxygen therapy (HBOT), growth factors, or bioengineered skin substitutes.
1.5. Rationale and Aim
2. Materials and Methods
2.1. Study Group
- -
- Grade 0: Skin intact, but the foot is “at risk” due to existing bony deformities;
- -
- Grade 1: Superficial ulcer, involving only the skin and subcutaneous tissue;
- -
- Grade 2: Deep ulcer with full-thickness extension;
- -
- Grade 3: Deep ulcer with abscess or osteomyelitis;
- -
- Grade 4: Partial gangrene of the foot;
- -
- Grade 5: Extensive gangrene.
2.2. Study Design
- Initial session: colon hydrotherapy, followed by rectal ozone insufflation (20 µg/mL, one session/week for 3 weeks, then every 2 weeks for 4 weeks), administration of oral probiotics, and adoption of an anti-inflammatory alkaline diet.
- Subsequent sessions (twice weekly):
- -
- Wound antisepsis and lavage with ozonized water;
- -
- Wound debridement (performed only at the first session);
- -
- Antibiotic therapy strictly guided by antibiogram results;
- -
- Local ozone therapy: limb bagging (70 µg/mL for 3–4 sessions, then gradually reduced to 40 µg/mL) and perilesional infiltrations (5–10 µg/mL);
- -
- Systemic ozone therapy: major autohemotherapy with 120–150 mL venous blood ozonated at 25–35 µg/mL, provided systolic BP ≤ 160 mmHg;
- -
- Wound dressing with sterile compresses and ozonated olive oil at the end of each procedure;
- -
- Pulsed electromagnetic field therapy (20–70 Hz, 10–15 Gauss, 30 min/session, 2 sessions/week), adapted to wound-healing phase.
- Phase I—Inflammatory phase: 70–100 Hz, providing anti-inflammatory and analgesic effects by decreasing pro-inflammatory cytokines (TNF-α, IL-1β) and improving microcirculation (~1 week) [56].
- Phase III—Tissue maturation and remodeling: 20–30 Hz to promote cellular metabolism and collagen synthesis; toward the final stage, 70 Hz was applied for its anti-inflammatory effect and to improve collagen quality, extracellular matrix remodeling, and re-epithelialization (>3–4 weeks) [59].
2.3. Statistical Analysis
3. Results
3.1. Participant Flow and Analysis Set
3.2. Baseline Characteristics
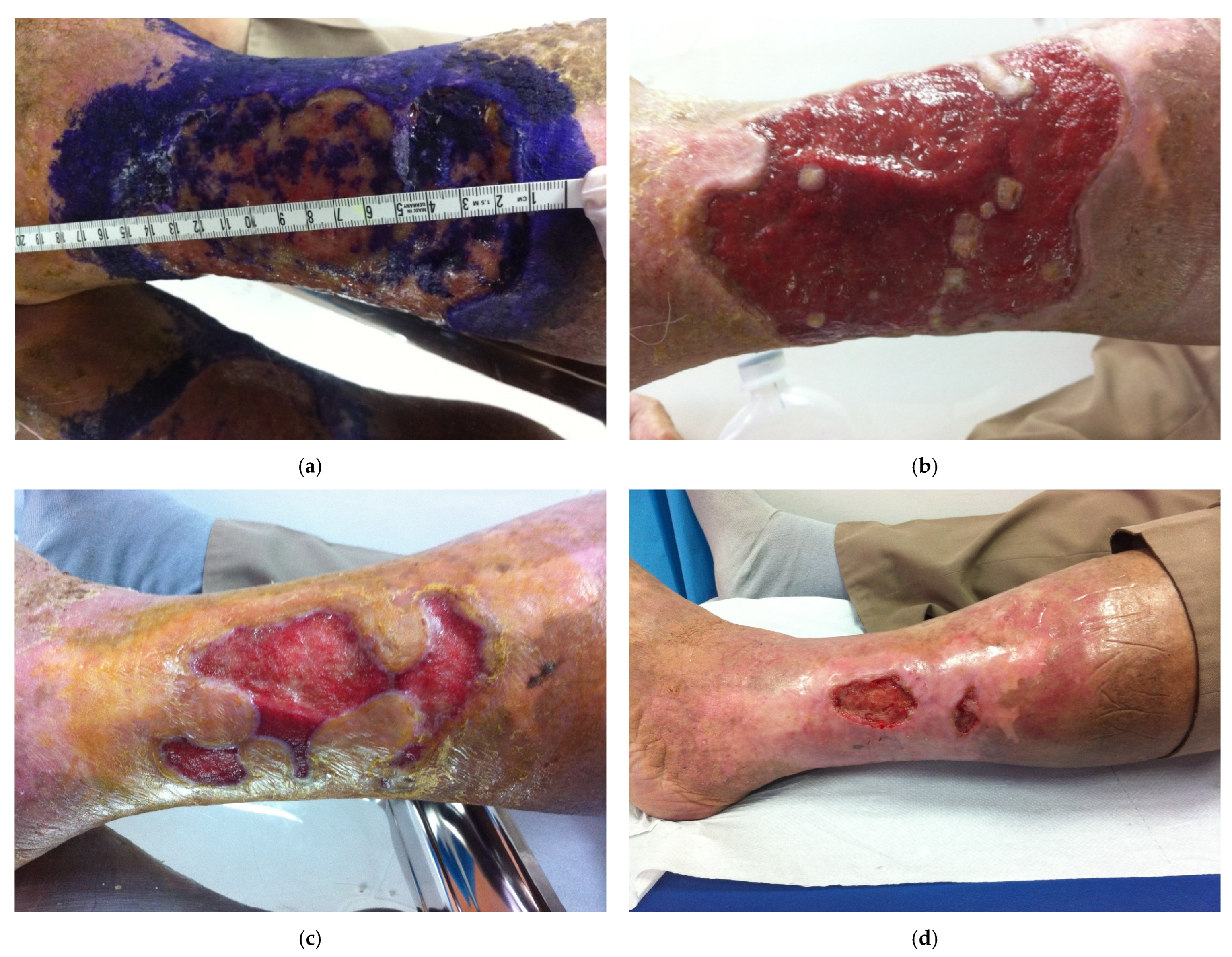
3.3. Primary Outcome: Ulcer Healing
3.4. Secondary Outcomes
3.4.1. Glycemic Control (Fasting Blood Glucose)
