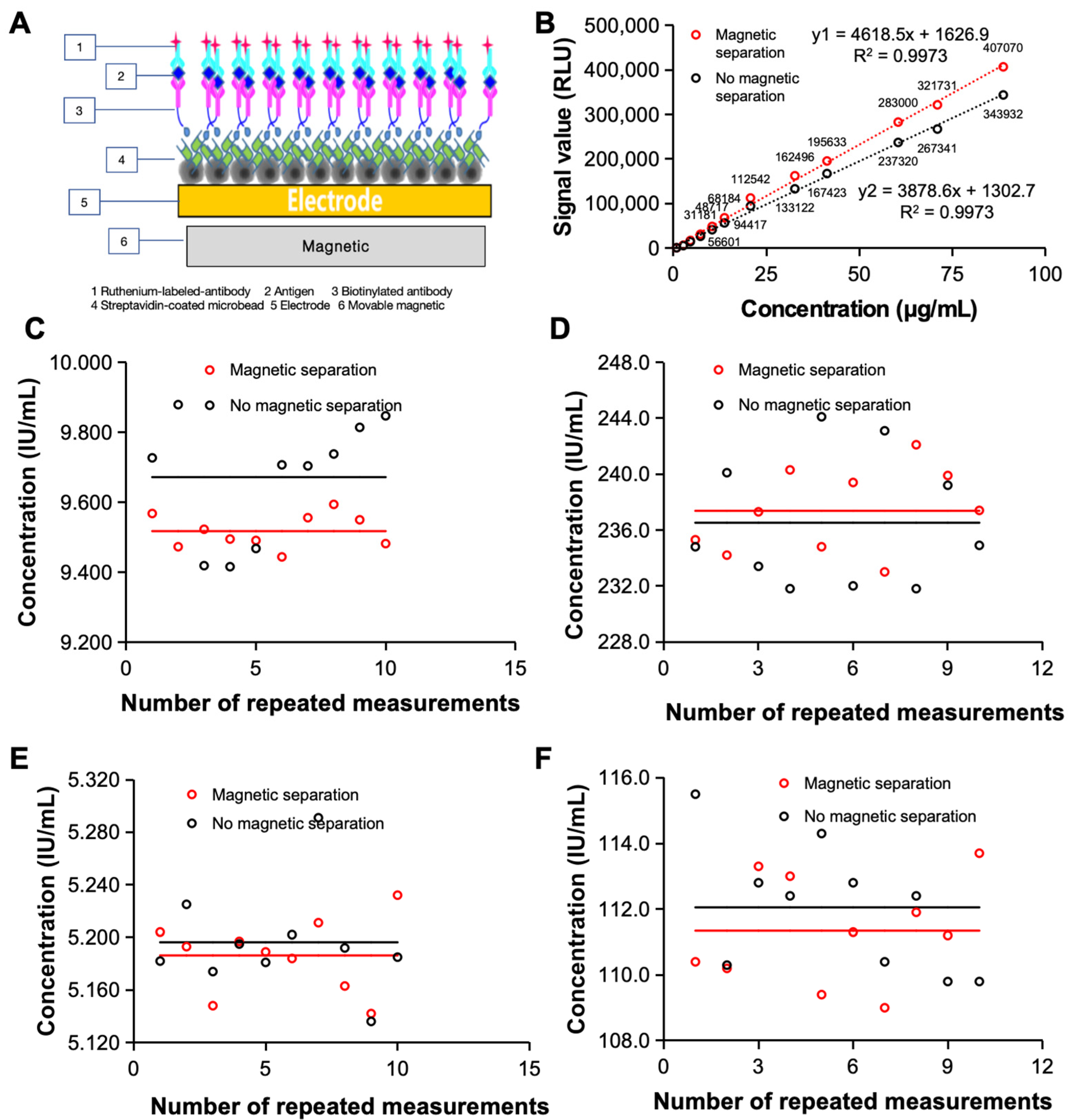
3.1. Magnetic Separation Module to Improve Detection Accuracy
Tested samples are often complex and contain the target substance and various other substances, which can influence the test process and reduce the accuracy of the test. To improve the possibility of clinical application of the electrochemiluminescence immunoassay system, it is important to further improve the accuracy of the assay to meet the clinical testing requirements. We therefore designed a magnetic separation module for the system based on permanent magnets and magnetic beads to separate interferents in the sample from the sample–reagent conjugate during analysis to achieve purification and enrichment of the target and eventually improve the precision of sample detection [
Figure 1A].
Magnetic beads are a major component of magnetic separation modules, and their design is important for achieving good magnetic separation with magnetic enrichment capabilities. The large surface area of the magnetic particles and the grooves on their surface increase the surface molecular loading (10 μg of biotinylated antibody per mg of streptavidin-modified magnetic bead surface). The high adsorption efficiency of synthetic magnetic particles therefore reduces the use of samples and increases the sensitivity of the assay, providing a basis for clinical applications.
