Abstract
The regenerative capacity of the peripheral nervous system is limited, and peripheral nerve injuries often result in incomplete healing and poor outcomes even after repair. Transection injuries that induce a nerve gap necessitate microsurgical intervention; however, even the current gold standard of repair, autologous nerve graft, frequently results in poor functional recovery. Several interventions have been developed to augment the surgical repair of peripheral nerves, and the application of functional biomaterials, local delivery of bioactive substances, electrical stimulation, and allografts are among the most promising approaches to enhance innate healing across a nerve gap. Biocompatible polymers with optimized degradation rates, topographic features, and other functions provided by their composition have been incorporated into novel nerve conduits (NCs). Many of these allow for the delivery of drugs, neurotrophic factors, and whole cells locally to nerve repair sites, mitigating adverse effects that limit their systemic use. The electrical stimulation of repaired nerves in the perioperative period has shown benefits to healing and recovery in human trials, and novel biomaterials to enhance these effects show promise in preclinical models. The use of acellular nerve allografts (ANAs) circumvents the morbidity of donor nerve harvest necessitated by the use of autografts, and improvements in tissue-processing techniques may allow for more readily available and cost-effective options. Each of these interventions aid in neural regeneration after repair when applied independently, and their differing forms, benefits, and methods of application present ample opportunity for synergistic effects when applied in combination.
1. Introduction
In the opening paragraph of a review covering peripheral nerve repair published in 1944, Weiss included the following comment on arterial sleeve cuffing, an emerging technique trending toward clinical adoption at the time:
“But further application of the lessons thus learned gives promise of even more substantial improvements, and sleeve splicing may eventually be superseded by some other, more meritorious procedure incorporating its experiences. The emphasis lies more on the principle than on the current form of its application…”[1]
Relative to the technique described—namely, connecting severed nerve ends with donor infant aorta without suture—methods of repair for peripheral nerve injury (PNI) have advanced greatly, particularly concerning the available materials employed to guide axonal regeneration. Arterial sleeve cuffing is no longer applied in nerve repair, and the prediction made by Weiss appears true 80 years later, as the introduction and widespread adoption of biomaterials into clinical use and research has allowed for recent advancements and even greater potential for success in the future of nerve reconstruction after injury.
Unfortunately, the management of peripheral nervous system injuries remains challenging, with approximately one-third of patients with PNI exhibiting incomplete recovery and poor outcomes such as chronic pain, deficits in motor and sensory function, and muscle atrophy [2]. These outcomes are even worse in cases where a large gap exists between the proximal and distal stumps to be reconnected, and the use of an interposed graft or other nerve substitute is required to achieve a tension-free repair.
In mammalians, unlike in cold-blooded organisms, the nerves of the central nervous system (CNS) fail to regenerate after injury. In contrast, the nerves of the peripheral nervous system (PNS) retain their regenerative capacity, which mostly relies on the activity of the peripheral glial cells—the axon-ensheathing myelinating and nonmyelinating Schwann cells (SCs) [3].
Upon transection of a peripheral nerve, axons become disconnected from their cell bodies, triggering Wallerian degeneration distal to the injury. This begins with the breakdown of the myelin sheath through SC autophagy and macrophage recruitment, with subsequent denervation of the SCs associated with it. Once denervated, changes in SC gene expression lead to their transdifferentiation to a phenotype better equipped for supporting axonal growth, regeneration, and eventual reinnervation of target tissues [3,4,5]. Once transdifferentiated, SCs can perform repair functions, including the clearance of pathologic myelin to create a path for new axons, regulation of nutrient exchange with regenerating axons, and the promotion of new myelin production, among others [3,6,7]. A crucial aspect of this phenotypic shift and subsequent nerve healing is the upregulation of SC genes responsible for the secretion of several neurotrophic and chemotactic factors influencing cellular growth and migration [6].
In order for healing to take place across a nerve gap, numerous cells from both nerve stumps must be able to traverse the deficit in a well-defined order. This process begins with the secretion of factors and extracellular matrix (ECM) precursors from both transected nerve stumps, which solidify to a matrix of ECM cables, providing a bridge for SC crossing. SCs play a key role in guiding the remainder of axonal regeneration, as SC migration along the cables of this matrix is closely followed by the migration of endothelial cells and fibroblasts from both nerve stumps, and then axons from the proximal nerve stump, which are myelinated soon after. These processes were first described by Williams et al. in 1983 [8] and have since been delineated to five phases: (i) the fluid phase; (ii) the matrix phase; (iii) the cellular migration phase; (iv) the axonal phase; and (v) the myelination phase [8,9,10,11]. However, although possible, the process of neuronal regeneration in the PNS is often very slow and can be affected by several pathological processes associated with trauma, such as a large gap between the proximal and distal segments of a truncated nerve, local inflammation, ischemia, or background disorders, such as diabetes or congenital conditions [12,13].
Several therapies have been developed to affect the speed and quality of this natural recovery process with documented success in clinical application, and many more can be found in all phases of development. In general, this progression consists of first showing in vitro benefits through application to isolated and cultured cells. Next, an animal model is employed, most commonly the rat sciatic nerve due to its accessibility, size, and ease of functional recovery monitoring. After decades of confirmation by many groups, the therapy may be investigated in human trials of increasing rigor, size, and diversity, before consideration by regulatory authorities for its use outside of experimental platforms.
This review article provides a three-pronged comprehensive examination of (i) therapies that have undergone rigorous human clinical trials and are currently employed in clinical practice, (ii) therapies with characteristics that will allow particular ease of translation into clinical practice, and (iii) recently described, novel therapies that directly complement those already in use.
