Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer
Abstract
1. Introduction
2. Materials and Methods
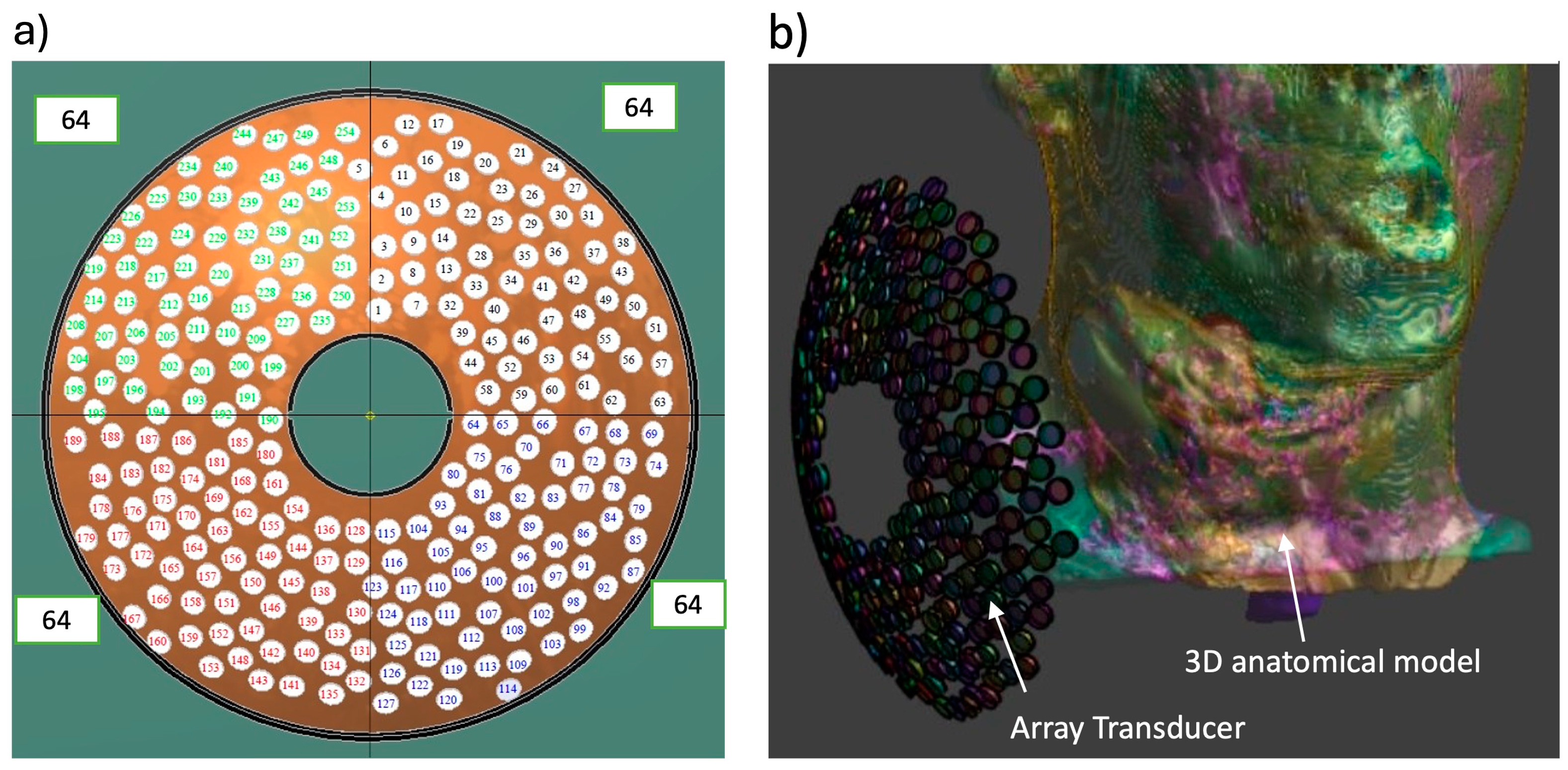
2.1. The Phased Array System
2.2. Phase Calculation
2.3. Acoustic and Biothermal Modeling
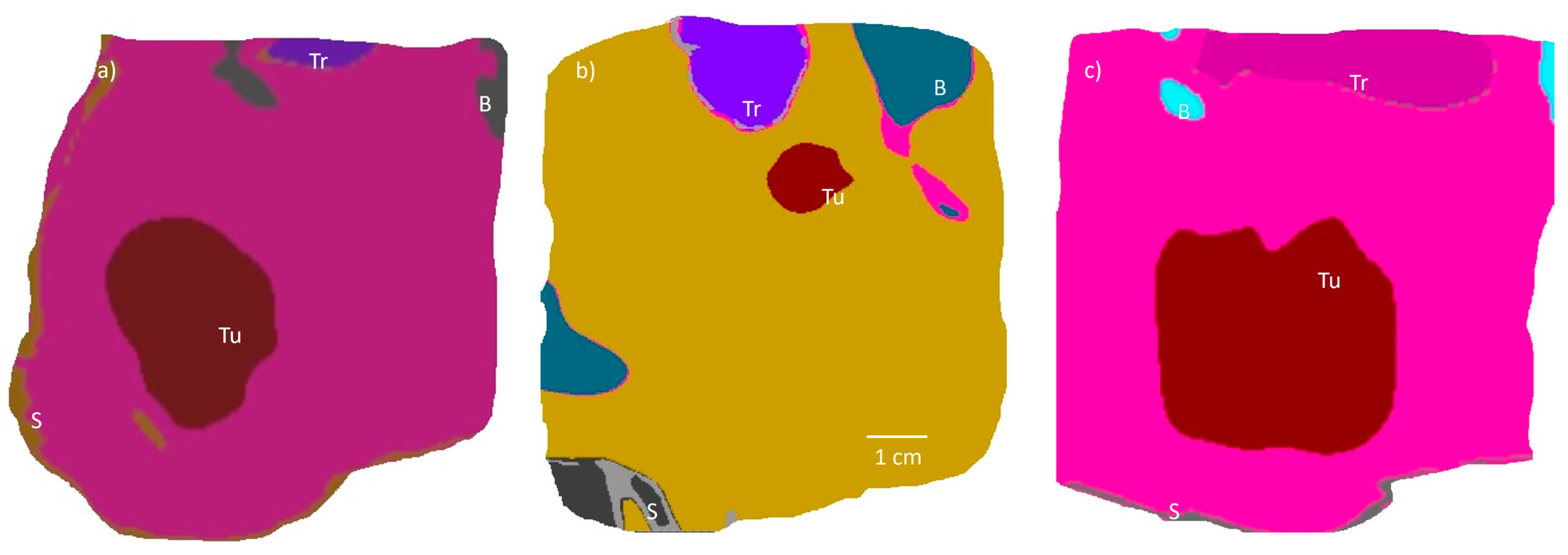
2.4. Patient-Specific Anatomical Models
3. Results
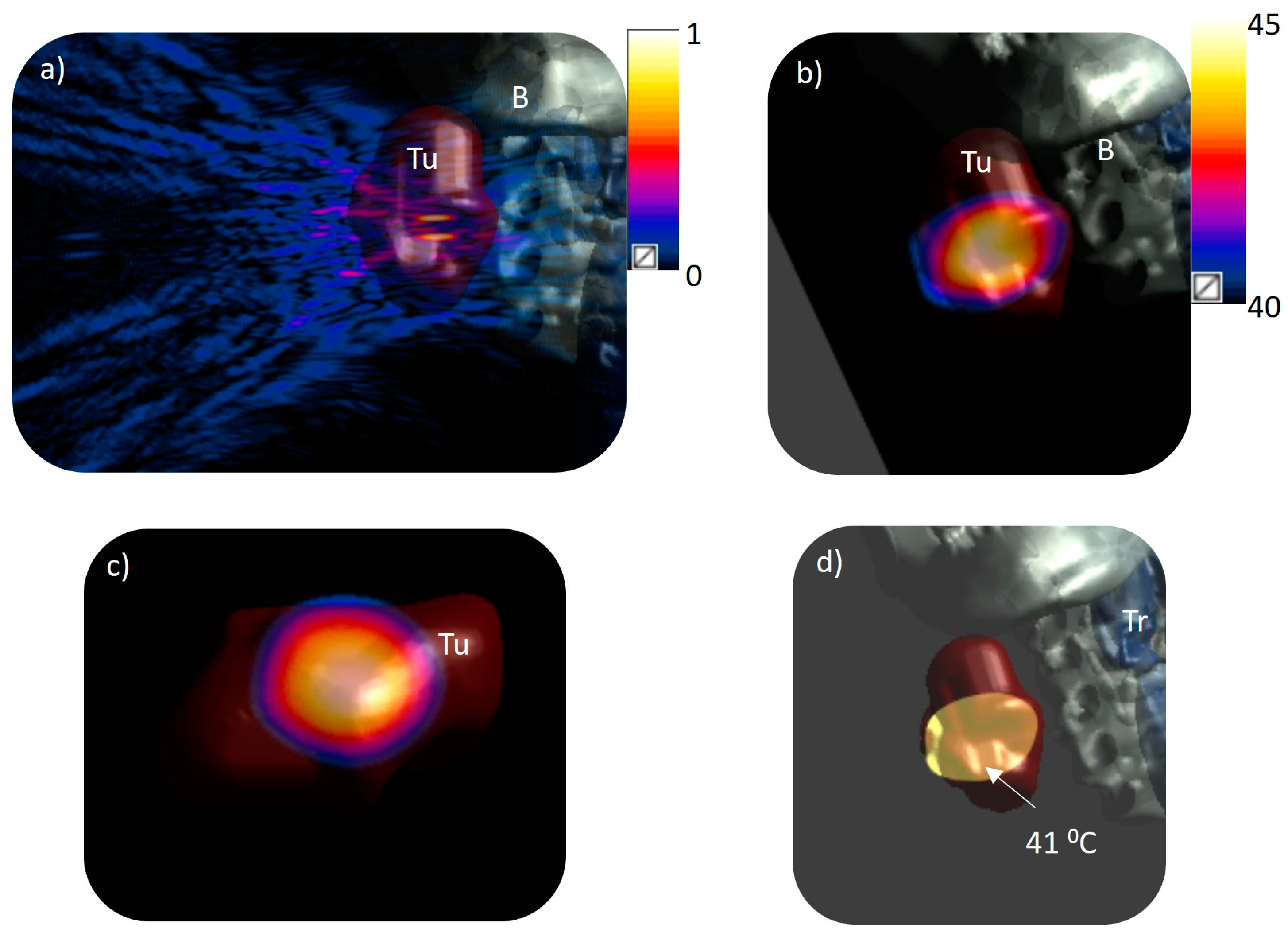
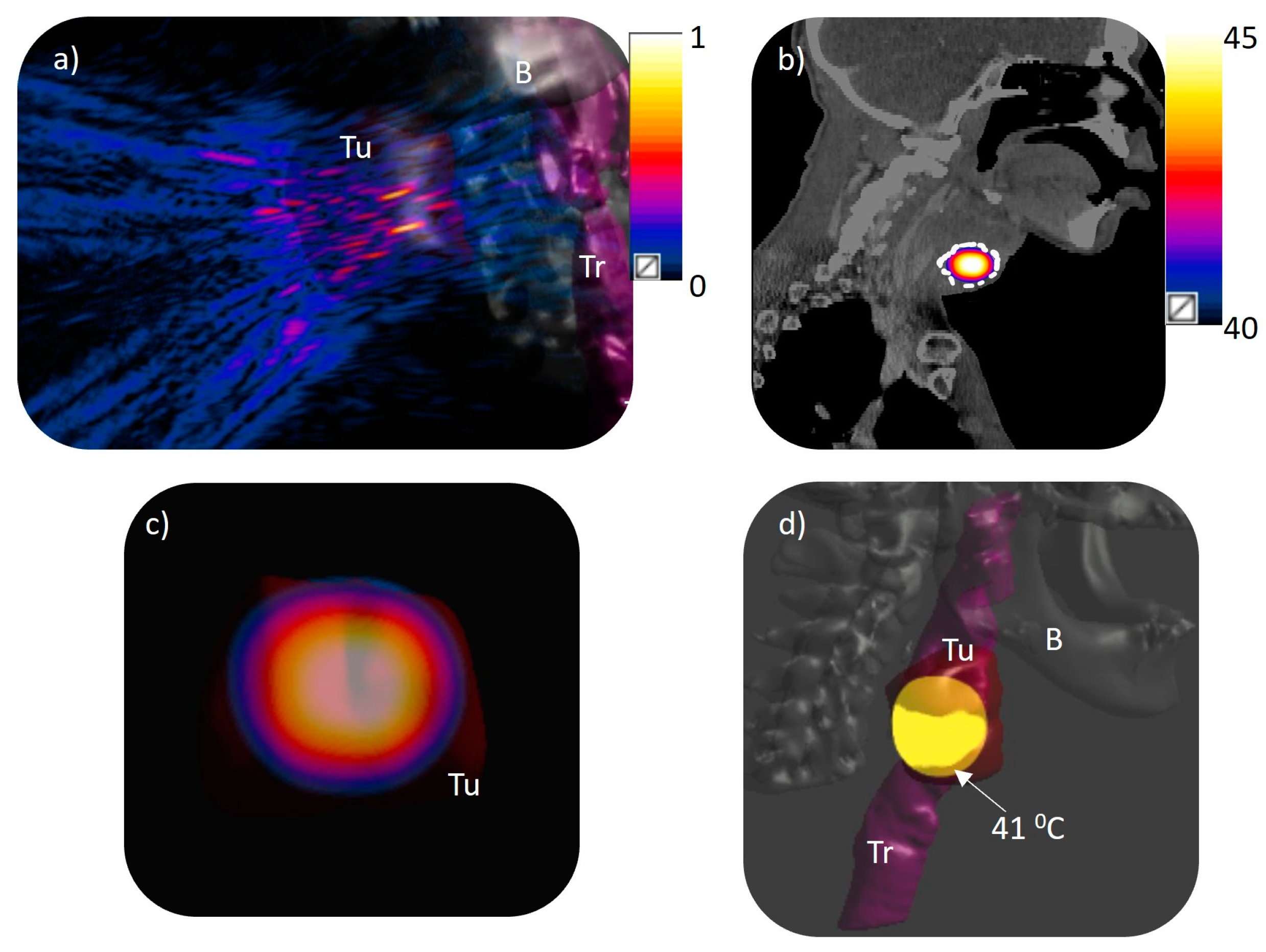
3.1. Acoustic Beam Profile
3.2. Volumetric Hyperthermia in Patient-Specific Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezra, E.W.C.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar]
- Lee, Y.-G.; Kang, E.J.; Keam, B.; Choi, J.-H.; Kim, J.-S.; Park, K.U.; Lee, K.E.; Kwon, J.H.; Lee, K.-W.; Kim, M.K.; et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: A nationwide retrospective cohort study (KCSG HN13–01). BMC Cancer 2020, 20, 813. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.-W.; Jang, J.-S.; Kim, M.-S.; Sun, D.-I.; Kim, B.-S.; Jung, S.-L.; Kang, J.-H.; Yoo, E.-J.; Yoon, S.-C.; Jang, H.-S.; et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1348–1355. [Google Scholar] [CrossRef]
- Yamazaki, H.; Kodani, N.; Ogita, M.; Sato, K.; Himei, K. Reirradiation of head and neck cancer focusing on hypofractionated stereotactic body radiation therapy. Radiat. Oncol. 2011, 6, 98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status quo and directions in deep head and neck hyperthermia. Radiat. Oncol. 2016, 11, 21. [Google Scholar] [CrossRef]
- Conley, B.A. Treatment of Advanced Head and Neck Cancer: What Lessons Have We Learned? J. Clin. Oncol. 2006, 24, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Grobholz, R.; Puric, E.; Bode-Lesniewska, B.; Lomax, N.; Khan, S.; Gaipl, U.S.; Fuchs, B.; Bodis, S. Enhanced tumour regression in a patient of liposarcoma treated with radiotherapy and hyperthermia: Hint for dynamic immunomodulation by hyperthermia. Int. J. Hyperth. 