The magnetic separation module is designed to separate the target material from the interfering material and to remove the remaining free fluorescent markers from the reaction system, further improving the accuracy of the analysis system. The introduction of a magnetic separation module can significantly improve the detection capability of the electrochemiluminescence immunoassay system. As shown in
Figure 1B, the standard curve of the FDP revealed that when the magnetic separation module was added, the detection signal value of the electrochemiluminescence immunoassay system was significantly greater than that of the non-magnetic separation module. The magnetic separation module can obtain higher signal values in both low- and high-concentration samples, which lays a foundation for the detection of low-abundance clinical samples.
In addition, the introduction of the magnetic separation module improved the detection repeatability of the electrochemiluminescence immunoassay system [
Figure 1C,D]. We first repeated 10 tests on AFP quality control samples with high and low concentrations and found that the CVs obtained by the electrochemiluminescence system with magnetic separation were 0.5% and 1.3%, respectively, after 10 repeated measurements of low-concentration samples. Both percentages were significantly greater than those without magnetic separation (1.8% and 2.0%). To further demonstrate the improvement in the detection repeatability of the magnetic separation module, we carried out the same measurements with clinical samples and carried out 10 independent repeated measurements on the same clinical sample. The results are shown in
Figure 1E,F for both high-concentration clinical samples and low-concentration clinical samples; the CV values (low-concentration samples: 0.5%, high-concentration samples: 1.5%) were significantly lower than those of non-magnetic separation electrochemiluminescence immunoassay systems (low-concentration samples: 0.8%, high-concentration samples: 1.7%).
3.2. A Compensation System for Current Output Photomultiplier Tubes to Improve Detection Sensitivity
Photomultiplier tubes are common detectors used in chemiluminescence signal detection techniques. Different chemiluminescence techniques differ in wavelength and signal strength due to the luminescent substances used, so photomultiplier tubes are selected for different signals. Typically, electrochemiluminescence immunoassay systems have a logarithmic amplifier to broaden the detection range of the circuit and ensure a comparable signal-to-noise ratio for data conversion over a large dynamic range, with the anode load resistance (RL) in the negative high-voltage system of the photomultiplier. However, this approach is limited by logarithmic curve compression: for inputs smaller than a certain value, the circuit outputs are negatively saturated. As a result, deviations in the interindividual consistency of the compression curve of the logarithmic amplifier circuit exist, which affects the results of small-signal measurements. We therefore optimized the design of a compensation system for current output photomultipliers to complement the variable signal with current to stabilize the input and ensure small signal detection capability.
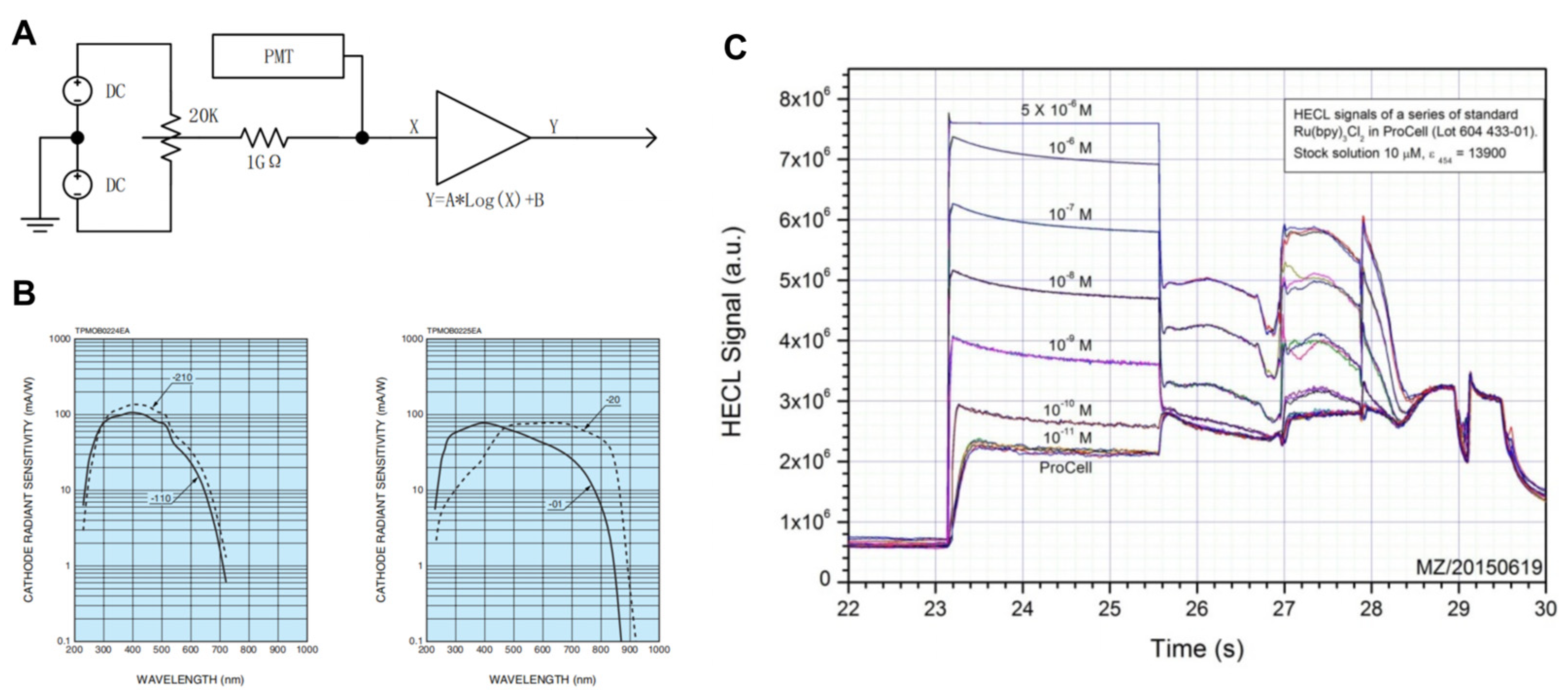
The compensation circuit is a variable constant current source, and the constant current circuit is shown in
