In Vivo Testing of a Second-Generation Prototype Accessory for Single Transapical Left Ventricular Assist Device Implantation
Abstract
1. Introduction

2. Materials and Methods
2.1. Device Design
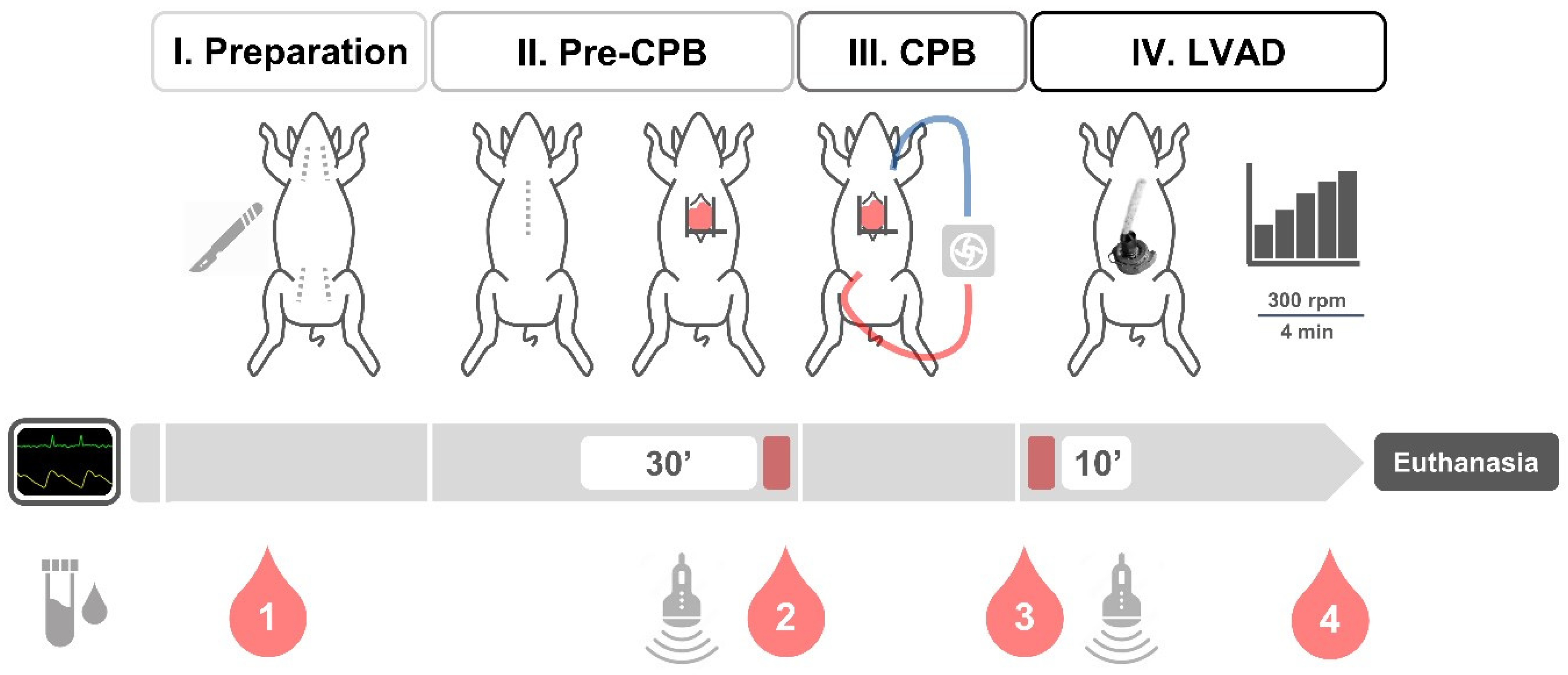
2.2. Animal Testing
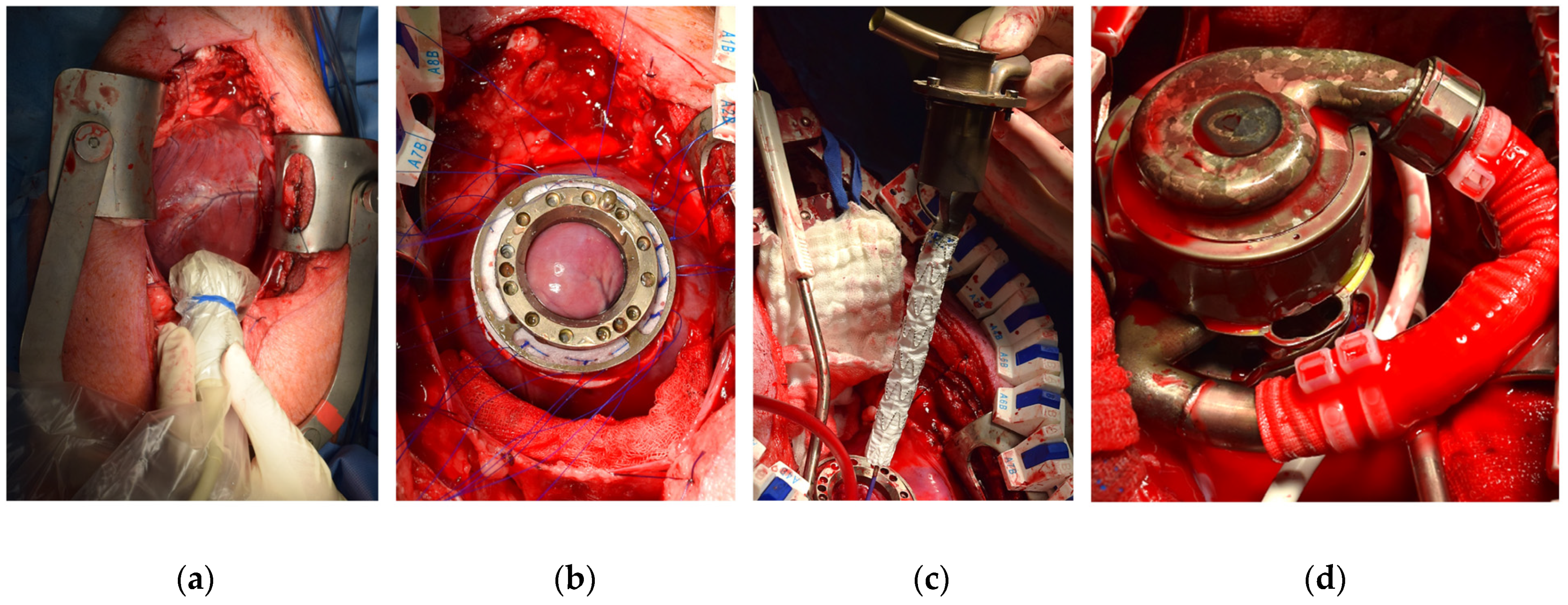
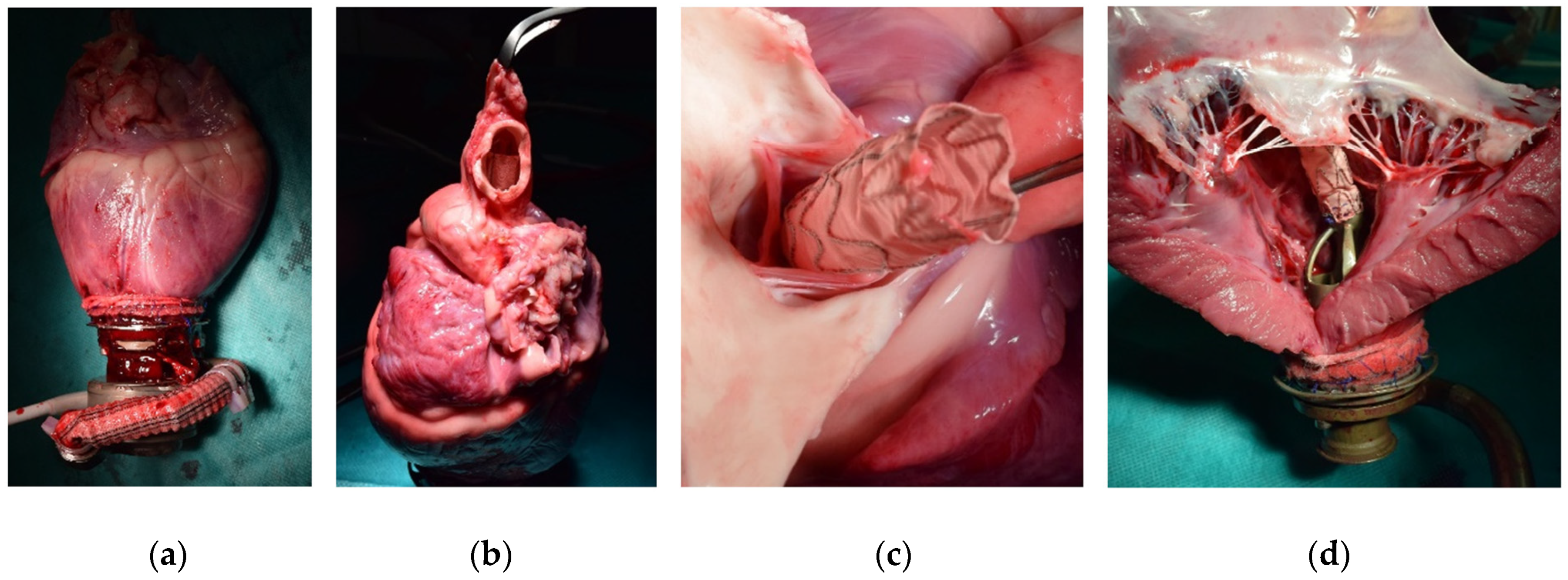
2.3. Surgical Procedure
2.4. Statistical Analysis
3. Results
3.1. Surgical Procedure
3.2. Hemodynamic Monitoring
3.3. Echocardiography
3.4. Hematology and Blood Chemistry
3.5. Post-Mortem Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Abbott HeartMate 3TM Left Ventricular Assist System: Instructions for Use. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160054C.pdf (accessed on 15 February 2023).
- Mehra, M.R. The Burden of Haemocompatibility with Left Ventricular Assist Systems: A Complex Weave. Eur. Heart J. 2019, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Jorde, U.P.; Saeed, O.; Koehl, D.; Morris, A.A.; Wood, K.L.; Meyer, D.M.; Cantor, R.; Jacobs, J.P.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann. Thorac. Surg. 2024, 117, 33–44. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Schmitto, J.D.; Krabatsch, T.; Damme, L.; Netuka, I. Less Invasive HeartMate 3 Left Ventricular Assist Device Implantation. J. Thorac. Dis. 2018, 10, S1692–S1695. [Google Scholar] [CrossRef]
- Karahan, M.; Kervan, Ü.; Kocabeyoğlu, S.S.; Sert, D.E.; Akdi, M.; Yılmaz, A.; Koçak, C.; Çatav, Z. Off-Pump Implantation of Left Ventricular Assist Device via Minimally Invasive Left Thoracotomy: Our Single-Center Experience. Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 37–44. [Google Scholar] [CrossRef]