2015, 31, 574–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.C.; Padanilam, J.; Holmes, W.; Ezzell, R.; Lee, R.; Tompkins, R.; Yarmush, M.; Toner, M. Dynamics of cell membrane permeability changes at supraphysiological temperatures. Biophys. J. 1995, 68, 2608–2614. [Google Scholar] [CrossRef]
- Staruch, R.M.; Ganguly, M.; Tannock, I.F.; Hynynen, K.; Chopra, R. Enhanced drug delivery in rabbit VX2 tumours using thermosensitive liposomes and MRI-controlled focused ultrasound hyperthermia. Int. J. Hyperth. 2012, 28, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Capitano, M.L.; Spernyak, J.A.; Schueckler, J.T.; Thomas, S.; Singh, A.K.; Evans, S.S.; Hylander, B.L.; Repasky, E.A. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 2011, 71, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Horsman, M.R. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int. J. Hyperth. 2010, 26, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Los, G.; van Vugt, M.J.; Pinedo, H.M. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br. J. Cancer 1994, 69, 235–241. [Google Scholar] [CrossRef]
- Elias, D.; Bonnay, M.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El Otmany, A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar] [PubMed]
- Ranjan, A.; Jacobs, G.C.; Woods, D.L.; Negussie, A.H.; Partanen, A.; Yarmolenko, P.S.; Gacchina, C.E.; Sharma, K.V.; Frenkel, V.; Wood, B.J.; et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release 2012, 158, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Staruch, R.M.; Hynynen, K.; Chopra, R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: Therapeutic effect in rabbit Vx2 tumours. Int. J. Hyperth. 2015, 31, 118–133. [Google Scholar] [CrossRef]