Figure 2A. It consists of an adjustable voltage source and a high-value resistor. The adjustable voltage source included a reference voltage source and a potentiometer. The adjustable source is generated by dividing the voltage of the potentiometer against the reference supply. A constant current source has a bipolar compensation capability and provides a current in the same direction as the photomultiplier current when the photomultiplier dark current is less than the minimum input for the amplifier circuit design. When the photomultiplier dark current is greater than the minimum current of the amplification circuit, the constant current source provides a compensation current in the opposite direction to the photomultiplier current. The compensation current provided by the compensation circuit is superimposed on the photomultiplier quiescent current so that the quiescent current input to the logarithmic amplifier circuit is constant for the purpose of selecting a suitable operating range for the logarithmic amplifier.
In the electrochemiluminescence immunoassay system used in this study, the measured luminescence area of the electroluminescence measuring cell was approximately 4 × 5 cm, which was determined by the distribution of magnetic beads and the opposite electrode/working electrode region. In theory, the closer the luminescent body, the greater the light collection efficiency, and the larger the photosensitive area of the photomultiplier tube, the greater the efficiency. Owing to the constraints of device cost and volume, H10721 (HAMAMATSU PHOTONICS K.K., Shizuoka, Japan) was finally selected as the photomultiplier tube used in the system. In addition, this type of photomultiplier tube has a multibase photocathode in the near-infrared region and has high sensitivity. H10721 has four kinds of current output photomultiplier tubes, which are suitable for the detection of different wavelengths. In the electrochemiluminescence immunoassay system, the central wavelength of the tripyridine ruthenium luminescence used is 620 nm, so H10721-20 (HAMAMATSU PHOTONICS K.K., Shizuoka, Japan) and H10721-01 (HAMAMATSU PHOTONICS K.K., Shizuoka, Japan) are more suitable for this electrochemical luminescence system. At the same time, the sensitivity of H10721-20 is high, and the sensitivity curve is flat near the central wavelength. However, the dark current of H10721-20 is greater than that of H10721-01, and the range is relatively large [
Figure 2B]. The electroluminescence is sensitive to low values, the dark current ratio is large and fluctuations affect the low SNR. Since the photomultiplier tube is a current output, the H10721-01 photomultiplier tube can be used directly in electrochemical luminescence immunoassays without the need for filters. In the tripyridine ruthenium–tripropylamine homogeneous solution test, the linear range of the system covered 10
5, and saturation occurred when the concentration was greater than 1 μM. The maximum output of the 0.5 μM photomultiplier was 65.14 μA, and the output of the 0.01 nM photomultiplier was 1.3 nA, which was close to the dark current of the photomultiplier. The optimal interval is 0.1 nM~0.5 μM, and the integral signal of the luminous interval is approximately 1000 to 5,000,000 [
Figure 2C].
Therefore, for the selection and testing of photomultiplier tubes, H10721-01 was selected as the photomultiplier device for the electrochemiluminescence immunoassay system. The design of the compensation circuit greatly improved the reception and detection of low detection signals, increasing the detection range of the electrochemiluminescence immunoassay system and providing it with the possibility to detect samples with low target substances.
3.3. Customized System Timing Module for High Test Throughput
After each component of the electrochemiluminescence immunoassay system was optimized, the properties of each component were maximized. Moreover, in order to achieve high-throughput results, the electrochemiluminescence immunoassay system requires optimization and precise control of the various steps in the analysis process to reduce various conflicts. The timing system module is a prerequisite for the orderly collaboration of the various parts of the analysis process, helps reduce conflicts between different actions, ensures the accuracy of the respective execution of parallel structures and is important for achieving high-throughput results.