- Centofanti, P.; La Torre, M.; Attisani, M.; Sansone, F.; Rinaldi, M. Rapid Pacing for the Off-Pump Insertion of the Jarvik Left Ventricular Assist Device. Ann. Thorac. Surg. 2011, 92, 1536–1538. [Google Scholar] [CrossRef]
- Potapov, E.V.; Kukucka, M.; Falk, V.; Krabatsch, T. Off-Pump Implantation of the HeartMate 3 Left Ventricular Assist Device through a Bilateral Thoracotomy Approach. J. Thorac. Cardiovasc. Surg. 2017, 153, 104–105. [Google Scholar] [CrossRef]
- Saeed, D.; Sixt, S.; Albert, A.; Lichtenberg, A. Minimally Invasive Off-Pump Implantation of HeartMate 3 Left Ventricular Assist Device. J. Thorac. Cardiovasc. Surg. 2016, 152, 1446–1447. [Google Scholar] [CrossRef]
- Kolff, W.J. Transapical Left Ventricular Bypass. Arch. Surg. 1971, 103, 656. [Google Scholar] [CrossRef]
- Kolff, W.J.; Norman, J.C. Transapical Left Ventricular Bypass. Chest 1971, 60, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Giridharan, G.A.; Tamez, D.; LaRose, J.; Sobieski, M.A.; Sherwood, L.; Koenig, S.C. Transapical Miniaturized Ventricular Assist Device: Design and Initial Testing. J. Thorac. Cardiovasc. Surg. 2011, 142, 668–674. [Google Scholar] [CrossRef][Green Version]
- Tamez, D.; LaRose, J.A.; Shambaugh, C.; Chorpenning, K.; Soucy, K.G.; Sobieski, M.A.; Sherwood, L.; Giridharan, G.A.; Monreal, G.; Koenig, S.C.; et al. Early Feasibility Testing and Engineering Development of the Transapical Approach for the HeartWare MVAD Ventricular Assist System. ASAIO J. 2014, 60, 170–177. [Google Scholar] [CrossRef][Green Version]