- Paulides, M.; Trefna, H.D.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency- and microwave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020, 163, 3–18. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sun, J.; Zhao, D.; He, H.; Wang, B.; Xu, L.; Shang, Y.; Ren, S.; Zhang, Y.; Wu, T. Modified-FOLFIRINOX combined with deep regional hyperthermia in pancreatic cancer: A retrospective study in Chinese patients. Int. J. Hyperth. 2019, 36, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Amichetti, M.; Romano, M.; Busana, L.; Bolner, A.; Fellin, G.; Pani, G.; Tomio, L.; Valdagni, R. Hyperfractionated radiation in combination with local hyperthermia in the treatment of advanced squamous cell carcinoma of the head and neck: A phase I–II study. Radiother. Oncol. 1997, 45, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Ma, S.; Fu, Z.; Hu, Q.; Wang, L.; Piao, Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int. J. Hyperth. 2011, 27, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Verduijn, G.M.; De Wee, E.M.; Rijnen, Z.; Togni, P.; Hardillo, J.A.U.; Ten Hove, I.; Franckena, M.; Van Rhoon, G.C.; Paulides, M. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma—A feasibility study. Int. J. Hyperth. 2018, 34, 994–1001. [Google Scholar] [CrossRef]
- Kroesen, M.; van Holthe, N.; Sumser, K.; Chitu, D.; Vernhout, R.; Verduijn, G.; Franckena, M.; Hardillo, J.; van Rhoon, G.; Paulides, M. Feasibility, SAR Distribution, and Clinical Outcome upon Reirradiation and Deep Hyperthermia Using the Hypercollar3D in Head and Neck Cancer Patients. Cancers 2021, 13, 6149. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized Trial of Hyperthermia and Radiation for Superficial Tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [PubMed]