History of Biomaterials in Nerve Repair
Less than 100 years passed between the development of the first plastic material in the 1860s and the first described use of a plastic polymer implanted in the human body in 1939, when a fibrosis-inducing wrap was used to mitigate the expansion of an arterial aneurysm [14,15]. Since this first described application, the widespread adoption of both natural and synthetic polymeric materials as biomaterials has revolutionized the field of surgery.
A biomaterial is defined as “a material designed to take a form that can direct, through interactions with living systems, the course of any therapeutic or diagnostic procedure” [16]. These materials have widely varying functions, strengths, and limitations owing to their diverse chemical formulations and fabrication methods. For the purpose of peripheral nerve repair, biomaterials have most commonly been formed into nerve conduits (NCs), which are tubular supports fashioned around areas where neural healing is to be encouraged. More specifically for this review, this latter definition will be the context in which biomaterials will be discussed. Interventions involving the manipulation of living or recently living tissues for bridging a deficit will be referred to generally as “grafts”. Finally, “nerve guide” will be used as an umbrella term to include both NC and graft approaches, though many groups use these terms interchangeably in their descriptions.
The origin of biomaterial-based nerve guides is often cited as the use of decalcified bone tubes to facilitate nerve repair by Gluck in 1880 [17]. For many years following, research focused largely on the use of autogenous tissues as nerve guides, such as blood vessels (1891) [18], fascia (1915) [19], and skeletal muscle (1940) [20], though intermittently, researchers had leveraged nonbiologic materials including magnesium (1900) [21], gelatin (1901) [22], and galalith (1915) [23]. In 1946, Weiss and Taylor bridged nerve gaps in animal models with collagen sleeves, a material still in use today, though cited rapid resorption of the material as a reason for only rare success [24]. As a direct result of these early studies and the long history of poor outcomes in nerve repair, decades of research have explored what properties make up the ideal nerve guide, with novel biomaterials accounting for recent success in addressing the limitations delineated from earlier attempts. The five phases of neural regeneration are illustrated in Figure 1A, along with interventions currently in use or under study to enhance these phases (Figure 1B–L).
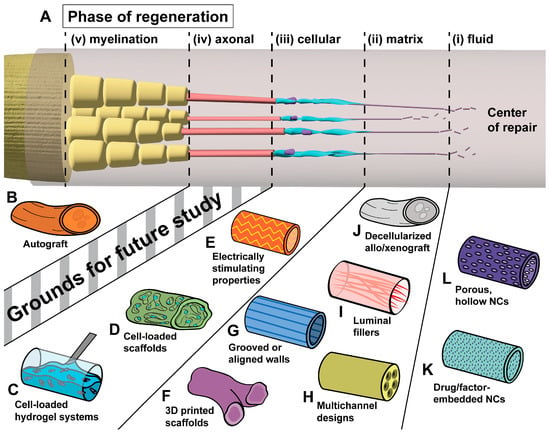
Figure 1.
The five phases of neural regeneration across a gap and selected implants to enhance regeneration in each. (A) Healing across a nerve deficit begins with (i) the secretion of ECM precursors, which (ii) coalesce to a matrix of ECM proteins, allowing (iii) cellular traversal of the deficit. (iv) These cells guide axonal growth, which is followed by (v) myelination [8,9,10,11]. (B) The current gold standard of repair across a deficit, autologous nerve graft, obtained through harvest of a patient sensory donor nerve. (C,D) Implantable scaffolds [25] and hydrogel systems [26] may be preloaded with cultured SC or SC-like cells [27]. (E) The incorporation of functional polymers that enhance electrical stimulation and conductivity allows for the preservation of denervated SC populations [27]. (F) Precise 3D printing of biocompatible scaffolds allows for the design of bifurcating and irregular scaffolds for improved topographic guidance [28]. (G–I) Topographic guidance features within walls [29] or intraluminal channels [30] and filaments [31] of a conduit encourage migration along its length. (J) Decellularization of allografts and xenografts allows for the removal of immunogenic components, leaving behind an ECM scaffold [32,33]. (K) The embedding of bioactive substances within conduit walls allows for the controlled, local release of the substance within the repair site [34]. (L) Porous, hollow conduits permit nutrient exchange while preventing cellular invasion [35].
2. Nerve Conduits
2.1. Structural Properties
NCs have been studied in a range of forms, and broad structural categories have been described as generations: first-generation conduits are hollow, tubular structures used for support and as a barrier to the environment; second-generation conduits are resorbable and biocompatible with specific wall structures to guide axonal growth topographically; and third-generation conduits incorporate other bioactive functions, such as luminal fillers, drugs to enhance regeneration, or cellular or extracellular components [36]. Within these large categories, NC structures can be further distinguished by their relative ability to degrade within the body, degree of porosity or permeability, and the specific topographic cues of their walls or intraluminal contents. The optimal characteristics among these have shifted greatly since early NC designs and continue to evolve as research progresses. Here, we offer a summary of how the field’s preferences have evolved, from the early concepts to where they currently lie.
2.1.1. Degradation
Nerve conduits in their simplest form exert their effects by directing elongating nerve fibers along a deficit to be bridged. This was the primary goal of early synthetic conduits composed of relatively inert, relatively poor-degrading materials such as silicone, which Lundborg et al. described in 1979 as the “chamber principle” of isolating a healing nerve [37,38]. It was theorized that enclosing the gap between two nerve ends could require fewer sutures, mitigate excess surgical trauma, prevent the invasion of the healing nerve deficit by fibrous scar tissue, and allow luminal accumulation of neurotrophic and neurite-promoting factors [9]. However, the use of a material that did not degrade within the body occasionally necessitated reoperation for removal due to delayed tissue reactions and fibrosis [37,38,39]. At the time of this early conduit’s use, the author noted that a second surgery was preferable to the use of material that would degrade and could induce inflammation and fibrosis, or interfere with environmental factors involved in healing [39].