- Yamazaki, K.; Umezu, M.; Koyanagi, H.; Outa, E.; Ogino, S.; Otake, Y.; Shiozaki, H.; Fujimoto, T.; Tagusari, O.; Kitamura, M. Development of a Miniature Intraventricular Axial Flow Blood Pump. ASAIO J. Am. Soc. Artif. Intern. Organs 1993, 39, M224–M230. [Google Scholar]
- Ali, J.; Catarino, P.; Abu-Omar, Y. Transcatheter Aortic Valve Implantation in Patients with a Left Ventricular Assist Device: A Word of Caution. Eur. J. Cardiothorac. Surg. 2020, 58, 1309–1310. [Google Scholar] [CrossRef]
- Singh, R.; Chandel, A.; Paras, J.; Lee, T.B.; Tang, D.G.; Shah, P.; Desai, M. Transapical Cannulation with a Dual Lumen Cannula for Mechanical Circulatory Support in Cardiogenic Shock. ASAIO J. 2022, 68, e215–e219. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Roberts, S.; Lampert, B.C.; Whitson, B.A. Temporary Extracorporeal Left Ventricular Support with Transapical ProtekDuo Cannula. JTCVS Tech. 2021, 5, 76–79. [Google Scholar] [CrossRef]
- Belani, K.; Saikus, C.E.; Schroder, J.N.; Klinger, R.Y. Transapical ProtekDuo Rapid Deployment Cannula as Temporary Left Ventricular Assist Device in a Jehovah’s Witness Patient. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3735–3742. [Google Scholar] [CrossRef]
- Capelli, J.; Emling, J.; Edwards, A.; Babu, A. Direct Apical Cannulation with Protek Duo Rapid Deployment Cannula via Mini Thoracotomy for Ambulatory Venoarterial-Extracorporeal Membrane Oxygenation. ASAIO J. 2024, 70, 565–569. [Google Scholar] [CrossRef]
- Schibilsky, D.; Scheumann, J.; Koester, P.J.; Demir, H.; Rausch, M.; Puiu, P.; Benk, C.; Maier, S.; Neudorf, S.; Diel, P.; et al. Development of a Novel Adapter to Enable Less-Invasive Left Ventricular Assist Device Implantation via the Left Ventricular Apex. ASAIO J. 2022, 68, e142–e144. [Google Scholar] [CrossRef] [PubMed]