Different scheduling algorithms are often used for different analytic systems (
Table 1), and common scheduling algorithms include first-come, first-served algorithms [
9,
10], the shortest job algorithm [
11,
12,
13], the shortest remaining time first algorithm [
14,
15,
16] and the time-slice rotation algorithm [
17]. The time-slice rotation algorithm is a system that allocates time slices to allocate resources to corresponding tasks in a fixed-length sequence. When the execution time of a task is complete, the system terminates the execution of that task and allocates the resources to the next task until all tasks have been executed. The time-slice rotation algorithm-based timing system therefore has good potential for application in multistep, multicomponent, multisystem synergistic electrochemiluminescence immunoassay systems.
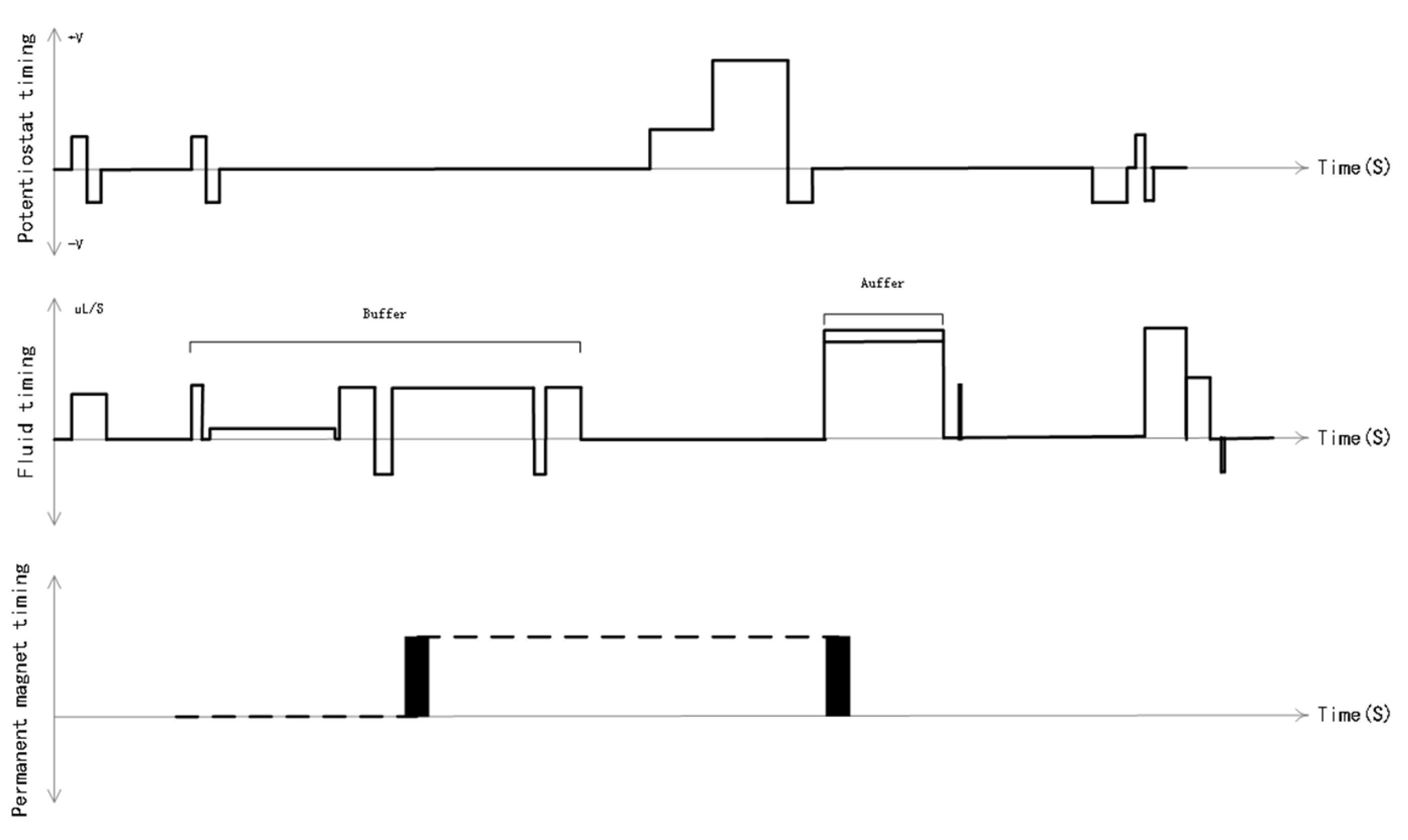
On this basis, we used a time-slice rotation algorithm to customize the timing system for the electrochemiluminescence immunoassay system, effectively combining all parts of the system to facilitate the generation of high-throughput results. The process of electrochemiluminescence immunoassay analysis requires the synergy of multiple systems, such as potential generation, fluid control, permanent magnet control and analysis reagent switching. The accuracy of the timing and the rationality of the parameters and of each system are highly demanding. On the basis of the requirements of the electrochemiluminescence analysis task, we designed constant potential timing, fluid timing and permanent magnet timing in concert [
Figure 3]. The design and customization of the timing of the system provides pipeline control of the various components of the electrochemiluminescence immunoassay system in accordance with the time rhythm, increasing the detection speed of the instrument to a maximum of 300 tests/hour, achieving a maximum unattended testing volume of 5.5 h and a maximum of 1575 samples, and ensuring a high degree of consistency between the luminescence moment and the electrode state, thus achieving a high-throughput result output. This enables high-throughput results to be output to meet the requirements of clinical applications.