3.4.2. Glycated Hemoglobin (HbA1c)
3.4.3. Inflammatory and Coagulation Markers
- CRP: 6.16 ± 0.54 mg/L vs. 7.54 ± 0.69 mg/L (p < 0.001) at 4 weeks, and 5.59 ± 0.46 mg/L vs. 7.55 ± 0.62 mg/L (p < 0.001) at 8 weeks.
- Fibrinogen: 329.0 ± 26.5 mg/dL vs. 403.9 ± 46.0 mg/dL (p < 0.001) at 4 weeks, and 293.1 ± 23.3 mg/dL vs. 406.3 ± 43.6 mg/dL (p < 0.001) at 8 weeks.
3.4.4. Weight and BMI
3.5. Microbiology and Antibiotic Use
3.6. Safety and Tolerability
4. Discussion
4.1. Summary of Main Findings
4.2. Mechanistic Interpretation
4.3. Comparison with Existing Literature
4.4. Clinical Implications
- -
- Reduce healing time and accelerate re-epithelialization.
- -
- Lower systemic inflammation and improve metabolic control.
- -
- Reduce antibiotic use and potentially decrease amputation risk.
5. Limitations
6. Suggestions for Research
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | diabetes mellitus |
| WHO | World Health Organization |
| IDF | International Diabetes Federation |
| DFS | diabetic foot syndrome |
| DFU | diabetic foot ulcers |
| MMPs | matrix metalloproteinases |
| AGEs | advanced glycation end-products |
| NO | nitric oxide |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| EGF | epidermal growth factor |
| KGF-2/FGF-10 | keratinocyte growth factor-2/paracrine growth factor 10 |
| bFGF/FGF-2 | basic fibroblast growth factor |
| NPWT | negative pressure wound therapy |
| HBOT | hyperbaric oxygen therapy |
| PEMF | pulsed electromagnetic field |
| VEGF | vascular endothelial growth factor |
| FBG | fibrinogen |
| ESR | erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| BMI | body mass index |
References
- World Health Organization. Diabetes—Fact Sheet. Available online: https://www.who.int/health-topics/diabetes (accessed on 23 August 2025).
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 23 August 2025).
- Societatea Română de Diabet, Nutriție și Boli Metabolice. Official Website. Available online: https://societate-diabet.ro/ (accessed on 23 August 2025).
- Societatea Română de Diabet, Nutriție și Boli Metabolice. Press Release—World Diabetes Day. 2024. Available online: https://societate-diabet.ro/2024/11/14/comunicat-de-presa-ziua-mondiala-a-diabetului-2024/ (accessed on 23 August 2025).