- Moros, E.G.; Roemer, R.B.; Hynynen, K. Simulations of scanned focused ultrasound hyperthermia. the effects of scanning speed and pattern on the temperature fluctuations at the focal depth. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1988, 35, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, G.; Poon, I.; Karam, I.; Higgins, K.; Pichardo, S.; Hynynen, K.; Enepekides, D. Magnetic resonance-guided high-intensity focused ultrasound combined with radiotherapy for palliation of head and neck cancer—A pilot study. J. Ther. Ultrasound 2016, 4, 12. [Google Scholar] [CrossRef]
- Pichardo, S.; Köhler, M.; Lee, J.; Hynnyen, K. In Vivo optimisation study for multi-baseline MR-based thermometry in the context of hyperthermia using MR-guided high intensity focused ultrasound for head and neck applications. Int. J. Hyperth. 2014, 30, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.D.; Lyon, P.C.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Focused Ultrasound Hyperthermia for Targeted Drug Release from Thermosensitive Liposomes: Results from a Phase I Trial. Radiology 2019, 291, 232–238. [Google Scholar] [CrossRef]
- Lyon, P.C.; Mannaris, C.; Gray, M.; Carlisle, R.; Gleeson, F.V.; Cranston, D.; Wu, F.; Coussios, C.C. Large-Volume Hyperthermia for Safe and Cost-Effective Targeted Drug Delivery Using a Clinical Ultrasound-Guided Focused Ultrasound Device. Ultrasound Med. Biol. 2021, 47, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.T.; Hynynen, K. Design and experimental evaluation of an intracavitary ultrasound phased array system for hyperthermia. IEEE Trans. Biomed. Eng. 1994, 41, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Diederich, C.J.; Hynynen, K. The feasibility of using electrically focused ultrasound arrays to induce deep hyperthermia via body cavities. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1991, 38, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K. The feasibility of interstitial ultrasound hyperthermia. Med. Phys. 1992, 19, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, X.; Gong, X.; Yang, C.; Zhang, R.; Chen, W.; Chen, J. Learning Curve of USgHIFU Ablation for Uterine Fibroids: A Multi-Center Prospective Study. J. Ultrasound Med. 2022, 41, 3051–3059. [Google Scholar] [CrossRef]
- Hsu, F.C.; Lee, H.-L.; Chen, Y.-J.; Shen, Y.-A.; Tsai, Y.-C.; Wu, M.-H.; Kuo, C.-C.; Lu, L.-S.; Yeh, S.-D.; Huang, W.-S.; et al. A Few-Shot Learning Approach Assists in the Prognosis Prediction of Magnetic Resonance-Guided Focused Ultrasound for the Local Control of Bone Metastatic Lesions. Cancers 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, K.G.; Yang, A.I.; Hitti, F.L.; Henry, L.; Heman-Ackah, S.M.; Chaibainou, H.; Baltuch, G.H. Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Essential Tremor Under General Anesthesia: Technical Note. Oper. Neurosurg. 2022, 22, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.; Ahmed, H.U. Reply to Francesco Montorsi, Armando Stabile, Elio Mazzone, Giorgio Gandaglia, and Alberto Briganti’s Letter to the Editor re: Deepika Reddy, Max Peters, Taimur, T. Shah, et al. Cancer Control Outcomes Following Focal Therapy Using High-intensity Focused Ultrasound in 1379 Men with Nonmetastatic Prostate Cancer: A Multi-institute 15-year Experience. Eur Urol 2022;81:407-13. Eur. Urol. 2022, 82, e74–e75. [Google Scholar]
- Diederich, C.J.; Stafford, R.J.; Nau, W.H.; Burdette, E.C.; Price, R.E.; Hazle, J.D. Transurethral ultrasound applicators with directional heating patterns for prostate thermal therapy: In vivo evaluation using magnetic resonance thermometry. Med. Phys. 2004, 31, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hand, J.W.; Shaw, A.; Sadhoo, N.; Rajagopal, S.; Dickinson, R.J.; Gavrilov, L.R. A random phased array device for delivery of high intensity focused ultrasound. Phys. Med. Biol. 2009, 54, 5675–5693. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Robert, J.D. 3D synthetic aperture imaging with a therapeutic spherical random phased array for transcostal applications. Phys. Med. Biol. 2021, 66, 035024. [Google Scholar]