3.4. Analytical Performance of the Electrochemiluminescence Immunoassay System
In this study, we validated the detection performance of the developed electroluminescence immunoassay system by detecting these three disease markers.
To evaluate the potential of an optimally designed high-throughput, high-sensitivity electrochemiluminescence immunoassay system for clinical application, we investigated the linearity, detection limits, signal-to-noise ratio, precision and accuracy of the system. First, we evaluated the linear relationship of the electrochemiluminescence immunoassay system. As shown in
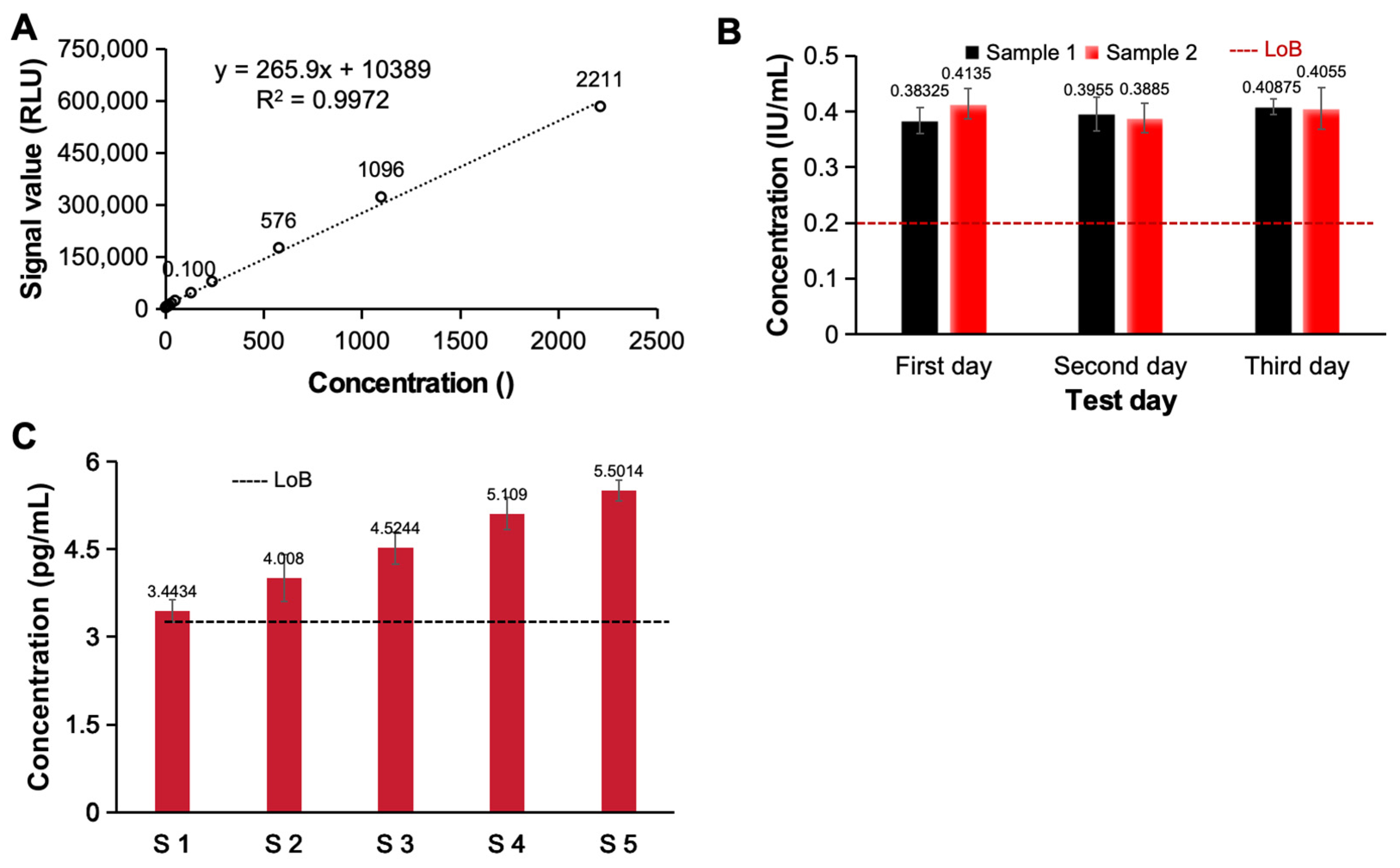
Figure 4A, as the concentration of the detected ACTH increased, the signal generated by the system also continuously increased. Even if the concentration exceeded 105 orders of magnitude, there was still an excellent linear relationship between the detected signal and the concentration (y = 265.9x + 10389, R
2 = 0.9972), so this analysis system has excellent potential for detecting samples with different concentrations. We subsequently analyzed and evaluated the detection limits of the constructed electrochemiluminescence immunoassay system. We tested two detection limit samples (four repetitions for each sample) on 3 test days and statistically analyzed the test results. As shown in
Figure 4B, ≥LoB (0.2 IU/mL) accounted for ≥87% of the total number of samples at the detection limit. Furthermore, five low-value samples with approximate detection limits (LoD ≤ 5 pg/mL) were tested, each sample was tested five times and the detection limits of the analysis system were evaluated. The results show that the number of test results below the blank limit (LoB ≤ 3 pg/mL) was less than or equal to 3, which meets the test requirements [
Figure 4C]. Based on the detection limits of the above two different analytical systems, the constructed electrochemiluminescence immunoassay system has a good detection limit and has the ability to detect low-abundance clinical samples.