- Bogdan-Andreescu, C.F.; Bănățeanu, A.-M.; Botoacă, O.; Defta, C.L.; Poalelungi, C.-V.; Brăila, A.D.; Damian, C.M.; Brăila, M.G.; Dȋră, L.M. Oral Wound Healing in Aging Population. Surgeries 2024, 5, 956–969. [Google Scholar] [CrossRef]
- Khan, M.S.; Jahan, N.; Khatoon, R.; Ansari, F.M.; Ahmad, S. An update on diabetic foot ulcer and its management modalities. Indian J. Microbiol. 2024, 64, 1401–1415. [Google Scholar] [CrossRef]
- Konarzewska, A.; Korzon-Burakowska, A.; Rzepecka-Wejs, L.; Sudoł-Szopińska, I.; Szurowska, E.; Studniarek, M. Diabetic Foot Syndrome: Charcot Arthropathy or Osteomyelitis? Part I: Clinical Picture and Radiography. J. Ultrason. 2018, 18, 42–49. [Google Scholar] [CrossRef]
- Morbach, S.; Lobmann, R.; Eckhard, M.; Müller, E.; Reike, H.; Risse, A.; Rümenapf, G.; Spraul, M. Diabetic Foot Syndrome. Exp. Clin. Endocrinol. Diabetes 2021, 129, S82–S90. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2021, 10, 29. [Google Scholar] [CrossRef]
- Dawi, J.; Tumanyan, K.; Tomas, K.; Misakyan, Y.; Gargaloyan, A.; Gonzalez, E.; Hammi, M.; Tomas, S.; Venketaraman, V. Diabetic Foot Ulcers: Pathophysiology, Immune Dysregulation, and Emerging Therapeutic Strategies. Biomedicines 2025, 13, 1076. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Mohsin, F.; Javaid, S.; Tariq, M.; Mustafa, M. Molecular Immunological Mechanisms of Impaired Wound Healing in Diabetic Foot Ulcers (DFU), Current Therapeutic Strategies and Future Directions. Int. Immunopharmacol. 2024, 139, 112713. [Google Scholar] [CrossRef]
- Petrescu, I.; Condrea, C.; Alexandru, A.; Dumitrescu, D.; Simion, G.; Severin, E.; Albu, C.; Albu, D. Diagnosis and Treatment Protocols of Cutaneous Melanoma: Latest Approach 2010. Chirurgia 2010, 105, 637–643. [Google Scholar]
- Bogdan-Andreescu, C.F.; Bănățeanu, A.M.; Albu, C.C.; Poalelungi, C.V.; Botoacă, O.; Damian, C.M.; Dȋră, L.M.; Albu, Ș.D.; Brăila, M.G.; Cadar, E.; et al. Oral mycobiome alterations in postmenopausal women: Links to inflammation, xerostomia, and systemic health. Biomedicines 2024, 12, 2569. [Google Scholar] [CrossRef]
- Lamers, M.L.; Almeida, M.E.S.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High Glucose-Mediated Oxidative Stress Impairs Cell Migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, Z.; Yang, C.; Zhou, P.; Jiang, H.; Chen, L.; Li, Y.; Li, Q. High Glucose Causes Distinct Expression Patterns of Primary Human Skin Cells by RNA Sequencing. Front. Endocrinol. 2021, 12, 603645. [Google Scholar] [CrossRef]
- RipszkyTotan, A.; Greabu, M.; Stanescu-Spinu, I.I.; Imre, M.; Spinu, T.C.; Miricescu, D.; Ilinca, R.; Coculescu, E.C.; Badoiu, S.C.; Coculescu, B.I.; et al. The Yin and Yang dualistic features of autophagy in thermal burn wound healing. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221125090. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.d.J.; Martinez-Blanco, M.d.R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current therapeutic strategies in diabetic foot ulcers. Medicina 2019, 55, 714. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gibbons, C.H.; Giurini, J.M.; Hilliard, M.E.; et al. Retinopathy, neuropathy, and foot care: Standards of care in diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S203–S215. [Google Scholar] [CrossRef]
- Chen, P.; Vilorio, N.C.; Dhatariya, K.; Jeffcoate, W.; Lobmann, R.; McIntosh, C.; Piaggesi, A.; Steinberg, J.; Vas, P.; Viswanathan, V.; et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3644. [Google Scholar] [CrossRef]