- Zubair, M.; Dickinson, R. Calculating the Effect of Ribs on the Focus Quality of a Therapeutic Spherical Random Phased Array. Sensors 2021, 21, 1211. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Dickinson, R.J. Simulation of a Modified Multielement Random Phased Array for Image Guidance and Therapy. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 1137–1140. [Google Scholar]
- Zubair, M.; Harput, S.; Dickinson, R. 3D Ultrasound Image Guidance and Therapy Through the Rib Cage with a Therapeutic Random Phased Array. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–9. [Google Scholar]
- Ebbini, E.S.; Cain, C.A. A spherical-section ultrasound phased array applicator for deep localized hyperthermia. IEEE Trans. Biomed. Eng. 1991, 38, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Adams, M.S.; Diederich, C.J. An endoluminal cylindrical sectored-ring ultrasound phased-array applicator for minimally-invasive therapeutic ultrasound. Med. Phys. 2023, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pennes, H.H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Adams, M.S.; Diederich, C.J. An Endoscopic Concentric Ring Sector-Vortex Ultrasound Phased Array Applicator for Pancreatic Tumor Ablation. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021. [Google Scholar]
- Zubair, M.; Adams, M.S.; Diederich, C.J. Deployable ultrasound applicators for endoluminal delivery of volumetric hyperthermia. Int. J. Hyperth. 2021, 38, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J. Physical Properties of Tissue: A Comprehensive Reference Book, edited by Francis, A. Duck. Med. Phys. 1991, 18, 834. [Google Scholar] [CrossRef]