Soon after, this view shifted. Nerve conduits are now designed to be biodegradable rather than inert to avoid the risks of retained foreign material at the repair site, as recognized by Merle et al. in 1989 [36,40,41]. At the same time, this degradation should not take place fast enough to trigger an inflammatory response or jeopardize the strength, shape, and axon-guiding ability of the NC [42]. The flexibility afforded by using biodegradable polymers in modern NCs addresses these concerns, with the added benefit of degradation often being tied to several strategies shown to aid in nerve healing, such as drug, growth factor, or cell delivery. Additionally, advancements toward the generation of functional materials, that can degrade in vivo, have allowed for the application of physical stimulation in innovative strategies, such as using biodegradable electrodes and nerve stimulators, discussed later in this review.
2.1.2. Topographic Features
Stemming from observations in 1912 that cells in culture moved along and adapted to the form of spider webs [43], “contact guidance” is a term used to describe the propensity of cells to adjust their orientation to align with groove-like patterns when growing on them [44]. Many NC fabrication techniques leverage this principle with topographical guidance cues, to enhance cellular alignment for migration and provide directionality to axonal tract regeneration. These include longitudinal topography introduced within conduit walls through aligned channels and/or grooves [29,45,46,47], or the generation of aligned fibers [48,49]. Additionally, aligned structural supports extending across the center of the conduit such as microchannels [50] or filaments of defined sizes and compositions [31,51,52] have been employed to encourage migration along their length. The function of these microfeatures may be enhanced by biochemical cues, such as those associated with extracellular matrix (ECM) proteins, using these approaches to promote migration [50]. The manipulation of these ECM proteins is also frequently cited as a method to tailor the “roughness” of a conduit’s surface to better allow native SC attachment and migration, or the survival of cells delivered with the NC [50,53,54].
The inclusion of hydrogels within some NC designs represents a distinct but related approach to topographic guidance. Hydrogels are composed of hyperhydrophilic polymer chains that exhibit a diverse set of structural characteristics, defined in part by their crosslinking degree and nature [55]. Through modulation of these crosslinks, hydrogels can be prepared as liquids and injected at a site or into a conduit prior to solidifying, via specific mechanisms such as changing temperature, pH, or time-dependent chemical gelation [55,56]. As a result, hydrogels often serve as a medium in which interventions such as drugs or cells are co-delivered with the NC materials, thereby also providing structural and topographic support within the lumen of an NC. Examples include the incorporation of ECM proteins into the gel matrix to be used as bioinstructive cues [56].
More recent advancements in NC fabrication techniques continue to allow for greater control over the topographic characteristics of all types of NCs. One example can be found in the application of shape memory nanofibers, applied in multichannel conduits of aligned and random orientations by Wang et al. in 2020 [30]. Additionally, the widespread advancement and adoption of 3D printing technology over the past decade has permitted a level of structural customization in nerve guides, that are not attainable by many other, more established NC production techniques. These include the fabrication of bifurcated and irregular conduits, reverse-engineered from 3D anatomical scans by Johnson et al. in 2015 [28], and many other potential functional integrations to allow for personalized therapies within PNI, as recently reviewed by Liu et al. [57].
2.1.3. Porous Structure and Permeability
Another early quality discovered to be vital in developing NCs was permeability and its associated porosity—defined as the ratio of the volume of interconnected void space divided by the total volume of a material [58]. This was exemplified by the use of an NC composed of expanded polytetrafluoroethylene (ePTFE) in 1998, which was designed to interact differentially with proteins in comparison to silicone, despite being relatively bio-stable like the latter material. Specifically, ePTFE allowed nutrient exchange as a result of its interconnected porous walls [59]. Other early notable porous NCs include some composed of biodegradable polymers such as polyglycolic acid (PGA) [60,61]. Permeability remains a key characteristic under study within nerve conduits to this day, with efforts to describe the ideal characteristics for neural regeneration in porosity, pore size, connectivity, uniformity, and three-dimensional morphology still ongoing [62].
Advancements in biomaterial fabrication processes have allowed conduits of different porous structures to be readily made, and several tissue-regeneration outcomes have been attributed to varying porosity [62], including improved proliferation and migration of SCs [63], as well as beneficial effects on macrophages [64,65] and angiogenesis [66]. Further improvements in fabrication processes will allow finer control of the porous structures of conduits and more precise regulation of cellular infiltration, nutrient exchange, and metabolic waste elimination at repair sites [62]. As an example, NCs with selective, unidirectional permeability afforded by asymmetric walls of small internal pores and larger external pores provide a promising approach toward spatially regulating substances delivered within the conduit, such as drugs or growth factors [67]. These build on earlier work showing pore size to be a crucial determining factor in allowing nutrients and other molecules to enter the lumen while limiting the entry of cells such as fibroblasts [35,68]. Other characteristics affected by porosity include mechanical performance, biodegradation rate, and cellular adhesion and proliferation [62].
2.1.4. Natural or Synthetic Composition
NCs can be fashioned from natural or synthetic materials or a combination of both as hybrid composite biomaterials. The characteristics of commonly employed materials in experimental nerve repairs and their relative advantages and disadvantages have been extensively reviewed, including several more recent published works [14,69,70,71,72,73,74,75]. Though critical to those developing and refining NCs, the intricacies of their properties are largely excluded from this review in the effort of providing a clinically focused discussion.
Briefly, naturally sourced materials used in NCs are numerous, and those that are well-studied as components for NCs either alone or in combination with another natural or synthetic polymer include collagen [76], chitosan [77], silk [78], gelatin [79], keratin [80], hyaluronic acid [81], alginate [82], fibrin [83], and agarose [83], among others. Natural materials are generally viewed as enabling less nondenaturing bioreactivity in the body, with generally better biocompatibility and a lower risk of degradation products exhibiting toxicity [84].