- O’Meara, S.; Al-Kurdi, D.; Ologun, Y.; Ovington, L.G.; Martyn-St James, M.; Richardson, R. Antibiotics and Antiseptics for Venous Leg Ulcers. Cochrane Database Syst. Rev. 2014, 2014, CD003557. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, K.; Guo, S.; Chang, R.; Zhang, C.; Guan, F.; Yao, M. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023, 155, 199–217. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, D.; Tan, L.; Huang, H. Silver Dressings for Healing Venous Leg Ulcers. Medicine 2020, 99, e22164. [Google Scholar] [CrossRef]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam Dressings for Treating Pressure Ulcers. Int. Wound J. 2014, 11, 445–455. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Garcia-Ojalvo, A.; Guillen-Nieto, G.; Ayala-Avila, M. Endogenous Biological Drivers in Diabetic Lower Limb Wounds Recurrence: Hypothetical Reflections. Int. J. Mol. Sci. 2023, 24, 10170. [Google Scholar] [CrossRef]
- Guo, Q. Comparison and evaluation of negative pressure wound therapy versus standard wound care in the treatment of diabetic foot ulcers. BMC Surg. 2025, 25, 208. [Google Scholar] [CrossRef]
- Bhutani, S.; Vishwanath, G. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg. 2012, 45, 316–324. [Google Scholar] [CrossRef]
- Pathault, E.; Sanchez, S.; Husson, B.; Vanhaecke, C.; Georges, P.; Brazier, C.; Mourvillier, B.; Viguier, M. Hyperbaric oxygen therapy enables pain reduction and healing in painful chronic wounds, including in calciphylaxis. Ann. Dermatol. Venereol. 2024, 151, 103325. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, K.S.; Chwa, E.S.; Weissman, J.P.; Garg, S.P.; Simmons, C.J.; Brandt, K.E.; Gosain, A.K. Practice Profile of Practicing Plastic Surgeons: A 20-year Review of Plastic Surgery Statistics. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5486. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone Therapy: A Clinical Review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Heng, H.; Liu, X.; Geng, H.; Liang, J. Evaluation of the Healing Potential of Short-Term Ozone Therapy in Diabetic Foot Ulcers (DFUs). Front. Endocrinol. 2024, 14, 1304034. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing. Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Ngeow, W.C.; Tan, C.C.; Goh, Y.C.; Deliberador, T.M.; Cheah, C.W. A Narrative Review on Means to Promote Oxygenation and Angiogenesis in Oral Wound Healing. Bioengineering 2022, 9, 636. [Google Scholar] [CrossRef]
- Lu, J.-Y.; Wang, X.-Q.; Fu, Z.-B.; Gao, L.-H.; Mannam, H.; Xiang, Y.-P.; Joo, Y.Y.; Zeng, J.-R.; Wang, D.; Paller, A.S. Topical Ozone Accelerates Diabetic Wound Healing by Promoting Re-Epithelialization through the Activation of IGF1R-EGFR Signaling. J. Investig. Dermatol. 2023, 143, 2507–2514.e6. [Google Scholar] [CrossRef]
- Białomyzy, A.; Kotrych, K.; Bogacz, A.; Podralska, M.; Górska, A.; Białecki, J.; Uzar, I.; Czerny, B.; Kamiński, A. Ozone therapy and negative pressure wound therapy in the treatment of difficult-to-heal wounds in diabetic foot syndrome and Charcot neuroarthropathy. J. Clin. Med. 2025, 14, 4017. [Google Scholar] [CrossRef]
- Haggerty, D.K.; Wahl, R.; Jones, N.; LaChance, J.; Hanna, M. Perceived Harm to Pet Health Associated with Human Quality of Life After a Public Health Disaster. Int. J. Environ. Res. Public Health 2025, 22, 250. [Google Scholar] [CrossRef]
- Wainstein, J.; Feldbrin, Z.; Boaz, M.; Harman-Boehm, I. Efficacy of Ozone–Oxygen Therapy for the Treatment of Diabetic Foot Ulcers. Diabetes Technol. Ther. 2011, 13, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Jafari-Oori, M.; Eftekhari, Z.; Jafari, N.J.; Maybodi, M.K.; Heydari, S.; Vahedian-Azimi, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. Effect of ozone therapy on diabetes-related foot ulcer outcomes: A systematic review and meta-analysis. Curr. Pharm. Des. 2024, 30, 2152–2166. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical Stimulation for Wound Healing: A Review of Evidence from In Vitro Studies, Animal Experiments, and Clinical Trials. Int J Low Extrem. Wounds. 2005, 4, 23–44. [Google Scholar] [CrossRef]