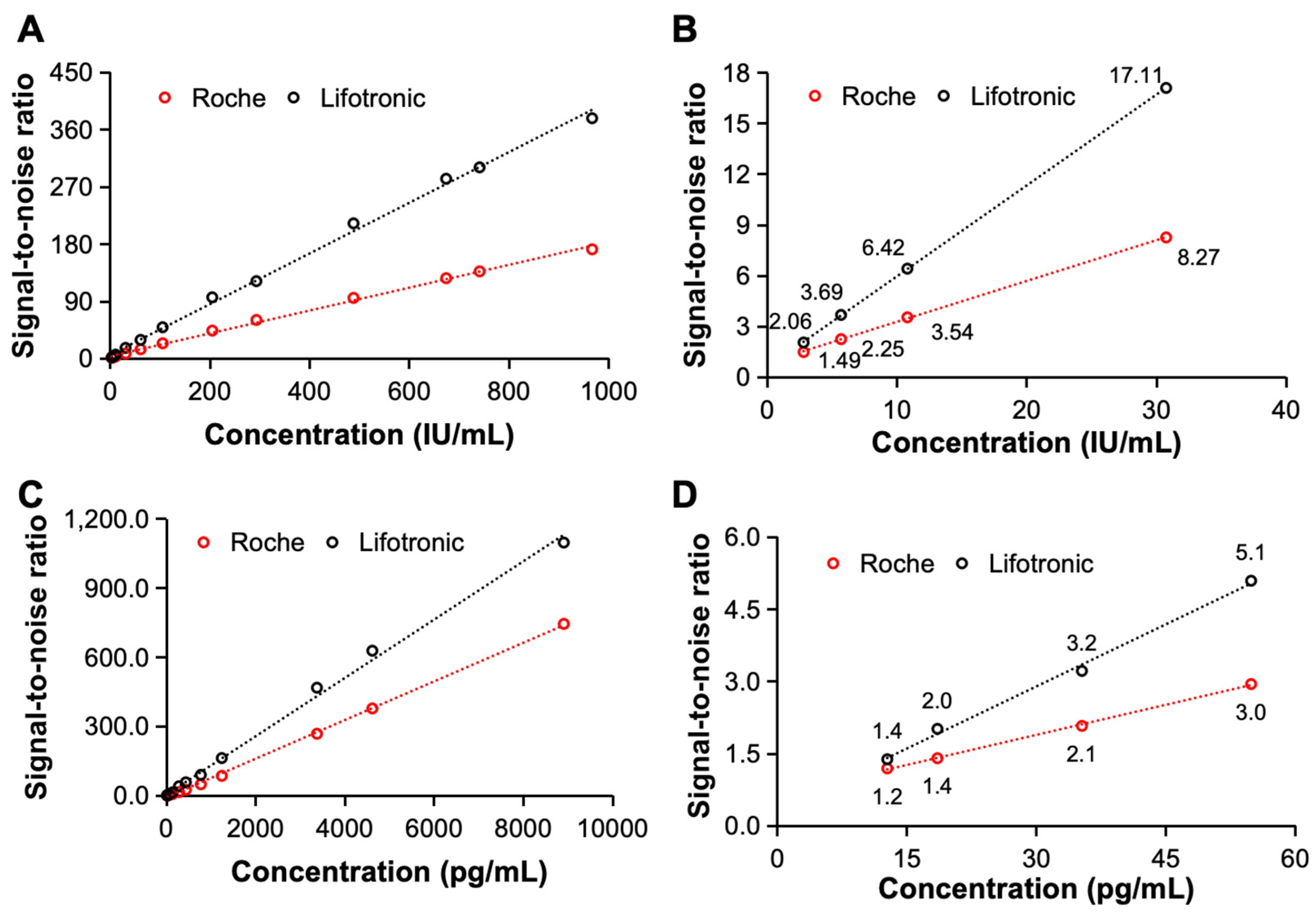
The signal-to-noise ratio is another important index for evaluating the detection capability of an electrochemiluminescence immunoassay system and reflects the strength of the received useful signal and the received interference signal. In this study, the signal-to-noise ratios of the constructed electrochemiluminescence immunoassay system for the detection of clinical samples containing AFP and hs-cTnT-STAT were compared with the SNR of the international leading international electrochemiluminescence immunoassay instrument of Roche. As shown in
Figure 5, the constructed system has a better signal-to-noise ratio when detecting both AFP and hs-cTnT-STAT, and there is a significant difference even when detecting low-concentration samples. Therefore, when detecting AFP and hs-cTnT-STAT, the constructed electrochemiluminescence immunoassay system has an excellent signal-to-noise ratio, good sensitivity and excellent detection performance.