Despite the above, NCs of exclusively natural composition have largely been replaced by synthetic or composite approaches, which leverage the manufacturability, strength, and customization allowed by synthetic polymers. Among synthetic options, well-studied biodegradable polymers include polyurethanes such as PCNU (polycarbonate urethane) and PEUU (poly(ester urethane) urea); polyesters such as PGA (poly(glycolic acid)), PLA (poly(lactic acid)), PLGA (poly(lactic acid-co-glycolic acid)), and PCL (polycaprolactone); along with many others. Nonbiodegradable polymers include, among others, silicone, poly(tetrafluoroethylene), and polystyrene [69].
2.2. Delivery of Therapeutic Agents
Modern NCs offer many functional applications in their composition, and a particularly translatable method of improving nerve regeneration after repair is the use of biomaterials for the delivery of locally acting therapeutics. The characteristics of the ideal drug delivery conduit are a subject of frequent review [10,41,58,85], and the generally agreed upon characteristics of implantable drug delivery devices for nerve regeneration include (i) biocompatibility, (ii) mechanical characteristics of flexibility, adequate strength, and suturability, (iii) porous, (iv) degradation products with low bioreactivity themselves relative to the target therapeutic’s function, (v) relatively low foreign body responses, and (vi) easily scaled to manufacturing.
The use of biodegradable synthetic polymers for drug delivery is particularly salient in nerve repair, as many promising bioactive substances shown to improve neural regeneration have either unknown or harsh side effects when administered systemically. Drug-loaded polymers are synthesized through many methods, and the increasing accessibility of the staples of NC fabrication techniques such as electrospinning, 3D printing, and bioprinting has opened the field to many research groups previously excluded without dedicated collaborators in biomedical engineering.
2.2.1. Cellular Approaches
As noted previously, SCs play a pivotal role in the healing of peripheral nerves, particularly when regeneration is required across a large nerve gap (>3 cm), such as those created by nerve transection and the subsequent retraction of nerve ends. Cultured SCs have been delivered to transected peripheral nerves in in vivo models since as early as 1979 [86,87], and the fabrication techniques of modern conduits aim to more closely mimic the extracellular environment to best allow SC proliferation and migration.
Direct delivery of SCs has shown success in many in vivo models, particularly in recent years, often through the injection of matrices seeded with cultured SCs [26,88,89], or through the inoculation of the walls for porous conduits [25]. These approaches have shown improved performance in metrics such as axonal regeneration, elongation, and myelination, occasionally approaching regeneration similar to autografts. Further illustrating this point, a recent systematic review by Vallejo et al. found that PNI repairs involving SC-loaded nerve guides over a gap of at least 10 mm showed similar results to autograft controls in histomorphometric and functional outcomes [90].
Unfortunately, inherent limitations exist in the use of primary SCs for delivery, particularly in PNI repair where acute trauma accounts for the vast majority of cases [91]. Transplantable primary SCs must be donor derived to avoid graft-versus-host interactions, and culturing cells to the quantity needed for human applications is time-consuming and often not feasible for adoption into widespread clinical use by current methods [92]. Though providing a supportive environment for the growth of endogenous SCs remains of vital interest, alternative cellular options have been considered for the delivery of exogenous cells, including SC precursors and stem cells of many types.
Stem-cell-based therapies represent a promising approach to the temporal limitations of culturing primary SCs. Ethical and safety concerns outside of the scope of this review are associated with the use of human embryonic stem cells given their source, despite their potential utility in the generation of SCs via rapid proliferation [93]. Induced pluripotent stem cells do not pose the same ethical issues and have shown promising results in generating large numbers of functional SCs through a SC precursor intermediate [94]. However, there are concerns with respect to their tumorigenic potential. Other prominent stem cell types under investigation for their role in traumatic peripheral nerve injury have been recently reviewed by Kubiak et al., with numerous in vivo studies showing potential for clinical application [95]. Oftentimes, these cells exert their effects by exhibiting a Schwann-cell-like phenotype or through the secretion of growth factors, and examples of frequently employed cell lines include adipose-derived stem cells (ADSCs) [83,96], bone-derived mesenchymal stem cells (BM-MSCs) [97], and neural stem cells [98], though long-term safety and efficacy studies are still needed to compare the wide range of considerations to determine which is superior. Several studies have compared ADSCs and BM-MSCs, with evidence suggesting that ADSCs may provide slight advantages in addition to greater ease of harvest, wide differentiation potential, and low immunogenicity [99,100]
2.2.2. Neurotrophic Agents/Growth Factors
An alternative to implanting entire cultured cells at a nerve repair site is to instead leverage only the growth factors they secrete. Numerous neurotrophic agents and their impacts on both in vitro and in vivo models have shown beneficial effects associated with their use, though none have been integrated into clinical practice for the treatment of peripheral nerve repair to date. Several of these factors are found at low concentrations physiologically; however, they are upregulated in neurons and denervated SCs upon nerve insults, leading to the frequent study of how their benefits can be applied or enhanced at sites of nerve repair [101]. Unfortunately, as proteins rather than shelf-stable pharmacologic agents, a particularly challenging aspect of applying neurotrophic factors as an adjunct therapy in nerve repair is their instability. Historical issues with their use include rapid inactivation by enzymes leading to short half-lives, as well as reaching adequate concentrations at their intended sites without triggering dose-limiting side effects [102].