- Ennis, W.J.; Lee, C.; Plummer, M.; Meneses, P. Current status of the use of modalities in wound care: Electrical stimulation and ultrasound therapy. Plast. Reconstr. Surg. 2011, 127 (Suppl. S1), 93S–102S. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.S.; Hagberg, S.; Gurfein, B.T. Veterinary Applications of Pulsed Electromagnetic Field Therapy. Res. Vet. Sci. 2018, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Strauch, B.; Herman, C.; Dabb, R.; Ignarro, L.J.; Pilla, A.A. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet. Surg. J. 2009, 29, 135–143. [Google Scholar] [CrossRef]
- Bassett, C.A.L. Beneficial Effects of Electromagnetic Fields. J. Cell. Biochem. 1993, 51, 387–393. [Google Scholar] [CrossRef]
- Luigi, C.; Pratellesi, T. Mechanisms of Action and Effects of Pulsed Electromagnetic Fields (PEMF) in Medicine. J. Med. Res. Surg. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Ross, C.L.; Zhou, Y.; McCall, C.E.; Soker, S.; Criswell, T.L. The Use of Pulsed Electromagnetic Field to Modulate Inflammation and Improve Tissue Regeneration: A Review. Bioelectricity 2019, 1, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Costantini, E.; Reale, M.; Amerio, P. Wound Repair and Extremely Low Frequency-Electromagnetic Field: Insight from In Vitro Study and Potential Clinical Application. Int. J. Mol. Sci. 2021, 22, 5037. [Google Scholar] [CrossRef] [PubMed]
- Goodall, R.J.; Langridge, B.; Lane, T.; Davies, A.H.; Shalhoub, J. A narrative review of the use of neuromuscular electrical stimulation in individuals with diabetic foot ulceration. Int. J. Low. Extrem. Wounds 2020, 19, 242–250. [Google Scholar] [CrossRef]
- Ma, K.C.; Baumhauer, J.F. Pulsed Electromagnetic Field Treatment in Wound Healing. Curr. Orthop. Pract. 2013, 24, 487–492. [Google Scholar] [CrossRef]
- Wade, B. A Review of Pulsed Electromagnetic Field (PEMF) Mechanisms at a Cellular Level: A Rationale for Clinical Use. Am. J. Health Res. 2013, 1, 51–55. [Google Scholar] [CrossRef]
- Luben, R.A. Effects of low-energy electromagnetic fields (pulsed and DC) on membrane signal transduction processes in biological systems. Health Phys. 1991, 61, 15–28. [Google Scholar] [CrossRef] [PubMed]
- International Working Group on the Diabetic Foot (IWGDF). IWGDF Guidelines 2023. Available online: https://iwgdfguidelines.org/guidelines-2023/ (accessed on 23 August 2025).
- Shah, P.; Inturi, R.; Anne, D.; Jadhav, D.; Viswambharan, V.; Khadilkar, R.; Dnyanmote, A.; Shahi, S. Wagner’s Classification as a Tool for Treating Diabetic Foot Ulcers: Our Observations at a Suburban Teaching Hospital. Cureus 2022, 14, e21501. [Google Scholar] [CrossRef]
- Choi, H.M.; Cheing, A.K.; Ng, G.Y.; Cheing, G.L. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS ONE 2018, 13, e0191074. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.L.C.; Wong, W.C.; Yip, S.L.; Chan, K.L.; Zheng, Y.P.; Cheing, G.L.Y. Pulsed Electromagnetic Field Therapy Promotes Healing and Microcirculation of Chronic Diabetic Foot Ulcers: A Pilot Study. Adv. Skin Wound Care 2015, 28, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, C.; Falldorf, K.; Sachtleben, J.; van Griensven, M. Low-frequency pulsed electromagnetic fields significantly improve time of closure and proliferation of human tendon fibroblasts. Eur. J. Med. Res. 2014, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Xie, C.; Luo, X.; Zhang, Q.; Xue, Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef]
- Astasio-Picado, Á.; Babiano, A.Á.; López-Sánchez, M.; Lozano, R.R.; Cobos-Moreno, P.; Gómez-Martín, B. Use of Ozone Therapy in Diabetic Foot Ulcers. J. Pers. Med. 2023, 13, 1439. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Tian, J.; Li, L.; Li, J.; Tian, J.H.; Yang, K. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2015, 2015, CD008474. [Google Scholar] [CrossRef]
- Travagli, V.; Iorio, E.L. The Biological and Molecular Action of Ozone and Its Derivatives: State-of-the-Art, Enhanced Scenarios, and Quality Insights. Int. J. Mol. Sci. 2023, 24, 8465. [Google Scholar] [CrossRef]