- Meissner, F.; Eichelkraut, D.; Schimmel, M.; Maier, S.; Vestner, H.; Schoen, M.; Czerny, M.; Bothe, W. Impact of an Accessory for Left Ventricular Assist Devices on Device Flow and Pressure Head In Vitro. Bioengineering 2023, 10, 486. [Google Scholar] [CrossRef]
- Meissner, F.; Eichelkraut, D.; Schimmel, M.; Buechsel, M.; Schoen, M.; Vestner, H.; Galbas, M.; Czerny, M.; Bothe, W. Impact of a Novel Accessory for Left Ventricular Assist Devices on Hemolysis and Degradation of Von Willebrand Factor In Vitro. J. Heart Lung Transplant. 2024, 43, S496–S497. [Google Scholar] [CrossRef]
- Dassault Systèmes. SolidWorks, Version 2021 SP3.0; Dassault Systèmes: Vélizy-Villacoublay, France, 2021.
- Osypka, A.; Meissner, F.; Ozturk, D.; Windisch, R.; Vestner, H.; Galbas, M.C.; Czerny, M.; Bothe, W. In Silico Analysis of Pressure Distribution and Flow Profiles Across a Novel Left Ventricular Assist Device Accessory. In Proceedings of the 38th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 6 October 2023. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Crick, S.J.; Sheppard, M.N.; Ho, S.Y.; Gebstein, L.; Anderson, R.H. Anatomy of the Pig Heart: Comparisons with Normal Human Cardiac Structure. J. Anat. 1998, 193 Pt 1, 105–119. [Google Scholar] [CrossRef]
- Galbas, M.C.; Meissner, F.; Asmussen, A.; Straky, H.C.; Schimmel, M.; Reuter, J.; Grundmann, S.; Czerny, M.; Bothe, W. A Systematic Methodology for Epicardial and Epiaortic Echocardiography in Swine Research Models. Health Sci. Rep. 2024, 7, e1777. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 2021.9.0.351; R Core Team: Vienna, Austria, 2021.