Advancements in culturing methods and the design of biocompatible scaffolds have allowed for the incorporation of growth factors into numerous biomaterial-based devices with promising results. As recently reviewed by Wan et al., nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), glial-derived neurotrophic factor (GDNF), insulin-like growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF) remain among the most widely examined factors for this purpose, having been employed in varieties of NCs, hydrogels, microspheres, nanoparticles, and exosomes [103].
Within the realm of peripheral nerve pathology, recombinant human nerve growth factor (rhNGF) has undergone evaluation at different phases of clinical trials as a treatment for diabetic neuropathy (phase III) [104], HIV-associated sensory neuropathy (phase II) [105], and most recently, neurotrophic keratopathy (NK) (phase IV) [106]. However, only topical rhNGF for NK has materialized as an FDA-approved therapy. Notably, the subcutaneous administration of rhNGF for diabetic neuropathy, to a large sample of 1019 patients, failed to show a significant benefit and resulted in unexpectedly high rates of injection site pain, hyperalgesia, myalgia, and peripheral edema when compared to that of the expected outcome from phase II testing [104]. As a result, over the past decade, the majority of research into the applications of biologic therapies has centered on local delivery, directly at the site of repair.
From the application of its existing indication for the treatment of NK, NGF may face fewer hurdles in regulatory agency approval and translation to nerve repair relative to other growth factors. Further study is necessary to elicit any adverse effects of local delivery, particularly any effects similar to those seen in the systemic administration for humans, relative to nerves. These include pain, myalgia, and edema, which may be difficult to appreciate in animal models without deliberate observation, or impossible altogether prior to human use.
2.2.3. Pharmacologic Agents
The delivery of existing pharmacologic agents to aid in nerve healing after repair offers several distinct advantages over the delivery of other substances such as neurotrophic factors and cells. In terms of logistics and the ease of translation into clinical practice, the use of well-studied drugs with years of successful application in other indicated uses should allow for faster translation into clinical practice, while simultaneously hosting readily available sources for manufacturing. Among the many drugs studied to aid peripheral nerve regeneration, immunosuppressants, corticosteroids, and drugs with antioxidant or anti-inflammatory effects have frequently been delivered in nerve conduits, scaffolds, and hydrogels.
Drugs can be better suited to endure the often harsh fabrication processes of conduits without losing function. In contrast, the fabrication of NCs containing biological agents such as proteins and cells often requires additional considerations in manufacturing, storage, and application to prevent their exposure to potentially damaging conditions such as high temperatures, organic solvents, adverse reactions with biomaterials or their degradation byproducts, and other conditions that deviate from near-physiologic conditions [56,102,107]. Preclinical outcomes from the successful embedding of cells and growth factors are representative of the great strides taken in regenerative medicine in recent decades. However, the introduction of established pharmaceuticals with well-known side effect profiles and interactions in the body into NCs likely remains closer to translation into surgical practice.
An example of the qualities described here as well as additional benefits provided by drug-specific delivery in nerve repair lies in the immunosuppressant tacrolimus (FK506). This drug is particularly relevant to peripheral nerve repair given its historical use in combatting the immunogenicity of nerve allografts prior to the introduction of acellular allografts, as discussed later in this review [108]. Numerous groups have examined this FDA-approved calcineurin inhibitor for its benefits in nerve healing since their description in 1994 [109], with several recent publications describing its application in a drug-releasing nerve wrap in in vivo models [34,110,111]. Local delivery of this drug has been shown to increase the number and size of myelinated nerve fibers, increase the number of regenerating motor and sensory neurons, and improve functional recovery, in addition to providing local immunosuppression at repair sites [34,112]. Tacrolimus is frequently prescribed for this purpose in organ transplant recipients, though its harsh side effects—including tremors, headache, nephrotoxicity, and diabetes mellitus—preclude its regular systemic administration as a surgical adjunct for peripheral nerve repair [113,114]. When incorporated into NCs, tacrolimus maintains its bioactive properties through the high voltages and often harsh solvents involved in electrospinning, and the drug–polymer matrix produced through this process is thermally stable at ambient and body temperatures, facilitating both storage and clinical application, respectively [34]. As such, tacrolimus-releasing conduits and wraps have emerged as promising and readily translatable therapies in peripheral nerve surgery.
The delivery of pharmacologic agents to injured nerves is yet another topic that is frequently reviewed in the literature [71,115,116]. However, comprehensive reviews of this subject often include pharmacologic agents that are not FDA-approved. Additionally, the heterogeneity seen in preclinical models of nerve injury adds further complexity to performing comparative studies, even when the same animal model and nerve are used. As such, we have assembled Table 1 below to include only drugs that (i) hold current FDA approval for any indication, (ii) have been delivered locally by a nerve conduit, hydrogel, or scaffold, and (iii) have been applied in a full transection and repair in vivo model. In doing so, we hope to highlight approaches that may be the closest to clinical adoption: those involving established drugs, for use in repairs across full transections, which frequently employ conduits.

Table 1.
Several pharmaceutical compounds currently approved for conditions unrelated to nerve repair have been delivered locally to in vivo transection and repair models to evaluate their effects on nerve healing across a deficit.