- Rathnayake, A.; Saboo, A.; Vangaveti, V.; Malabu, U. Electromechanical therapy in diabetic foot ulcers patients: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2023, 22, 967–984. [Google Scholar] [CrossRef]
- Kaadan, A.; Salati, S.; Cadossi, R.; Aaron, R. Regulation of Inflammatory Responses by Pulsed Electromagnetic Fields. Bioengineering 2025, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, M.; Polajžer, T.; Novickij, V.; Miklavčič, D. Determination of the Impact of High-Intensity Pulsed Electromagnetic Fields on the Release of Damage-Associated Molecular Pattern Molecules. Int. J. Mol. Sci. 2023, 24, 14607. [Google Scholar] [CrossRef]
- Flatscher, J.; Pavez Loriè, E.; Mittermayr, R.; Meznik, P.; Slezak, P.; Redl, H.; Slezak, C. Pulsed Electromagnetic Fields (PEMF)—Physiological Response and Its Potential in Trauma Treatment. Int. J. Mol. Sci. 2023, 24, 11239. [Google Scholar] [CrossRef]
- Cianni, L.; Di Gialleonardo, E.; Coppola, D.; Capece, G.; Libutti, E.; Nannerini, M.; Maccauro, G.; Vitiello, R. Current Evidence Using Pulsed Electromagnetic Fields in Osteoarthritis: A Systematic Review. J. Clin. Med. 2024, 13, 1959. [Google Scholar] [CrossRef]
- Abed Elrashid, N.A.; Ali, O.I.; Ibrahim, Z.M.; El Sharkawy, M.A.; Bin Sheeha, B.; Amin, W.M. A Double-Blinded Randomized Controlled Trial: Can Pulsed Electromagnetic Field Therapy Be a Novel Method for Treating Chronic Rhinosinusitis? Medicina 2024, 60, 1868. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-G.; Park, C.; Hwang, S.; Hong, J.-E.; Jo, M.; Eom, M.; Lee, Y.; Rhee, K.-J. Pulsed Electromagnetic Field (PEMF) Treatment Reduces Lipopolysaccharide-Induced Septic Shock in Mice. Int. J. Mol. Sci. 2022, 23, 5661. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- García-Jiménez, C.; Gutiérrez-Salmerón, M.; Chocarro-Calvo, A.; García-Martinez, J.M.; Castaño, A.; De la Vieja, A. From Obesity to Diabetes and Cancer: Epidemiological Links and Role of Gut Microbiota and Endotoxemia. J. Mol. Endocrinol. 2014, 52, R199–R212. [Google Scholar] [CrossRef]
- García-Jiménez, C.; Gutiérrez-Salmerón, M.; Chocarro-Calvo, A.; García-Martinez, J.M.; Castaño, A.; De la Vieja, A. From obesity to diabetes and cancer: Epidemiological links and role of therapies. Br. J. Cancer 2016, 114, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Defta, C.L.; Albu, C.-C.; Albu, Ş.-D.; Bogdan-Andreescu, C.F. Oral Mycobiota: A Narrative Review. Dent. J. 2024, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Abildinova, G.Z.; Benberin, V.V.; Vochshenkova, T.A.; Afshar, A.; Mussin, N.M.; Kaliyev, A.A.; Zhussupova, Z.; Tamadon, A. The gut-brain-metabolic axis: Exploring the role of microbiota in insulin resistance and cognitive function. Front. Microbiol. 2024, 15, 1463958. [Google Scholar] [CrossRef]
- Parekh, P.; Burleson, D.; Lubin, C.; Johnson, D.A. Colon Irrigation: Effective, Safe, and Well-Tolerated Alternative to Traditional Therapy in the Management of Refractory Chronic Constipation. J. Clin. Gastroenterol. Hepatol. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Aragona, S.E.; Spada, C.; De Luca, L.; Aragona, E.; Ciprandi, G.; COLONSTUDY Study Group. Probiotics for Managing Patients after Bowel Preparation for Colonoscopy: An Interventional, Double-Arm, Open, Randomized, Multi-Center, and National Study (COLONSTUDY). Minerva Gastroenterol. 2024, 70, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Piciucchi, M.; Rossi, A.; Satriano, A.; Manta, R. Is It Advisable to Use Probiotics Routinely after a Colonoscopy? A Rapid Comprehensive Review of the Evidence. Med. Sci. 2025, 13, 76. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. NCT02372201—Microbiome and Probiotics after Colonoscopy. Available online: https://clinicaltrials.gov/ct2/show/NCT02372201 (accessed on 23 August 2025).