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T.; et al. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; Version 3.4.2. Available online: https://cran.r-project.org/web/packages=ggplot2 (accessed on 13 August 2024).
- Lumley, T. Leaps: Regression Subset Selection; Version 3.1. Available online: https://cran.r-project.org/web/packages=leaps (accessed on 13 August 2024).
- Galbas, M.C.; Straky, H.C.; Meissner, F.; Reuter, J.; Schimmel, M.; Grundmann, S.; Czerny, M.; Bothe, W. Cardiac Dimensions and Hemodynamics in Healthy Juvenile Landrace Swine. Cardiovasc. Ultrasound 2024, 22, 3. [Google Scholar] [CrossRef]
- Galbas, M.; Meissner, F.; Straky, H.; Reuter, J.; Schimmel, M.; Czerny, M.; Bothe, W. Echocardiographic Assessment of a Novel Blood-Guiding Accessory for Left Ventricular Assist Devices in Swine. In Proceedings of the 38th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 6 October 2023. [Google Scholar]
- Schima, H.; Zrunek, P.; Stoiber, M.; LaRose, J.; Shambaugh, C.; Tamez, D.; Deckert, Z.; Plasenzotti, R.; Bergmeister, H.; Wieselthaler, G. Extended in Vivo Evaluation of a Miniaturized Axial Flow Pump with a Novel Inflow Cannula for a Minimal Invasive Implantation Procedure. J. Heart Lung Transplant. 2014, 33, 422–428. [Google Scholar] [CrossRef]
- Abiomed Home Page. Available online: https://www.abiomed.com/en-us (accessed on 9 July 2024).
- Higashi, H.; Nishimura, T.; Aono, J.; Sakaue, T.; Kurata, M.; Izutani, H.; Yamaguchi, O. Pathological Evidence of Native Aortic Valve Injury After Impella Support. Circ. Heart Fail. 2021, 14, e007571. [Google Scholar] [CrossRef]
- Ueda, K.; Yoshitani, K.; Hosotani, S.; Hayashi, H.; Fukushima, S.; Ohnishi, Y. Aortic Valve Insufficiency after Impella Device Insertion That Required Aortic Valve Replacement after Heart Mate III Left Ventricular Assist Device Implantation: A Case Report. J. Surg. Case Rep. 2021, 2021, rjab420. [Google Scholar] [CrossRef] [PubMed]
- Olsthoorn, J.R.; Goossens, E.A.C.; Lam, K.; Tonino, P.A.L.; Van Dantzig, J.-M. Aortic Valve Insufficiency as a Late Complication After Impella Device Implantation. JACC Cardiovasc. Interv. 2022, 15, e91–e93. [Google Scholar] [CrossRef] [PubMed]
- Use of the Impella BTRTM in Patients with Heart Failure: An Early Feasibility Study (BTR EFS). Available online: https://clinicaltrials.gov/study/NCT05291884 (accessed on 9 July 2024).
- World’s First Patient Implanted with Impella BTR Minimally Invasive Heart Pump. Available online: https://www.abiomed.com/en-us/about-us-/news-and-media/press-releases/worlds-first-patient-implanted-with-impella-btr-minimally-invasive-heart-pump (accessed on 9 July 2024).
- Abiomed Recalls All Impella Left Sided Blood Pumps for Risk of Motor Damage After Contact with Transcatheter Aortic Valve Replacement (TAVR) Stent. Available online: https://www.fda.gov/medical-devices/medical-device-recalls/abiomed-recalls-all-impella-left-sided-blood-pumps-risk-motor-damage-after-contact-transcatheter (accessed on 13 August 2024).
- Barandon, L.; Nubret, K.; Haddadi, M.; Garrigue, S. In-Vivo Assessment of A Novel Ventricular Systole-Synchronized, Intraventricular Propelling, Left Ventricular Assist Device For Advanced Heart Failure. In Proceedings of the 66th Annual Conference of the American Society for Artificial Internal Organs, New Orleans, LA, USA, 10 June 2021. [Google Scholar]
- FineHeart Obtains Czech Health Authority Approval to Commence First in Human Clinical Study. Available online: https://fineheart.fr/2023/10/03/fineheart-obtains-czech-health-authority-approval-to-commence-first-in-human-clinical-study/ (accessed on 10 March 2024).