In addition to passive drug delivery based on the degradation of polymers, advances in biomaterials have allowed for systems with finer control, with one example being the use of magnetic nanoparticles (MNPs). These functions are achieved by either embedding MNPs within hydrogel networks or electrospinning fibers of other, more biocompatible polymers infused with MNPs [127]. Potential applications to the peripheral nervous system include neural cell manipulation and guidance, as well as the spatially precise delivery of bioactive compounds [127]. For instance, Giannaccini et al. were able to conjugate NGF and VEGF to MNPs prior to injection into a nerve conduit with a strip of magnetic tape around their center. These growth factors have short half-lives which limit their use, and their distributions are nonuniform within regenerating nerves due to being secreted from the proximal and distal nerve stumps. Through the use of NMPs for delivery, these growth factors were maintained at a higher concentration within the conduit, extending their use, and were particularly concentrated in the region of the nerve deficit most in need, the center [128]. Though promising in theory, the distribution of magnetic metals in a high surface area to volume nanoparticle form within the body will require significant additional study and proof of safety before translation into human applications. MNPs pose significant risks to multiple body systems upon exposure, and well-established interactions include the activation of oxidative stress, inflammation, and indirect DNA damage [129]. As such, their use remains a contested topic within the discussion of drug delivery, as the benefits of sequestering the delivered drug must outweigh the inherent inflammatory reactivity of introducing such metallic materials into the body.
In addition to precise spatial delivery, recent advancements in drug encapsulation within biomaterials allow greater temporal control, such as through ultrasound-responsive delivery systems employing multiple bioactive compounds. This was recently demonstrated by Shan et al. by encapsulating a hydrogel network directly with a drug shown to mitigate neuroinflammation in the early stages of repair (vitamin B12), as well as with microspheres loaded with nanoparticles, which are loaded with factors capable of promoting long-term regeneration (NGF) [130]. Drug release is then regulated by ultrasonic stimulation, for the regulation of both rapid release from the loose hydrogel structure and prolonged release from the more dense triple-encapsulated system according to the ideal therapeutic time window [130].
3. Intraoperative Electrical Stimulation
3.1. Current Approach
The electrical stimulation (ES) of nerves to promote axonal regeneration is a relatively recent addition to the study of nerve repair and healing, with few clinical trials published thus far [131]. The application of electrical currents to nerves has been leveraged by many medical fields, with recent and exciting results in interventions ranging from modulating pain [132] to improving memory [133]. Within the realm of nerve repair, the foundational work of using ES to accelerate axonal regeneration is often attributed to studies by Hoffman [134], Nix and Hopf [135], and Pockett and Gavin [136].
The most salient application of ES to surgical nerve repair lies in the stimulation of nerves through direct contact with electrodes to generate its beneficial effects. However, as this method often uses the relatively non-specific phrase of “electrical stimulation” in its publications, a discussion of other, similarly named therapies is warranted. In humans, therapeutic electrical stimulation has been applied to patients with peripheral nerve pathology in four particularly similar methods: (i) at low intensity through the skin, largely to treat pain and neuropathy (transcutaneous electrical nerve stimulation, TENS) [137]; (ii) also through the skin, however directed at denervated muscles following peripheral nerve injury, largely to mitigate atrophy and preserve function upon reinnervation (electrical stimulation of denervated muscle, ESDM) [138]; (iii) stimulation through implants in downstream denervated musculature after nerve injury for the same purpose as the previous method [139]; and (iv) directly to injured nerves to promote reinnervation of downstream targets, as discussed in the remainder of this review (commonly referred to simply as electrical stimulation, ES). Of note, nomenclature regarding approaches (ii), (iii), and (iv) varies frequently in reporting and would benefit greatly from standardization within the future literature. The use of 20 Hz neuronal electrical stimulation is suggested.
ES in the context of this discussion is applied proximal to a repaired nerve site, either intraoperatively before skin closure [140,141,142] or in the immediate postoperative period [143]. It is performed most commonly at a frequency of 20 Hz for one hour, a frequency and duration first shown effective by Al-Majed et al., which has since been confirmed by numerous animal [144,145,146] and human [140,141,142,143] studies. This significantly increases the levels of cyclic adenosine monophosphate (cAMP) within neurons [147,148,149], upregulates neurotrophic factors and their receptors on both SCs [150,151,152] and neurons [153], and increases the levels of growth-associated genes and cytoskeletal proteins necessary for growth [145,154,155]. The specific directionality of the stimulus is applied to trigger a cascade of action potentials toward the cell body (antidromic), as this has been shown to be the site of action of ES’s benefits [156].
The first randomized control trials of one-time, intraoperative ES took place in 2010 by Gordon et al. and examined intraoperative ES as a method to improve outcomes in patients with median nerve compression secondary to carpal tunnel syndrome (CTS) [142]. With the application of 20 Hz of stimulation for one hour during carpal tunnel release, motor and sensory reinnervation significantly improved, though a benefit to functional recovery was not found. The next major trial of ES involved patients undergoing surgical repair of complete transection injuries to digital nerves and found that the same ES parameters significantly improved sensory outcomes in all methods tested, though did not show a significant disability improvement as assessed by the Disability of the Arm, Shoulder, and Hand questionnaire (DASH) compared to the control, sham ES [141].
Further study has led to intraoperative ES being applied to oncologic neck dissection to mitigate postoperative shoulder dysfunction [140], as well as decompression operations to treat cubital tunnel syndrome [143,157]. These studies have further supported the efficacy of ES shown in prior work, in addition to providing evidence of functional benefits to the disability and recovery of muscle strength.
To our knowledge, only one human study, by Wong et al., discussed above, has involved the use of ES in the treatment of a complete transection and repair of a peripheral nerve [141], with the remainder involving comparatively minor injuries from compression [142,143,157] or axonal injury due to devascularization and retraction [140]. Less severe nerve insults such as those that are close in proximity to their targets of innervation are less likely to experience the poor outcomes ES has been developed to address, and as a result, its full effects may be revealed only in severe injuries with historically worse healing. Though Wong et al. applied ES in a population meeting these characteristics [141], transections of nerves in the compact space of the digits often also involve concomitant tendon, artery, or vein injuries, and this heterogeneity of patients may have reduced the study’s power to detect a significant difference. As such, further study in more severe injuries which are further from their reinnervation targets, and with more homogenous patient populations, will be valuable to establish the true efficacy of ES in improving peripheral nerve repair outcomes.