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; Gravenor, M.B.; et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: A multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE). Health Technol. Assess. 2013, 17, 1–140. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and Type 2 Diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Caciano, S.L.; Inman, C.L.; Gockel-Blessing, E.E.; Weiss, E.P. Effects of Dietary Acid Load on Exercise Metabolism and Anaerobic Performance. J. Sports Sci. Med. 2015, 14, 364–371. [Google Scholar]
- Karakousis, N.D.; Pyrgioti, E.E.; Georgakopoulos, P.N.; Apergi, K.; Papanas, N. Magnesium and Diabetic Foot Ulcers: A Mini Review. Int. J. Low. Extrem. Wounds 2023, 22, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Pena, G.; Kuang, B.; Cowled, P.; Howell, S.; Dawson, J.; Philpot, R.; Fitridge, R. Micronutrient status in diabetic patients with foot ulcers. Adv. Wound Care 2020, 9, 9–15. [Google Scholar] [CrossRef]
- Erdem, H.A.; Yalçın, N.; Kaya, A.; Taşbakan, M. Vitabiotic: An Alternative Approach to Diabetic Foot. Wound Repair Regen. 2024, 32, 890–894. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Beltrán-Velasco, A.I.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Valcárcel-Martín, R.; Tornero-Aguilera, J.F. Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants 2025, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline Precursors and Collagen Synthesis: Biochemical Challenges of Nutrient Supplementation and Wound Healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Fatima, M.T.; Bhat, A.A.; Nisar, S.; Fakhro, K.A.; Al-Shabeeb Akil, A.S. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon 2022, 9, e12698. [Google Scholar] [CrossRef]
- Qi, L.; Hu, F.B. Dietary Glycemic Load, Whole Grains, and Systemic Inflammation in Diabetes. Curr. Opin. Lipidol. 2007, 18, 3–8. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J. Low Glycaemic Index/Load Diets for Diabetes Mellitus. Cochrane Database Syst. Rev. 2009, 2009, CD006296. [Google Scholar] [CrossRef]
- Burcea, A.; Mihai, L.L.; Bechir, A.; Suciu, M.; Bechir, E.S. Clinical Assessment of the Hyperbaric Oxygen Therapy Efficacy in Mild to Moderate Periodontal Affections: A Simple Randomised Trial. Medicina 2022, 58, 234. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.H.; Kim, K.B.; Kim, H.S.; Lee, Y.K. Factors Influencing Wound Healing in Diabetic Foot Patients. Medicina 2024, 60, 723. [Google Scholar] [CrossRef]
- Krupa, K.N.; Fritz, K.; Parmar, M. Omega-3 Fatty Acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564314/ (accessed on 23 August 2025).