- Meissner, F.; Szvetics, S.; Galbas, M.C.; Russe, M.; Schibilsky, D.; Kaier, K.; Czerny, M.; Bothe, W. Longitudinal Cardiac Dimensions in Patients Undergoing LVAD Implantation. Artif. Organs 2024, 48, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kaul, P. Sternal Reconstruction after Post-Sternotomy Mediastinitis. J. Cardiothorac. Surg. 2017, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Pilz, P.M.; Ward, J.E.; Chang, W.-T.; Kiss, A.; Bateh, E.; Jha, A.; Fisch, S.; Podesser, B.K.; Liao, R. Large and Small Animal Models of Heart Failure with Reduced Ejection Fraction. Circ. Res. 2022, 130, 1888–1905. [Google Scholar] [CrossRef] [PubMed]


| Pre-CPB | LVAD | MD | p | |
|---|---|---|---|---|
| HR (bpm) | 101.0 ± 29.4 | 126.0 ± 29.2 | 25.0 | 0.032 |
| SBP (mmHg) | 98.9 ± 19.8 | 96.4 ± 35.2 | −2.5 | 0.940 |
| DBP (mmHg) | 59.7 ± 15.8 | 35.0 ± 27.7 | −24.7 | 0.018 |
| MBP (mmHg) | 72.9 ± 14.7 | 55.8 ± 25.5 | −17.0 | 0.045 |
| sPAP (mmHg) | 25.9 ± 5.6 | 31.0 ± 13.0 | 5.2 | 0.097 |
| dPAP (mmHg) | 14.3 ± 3.9 | 20.1 ± 8.8 | 5.8 | 0.063 |
| mPAP (mmHg) | 19.4 ± 4.1 | 25.4 ± 11.1 | 6.0 | 0.080 |
| CVP (mmHg) | 9.4 ± 3.4 | 9.9 ± 4.3 | 0.5 | 0.321 |
| CO (L/min) | 7.9 ± 2.3 | 5.5 ± 1.2 | −2.4 | 0.120 |
| BT (°C) | 36.6 ± 1.1 | 35.3 ± 0.8 | −1.2 | 0.008 |
| CCA flow (L/min) | 0.49 ± 0.17 | 0.30 ± 0.15 | −0.19 | 0.016 |
| Flow (L/min) | 3.11 ± 1.28 | |||
| Speed (rpm) | 5029 ± 892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meissner, F.; Galbas, M.C.; Straky, H.; Vestner, H.; Schoen, M.; Schimmel, M.; Reuter, J.; Buechsel, M.; Dinkelaker, J.; Cristina Schmitz, H.; et al. In Vivo Testing of a Second-Generation Prototype Accessory for Single Transapical Left Ventricular Assist Device Implantation. Bioengineering 2024, 11, 848. https://doi.org/10.3390/bioengineering11080848
Meissner F, Galbas MC, Straky H, Vestner H, Schoen M, Schimmel M, Reuter J, Buechsel M, Dinkelaker J, Cristina Schmitz H, et al. In Vivo Testing of a Second-Generation Prototype Accessory for Single Transapical Left Ventricular Assist Device Implantation. Bioengineering. 2024; 11(8):848. https://doi.org/10.3390/bioengineering11080848
Chicago/Turabian StyleMeissner, Florian, Michelle Costa Galbas, Hendrik Straky, Heiko Vestner, Manuela Schoen, Marius Schimmel, Johanna Reuter, Martin Buechsel, Johannes Dinkelaker, Heidi Cristina Schmitz, and et al. 2024. "In Vivo Testing of a Second-Generation Prototype Accessory for Single Transapical Left Ventricular Assist Device Implantation" Bioengineering 11, no. 8: 848. https://doi.org/10.3390/bioengineering11080848
APA StyleMeissner, F., Galbas, M. C., Straky, H., Vestner, H., Schoen, M., Schimmel, M., Reuter, J., Buechsel, M., Dinkelaker, J., Cristina Schmitz, H., Czerny, M., & Bothe, W. (2024). In Vivo Testing of a Second-Generation Prototype Accessory for Single Transapical Left Ventricular Assist Device Implantation. Bioengineering, 11(8), 848. https://doi.org/10.3390/bioengineering11080848