- Hijnen, N.M.; Heijman, E.; Köhler, M.O.; Ylihautala, M.; Ehnholm, G.J.; Simonetti, A.W.; Grüll, H. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-u. Int. J. Hyperth. 2012, 28, 141–155. [Google Scholar] [CrossRef]
- Partanen, A.; Tillander, M.; Yarmolenko, P.S.; Wood, B.J.; Dreher, M.R.; Köhler, M.O. Reduction of peak acoustic pressure and shaping of heated region by use of multifoci sonications in MR-guided high-intensity focused ultrasound mediated mild hyperthermia. Med. Phys. 2012, 40, 013301. [Google Scholar] [CrossRef]
- Fjield, T.; Fan, X.; Hynynen, K. A parametric study of the concentric-ring transducer design for MRI guided ultrasound surgery. J. Acoust. Soc. Am. 1996, 100, 1220–1230. [Google Scholar] [CrossRef]
- Tillander, M.; Hokland, S.; Koskela, J.; Dam, H.; Andersen, N.P.; Pedersen, M.; Tanderup, K.; Ylihautala, M.; Köhler, M. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med. Phys. 2016, 43, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M. Ultrasound Image Guidance and Therapy with Random Phased Arrays. Ph.D. Thesis, Imperial College London, London, UK, 2019. [Google Scholar]


| Tissue | Density (kg/m3) | Attenuation (Np/m) | Sound Speed (m/s) | Thermal Conductivity (W/m/°C) | Specific Heat (J/kg/°C) | Perfusion Rate (kg/m3/s) |
|---|---|---|---|---|---|---|
| Skin | 1109 | 21.16 | 1624 | 0.37 | 3391 | 2.05 |
| Tumor | 1090 | 8.75 | 1588.4 | 0.49 | 3421 | 0.35 |
| Bone | 1908 | 185.4 | 2770.3 | 0.32 | 1313 | 0.3 |
| Soft tissue | 1090 | 7 | 1588.4 | 0.49 | 3421 | 0.7 |
| Fat | 911 | 4.35 | 1440.2 | 0.21 | 2348 | 0.52 |
| Trachea | 1080 | 0.44 | 1639.6 | 0.49 | 3568 | 0.66 |
| Model | Tumor Volume (cm3) | Tumor Dimensions (cm) | Depth of Tumor Margin from Skin (cm) | Minimum Distance of Tumor Margin from Trachea (cm) |
|---|---|---|---|---|