- Wu, T.; Seaver, P.; Lemus, H.; Hollenbach, K.; Wang, E.; Pierce, J.P. Associations between Dietary Acid Load and Biomarkers of Inflammation and Hyperglycemia in Breast Cancer Survivors. Nutrients 2019, 11, 1913. [Google Scholar] [CrossRef] [PubMed]
| Group | Intervention | No. of Patients |
|---|---|---|
| G1-Control | Standard treatment (debridement, dressings, antibiotics when indicated by wound culture and antibiogram) + local and general ozone therapy | 14 |
| G2-Protocol | Standard treatment + local and general ozone therapy + probiotic hydrocolonotherapy + alkaline diet + PEMF (20–70 Hz) | 14 |
| Parameter | Control Group (n = 14) | Intervention Group (n = 14) | p-Value |
|---|---|---|---|
| Age (years) | 61.4 ± 8.2 | 60.7 ± 7.9 | 0.78 |
| Male/Female | 8/6 | 9/5 | 0.68 |
| Diabetes type I/II | 4/10 | 5/9 | 0.71 |
| Duration of diabetes (years) | 12.3 ± 6.1 | 13.0 ± 5.8 | 0.65 |
| Ulcer area (cm2) | 11.79 ± 5.41 | 13.00 ± 6.04 | 0.58 |
| Fasting blood glucose (mg/dL) | 191.1 ± 25.4 | 183.8 ± 21.6 | 0.42 |
| HbA1c (%) | 7.67 ± 2.09 | 8.02 ± 0.75 | 0.56 |
| Weight (kg) | 84.4 ± 4.0 | 83.8 ± 4.0 | 0.67 |
| BMI (kg/m2) | 25.9 ± 10.1 | 30.0 ± 1.6 | 0.15 |
| CRP (mg/L) | 7.49 ± 0.64 | 7.05 ± 0.53 | 0.06 |
| Fibrinogen (mg/dL) | 399.9 ± 48.1 | 369.5 ± 32.2 | 0.06 |
| Timepoint | Control | Intervention | p-Value |
|---|---|---|---|
| Baseline | 11.79 ± 5.41 | 13.00 ± 6.04 | 0.58 |
| 4 weeks | 7.93 ± 4.14 | 5.79 ± 2.19 † | 0.009 |
| 8 weeks | 4.93 ± 3.41 | 1.79 ± 1.67 ‡ | 0.005 |
| Parameter | Control 4w | Intervention 4w | p-Value | Control 8w | Intervention 8w | p-Value |
|---|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | 216.6 ± 37.2 | 162.4 ± 16.8 § | <0.001 | 220.4 ± 36.5 | 136.6 ± 9.6 § | <0.001 |
| HbA1c (%) | 9.17 ± 1.29 | 6.86 ± 1.86 ‡ | 0.001 | 8.58 ± 2.44 | 6.45 ± 0.45 ‡ | 0.004 |
| CRP (mg/L) | 7.54 ± 0.69 | 6.16 ± 0.54 § | <0.001 | 7.55 ± 0.62 | 5.59 ± 0.46 § | <0.001 |
| Fibrinogen (mg/dL) | 403.9 ± 46.0 | 329.0 ± 26.5 § | <0.001 | 406.3 ± 43.6 | 293.1 ± 23.3 § | <0.001 |
| Weight (kg) | 85.3 ± 4.0 | 82.0 ± 3.8 ‡ | 0.004 | 85.3 ± 3.7 | 79.7 ± 3.7 § | 0.001 |
| BMI (kg/m2) | 27.85 ± 7.69 | 29.38 ± 1.56 | 0.475 | 27.66 ± 7.6 | 26.69 ± 7.0 | 0.729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubulac, L.; Gheorghe, I.-R.; Ungureanu, E.; Bogdan-Andreescu, C.F.; Albu, C.-C.; Gheorghe, C.-M.; Mușat, O.; Eremia, I.A.; Panea, C.A.; Burcea, A. Promoting Re-Epithelialization in Diabetic Foot Wounds Using Integrative Therapeutic Approaches. Bioengineering 2025, 12, 1053. https://doi.org/10.3390/bioengineering12101053
Bubulac L, Gheorghe I-R, Ungureanu E, Bogdan-Andreescu CF, Albu C-C, Gheorghe C-M, Mușat O, Eremia IA, Panea CA, Burcea A. Promoting Re-Epithelialization in Diabetic Foot Wounds Using Integrative Therapeutic Approaches. Bioengineering. 2025; 12(10):1053. https://doi.org/10.3390/bioengineering12101053
Chicago/Turabian StyleBubulac, Lucia, Iuliana-Raluca Gheorghe, Elisabeth Ungureanu, Claudia Florina Bogdan-Andreescu, Cristina-Crenguța Albu, Consuela-Mădălina Gheorghe, Ovidiu Mușat, Irina Anca Eremia, Cristina Aura Panea, and Alexandru Burcea. 2025. "Promoting Re-Epithelialization in Diabetic Foot Wounds Using Integrative Therapeutic Approaches" Bioengineering 12, no. 10: 1053. https://doi.org/10.3390/bioengineering12101053
APA StyleBubulac, L., Gheorghe, I.-R., Ungureanu, E., Bogdan-Andreescu, C. F., Albu, C.-C., Gheorghe, C.-M., Mușat, O., Eremia, I. A., Panea, C. A., & Burcea, A. (2025). Promoting Re-Epithelialization in Diabetic Foot Wounds Using Integrative Therapeutic Approaches. Bioengineering, 12(10), 1053. https://doi.org/10.3390/bioengineering12101053