3.2. Novel Biomaterial Applications to Electrical Stimulation
As the effects of electrical stimulation on axonal regeneration have been shown in animal studies since as early as the 1950s [134], many groups have developed biomaterial-based approaches for its delivery, with many of these progressing to in vivo studies with success. However, very few, if any, have successfully made it to human use, as electrically conductive polymers for highly reactive materials are relatively nondegradable, and thereby pose long-term foreign-body response issues for patients at this time. Among others, these include conductive, self-powered, and wireless electrically stimulating nerve conduits and devices.
3.2.1. Conductive Polymers
Numerous electrically conductive NCs have been studied in animal models, with particularly promising results when combined with ES, though none have reached human implementation at this time due to concerns largely related to biocompatibility [158]. Materials used for this purpose include polypyrrole (PPy) [27,159,160,161], polyaniline (PANI) [162,163], and poly(3,4-ethylenedioxythiophene) (PEDOT) [164], though these materials are largely limited to integration within another biocompatible natural or synthetic polymer at this time. Carbon nanotubes (CNTs) and graphene (GO) represent additional materials being studied for their applications as conductive additives for enhancing neural growth and regeneration, though their safety profiles with respect to in vivo application are less clear at this time [158]. Notably, graphene-based materials have also been studied for their ability to induce the transdifferentiation of MSCs to SC-like phenotypes through the delivery of electrical stimulation alone, in the absence of chemical growth factors [165].
3.2.2. Self-Powered Conduits
Similar to conductive NCs in intended effect, self-powered conduits incorporating nanogenerators, which are able to generate electric potentials on their surface upon mechanical deformation, represent a potential source of therapeutic electrical stimulation that would not require an external electric source. Instead, the conduits may generate an electric stimulus through activation by forces such as natural body movement, noninvasive ultrasound waves, or magnetic fields, as recently reviewed by several groups [166,167]. Other examples of the potential applications of self-powered conduits include the incorporation of piezoelectric polymers to generate electrical power from mechanical deformation caused by natural rat body movement [168] or from physiologic actions such as breathing [169].
Promising materials under study for these applications are numerous [166], with examples including piezoceramics such as zinc oxide (ZnO) [168], as well as synthetic piezopolymers such as the nonbiodegradable polyvinylidene fluoride (PVDF) [170] and biodegradable (PHBV) [171] and poly(L-lactic acid) (PLLA) [172]. Similar to concerns discussed previously in regard to magnetic nanoparticles, the use of some conductive and piezoelectric materials in nanoparticle form will require extensive study for proof of safety before implementation in humans. As an example, ZnO nanoparticles, even at low concentrations, can induce oxidative stress and damage DNA in cells [173]. This may be mitigated by implementing the gradual release of such particles when incorporated into slowly degrading polymer substrates [168], though highlights the risks involved in the introduction of functional exogenous substances into the body, even locally.
Some natural biopolymers host relatively strong piezoelectricity including cellulose, chitosan, and collagen [174], and continued study of these compounds in this context is likely warranted.
3.2.3. Wireless Nerve Stimulators
A limitation of the most commonly employed ES protocol (20 Hz, 1 h) at this time is the need to extend a completed operation by a full hour in order to allow stimulation by direct contact with nerves. This increases operating times, as well as risks such as exposing the patient to greater amounts of anesthetic and the potential for complications. As one method to combat this, biocompatible electrode advancements have allowed the development of wireless, resorbable nerve stimulators that can be implanted at a nerve repair site immediately prior to wound closure and activated when most appropriate. These stimulators are able to stably deliver therapeutic levels of ES (20 Hz for 1 h) prior to the rapid degradation of its electronic components within 3 days and total degradation of the device within 10 days [175]. As the optimal ES frequency and duration are still debated, other groups have designed wireless nerve stimulators that are able to function for up to 6 days prior to resorption within 25 days [176], as well as up to 30 days with degradation within 50 days [177].
At this time, the utility of ES outside of the immediate postsurgical period is still debated; however, if greater efficacy is shown by extended or continuous ES, these implantable devices will likely be crucial to effective clinical translation.
4. Nerve Grafts
4.1. Currently Available Nerve Grafts
In cases of severe nerve deficits where joining the distal and proximal stumps cannot be accomplished without tension, the use of a grafted nerve segment interposed between the transected nerve stumps may be necessary. Clinically, current options include the gold-standard autologous nerve autograft or commercially available processed acellular allograft.
4.1.1. Autograft
The first described use of successful autografting in an animal model was by Philipeaux and Vulpian in 1870, using an autologous lingual nerve graft to repair a hypoglossal nerve deficit in a dog [178] (per [179]). The first successful human autograft is less clear, though may have been by Robson in 1889, who bridged a median nerve gap with a popliteal nerve graft [180,181]. Repair by autograft remains the current gold standard due to the graft’s innate architecture and resident SCs releasing growth factors providing the adequate environment necessary to facilitate nerve regeneration [182,183].
Though replacing a deficit with a freshly harvested, autologous nerve has a proven history of success, this carries distinct disadvantages in application. Along with the inherent morbidity of removing a functional nerve used as a donor, this second surgical site presents additional opportunities for infection, can require intraoperative repositioning to access, prolongs time under anesthesia, and increases the potential for painful neuroma formation at the donor site [184]. According to a recent review evaluating 214 sural nerve harvests for grafts, 92.5% of these patients experienced sensory deficits, 22.9% chronic pain, 1.4% wound infections, and 7.0% wound complications other than infection [185]. Additionally, necrosis of resident SCs as a result of decreased perfusion to the graft can take place after transfer, which may be problematic in particularly long repairs [186].