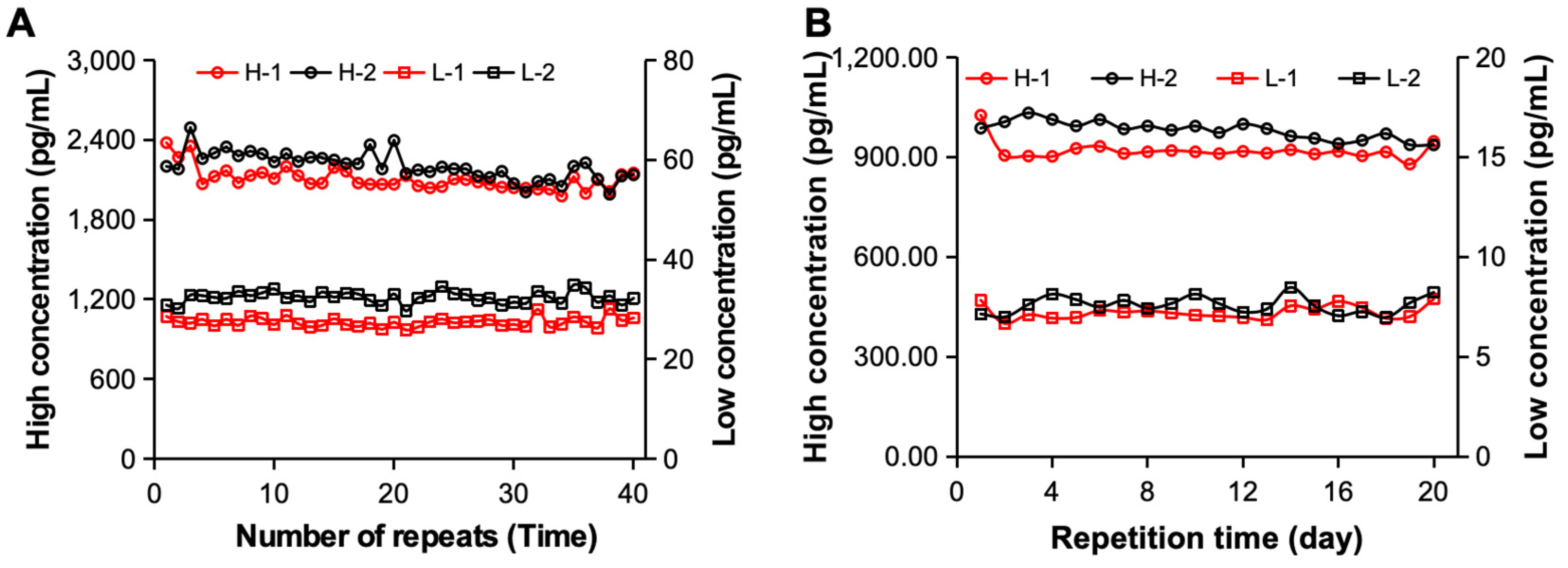
To evaluate whether the constructed electrochemiluminescence immunoassay system can be used for the detection of clinical samples, we further analyzed its detection precision. First, the samples containing high-concentration and low-concentration hs-cTnT STAT substances were tested independently 40 times via two sets of electrochemiluminescence immunoassay systems [
Figure 6A]. The statistical results revealed that when standard substance H was detected, the CV values of the two systems were 4.02% and 4.70%, respectively. The CVs of standard substance L were 3.46% and 3.62%, respectively, and both were <5%, meeting the requirements. Furthermore, we examined different clinical samples from 20 consecutive days to analyze their precision over time. As shown in
Figure 6B, both sets of analysis systems achieved excellent detection precision for different samples during the 20-day detection period. When clinical sample H was tested, its CV was 4.67% and 3.13%, and when clinical sample L was tested, its CV was 4.92% and 2.86%, respectively. Therefore, the excellent detection precision of the constructed electrochemiluminescence immunoassay system was determined by evaluating the reproducibility of the measurements.