| 1 | 22.6 | 5.0 × 3.0 × 2.7 | 2 | 3.4 |
| 2 | 0.78 | 1.4 × 1.1 × 0.9 | 5.4 | 0.5 |
| 3 | 43.8 | 4.0 | 1.7 | 2.6 |
| Model | No. of Foci | Position of Foci (x, y, z) mm | Applied Acoustic Power (W) | I0 (W/cm2) | Peak T (°C) | V43, V41, V40 (cm3) | Dmax (43, 41, 40 °C) (mm) | Dmin (43, 41, 40 °C) (mm) | L/D Ratio (43, 41, 40 °C) | PHV > 40 °C (%) | T10,T50, T90 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | (±5, ±5, 130) | 1.064 | 0.018 | 45 | 2.61 8.9 15 | 42 49 52 | 20 15 12 | 1.2 1.36 1.42 | 68 | 43.05, 41.1, 40.2 |
| 2 | 1 | (0, 0, 130) | 1.32 | 0.014 | 45 | 0.1 0.35 0.89 | 55 57 58 | 46 42 39 | 1.8 1.87 1.82 | 100 | 41.5, 39.6, 38.9 |
| 3 | 4 | (±5, ±5, 130) | 1.34 | 0.0142 | 45 | 4.36 13.4 23 | 46 51 55 | 18 13 10 | 1.62 1.6 1.43 | 52 | 43.3, 42.2, 40.8 |
| 3 | 8 | (±5, 0, 130), (0, ±5, 130) (±10, ±10, 130) | 9.56 | 0.097 | 45 | 8.32 27 48 | 42 52 59 | 15 10 7 | 1.12 1.23 1.24 | 100 | 43, 40.5, 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Uddin, I.; Dickinson, R.; Diederich, C.J. Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer. Bioengineering 2025, 12, 14. https://doi.org/10.3390/bioengineering12010014
Zubair M, Uddin I, Dickinson R, Diederich CJ. Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer. Bioengineering. 2025; 12(1):14. https://doi.org/10.3390/bioengineering12010014
Chicago/Turabian StyleZubair, Muhammad, Imad Uddin, Robert Dickinson, and Chris J. Diederich. 2025. "Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer" Bioengineering 12, no. 1: 14. https://doi.org/10.3390/bioengineering12010014
APA StyleZubair, M., Uddin, I., Dickinson, R., & Diederich, C. J. (2025). Delivering Volumetric Hyperthermia to Head and Neck Cancer Patient-Specific Models Using an Ultrasound Spherical Random Phased Array Transducer. Bioengineering, 12(1), 14. https://doi.org/10.3390/bioengineering12010014