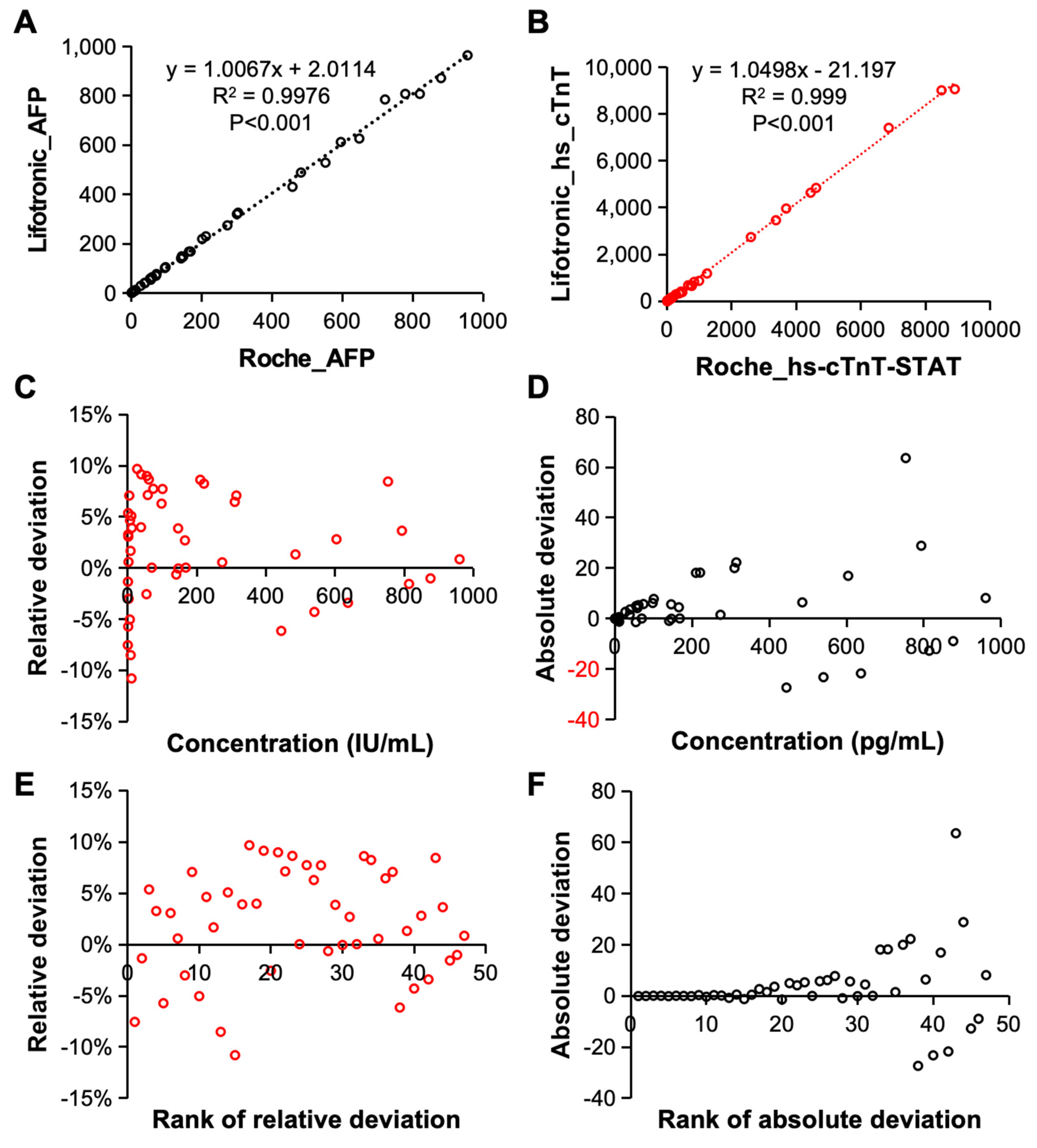
Finally, the detection accuracy of the constructed electrochemiluminescence immunoassay systems was evaluated. The detection results for AFP [
Figure 7A] and hs-cTnT-STAT [
Figure 7B] which were obtained by the system were compared with those of the Roche electrochemiluminescence immunoassay instrument. First of all, when the analysis system detected AFP and hs-cTnT-STAT, its detection results were in good agreement with those of the international Roche system. The correlations between the detection results of the two systems were 0.9976 and 0.999; thus, there was no significant difference between the detection results of the two systems. Therefore, the constructed electrochemiluminescence immunoassay system has excellent detection accuracy and can reach the leading international level. The relative deviation, absolute deviation, relative deviation ranking and absolute deviation ranking of the AFP detected by the system were analyzed. As shown in
Figure 7C–F, both the relative deviation and the absolute deviation reflect the detection accuracy of the analysis system.
In summary, the high-throughput and high-sensitivity electrochemiluminescence immunoassay system shows superior performance in terms of the following basic properties: online relationship, detection limit, signal-to-noise ratio, precision and accuracy. Therefore, the system is expected to become an alternative method for clinical analysis, achieving high throughput and high sensitivity, and provide a reference for clinical disease diagnosis.