Nerve autografts can be size-matched to the recipient nerve diameter by choice of donor nerve, as well as by using single, cable, trunk, or interfascicular techniques [182,187]. The donor nerves most commonly used for this include the sural nerves, medial and lateral antebrachial cutaneous nerves, superficial branches of the radial nerve, dorsal cutaneous branches of the ulnar nerve, superficial and deep peroneal nerves, intercostal nerves, and the posterior and lateral femoral cutaneous nerves [185,188].
A recent systematic review comparing available, FDA-approved NCs and wraps against direct repair or autograft in upper limb peripheral nerve repairs found that the use of such devices may be associated with a higher rate of adverse events and greater need for revision surgery, though the evidence was noted to be very uncertain [189]. There was little or no difference in mean sensory recovery or integrated functional outcome scores at 2 years between the groups, but once again, both conclusions were stated to be by very uncertain evidence. Though five-year functional outcomes may be slightly improved with device use, this was additionally with low-certainty evidence [189].
4.1.2. Allograft
In addition to their animal experimentation in autografting, Philipeaux and Vulpian also conducted early, though unsuccessful, work in allografts in 1863 [179]. The first attempted use of an allograft in humans was likely by Albert in 1878, using nerve material from a recently amputated leg of another man to bridge a gap in the median nerve of a patient, which became necrotic and required removal [190] (per [181]).
Allografts only became a viable clinical option with the development and further understanding of therapeutic immunosuppression, though the long-term systemic immunosuppression needed to prevent rejection of a cadaveric nerve caused concern for other pathologies, including organ toxicity, opportunistic infections, and neoplastic processes [191]. Fresh allograft use has typically been described only in severe cases where the extent of nerve deficit would be otherwise irreparable by available donors. This is illustrated by the bridging of a 23 cm sciatic nerve defect using 10 cabled fresh allografts followed by immunosuppression in an 8-year-old boy by Mackinnon et al. in 1988 [192]. Similar repairs were performed in seven additional patients who required immunosuppression for an average of 18 months [108]. This was circumvented by the introduction of processed acellular nerve allografts (ANAs) to clinical practice in 2007, which do not require systemic immunosuppression, and had spread in use to approximately 70% of hand surgery practices in the United States by 2018 [193].
Processed, in this setting, describes the removal of immunogenic components from an initially cellular nerve graft, leaving behind a highly organized extracellular matrix capable of serving as a scaffold that closely mimics the native tissue architecture [194]. Nerve guides containing acellularized epineurium, fascicles, and endoneurium to support growth, migration, and angiogenesis provide a platform for nerve regeneration [184,195]. Though necessary in order to fully combat immunogenicity, this process also removes SCs from the graft, resulting in the ANA still ultimately being reliant upon in situ SC migration similar to other non-SC-loaded nerve conduits [196,197]. Though ANAs are architecturally primed for this migration, the lack of adequate functional SCs may be one of many limiting factors for their application to longer nerve deficits than their current maximum factory-available length of 7 cm [188,196].
Another established limitation of ANAs in bridging nerve gaps of greater lengths is the natural senescence SCs undergo when exposed to environmental stress, aging, or chronic denervation [198,199,200]. Strategies to mitigate the senescence of in situ SCs within ANAs as they proliferate and migrate long distances to reinnervation targets are currently under study, including the neurotrophic effects of side-to-side bridge grafting [201] and reverse end-to-side (RETS) nerve transfers [202,203], which have shown benefits in muscle recovery. Both tacrolimus and electrostimulation, discussed previously, have also been suggested to promote earlier SC reinnervation in the distal stump of transected nerves [199]. Finally, the use of TGFβ, interleukin-10 (IL10), and other cytokines mediating the interactions between SCs and macrophages recruited to the repair site are under study for their abilities to help maintain the repair-oriented SC phenotype, particularly in delayed PNI repair [199,204].
4.2. Advancements in Grafts
4.2.1. Xenografts
Xenografting refers to the process of transferring donor tissue of one species into a recipient of another species. Similar to allografts, improved tissue-processing and immunosuppression techniques in recent decades have allowed xenografts to emerge as a potential substitute for autografts in peripheral nerve repair. In addition to eliminating concerns of donor site morbidity and the need for immunosuppression previously solved by allografts, xenografts host the advantages of being easily scaled to manufacturing, abundant in sourcing, and cost-effective in comparison to ANAs which require cadaveric donor tissue [205].
Xenograft donor tissue is often from bovine or porcine sources, with the latter often favored due to widespread availability, relative genetic similarity to humans, and a thorough understanding of porcine physiology through decades of biomedical study as a model organism. In addition to this, they are largely anatomically compatible with humans, sharing a similar nervous system structure [33]. Further evidence for the utility of porcine tissue as it applies to nerve healing lies in the currently FDA-approved nerve conduit Surgisis nerve cuff, which is composed of a cell-free collagen matrix sourced from porcine small intestinal submucosa [206].
Several studies have been performed in animal models over the past decade showing promising results, including the repair of a 6 mm facial nerve deficit in rats using an acellularized rabbit xenograft, which showed comparable results to autografts [207]. In another similarly designed study bridging a 1 cm deficit, it was found that autografts outperformed xenografts and allografts, with these two options performing similarly [32]. Recently, experiments have shown autografts to be superior to allografts, which in turn are superior to xenografts when applied to a 15 mm deficit. Xenografting was achieved in this latter study by utilizing processed human donor tissue in a rat nerve deficit [208].
Decellularization techniques are still being modified, showing improved results that may eventually match or overtake results from the gold-standard autologous graft. One such example includes the use of a novel supercritical carbon dioxide extraction technique, which was applied in tandem with the well-established chemical decellularization seen in commercial acellular nerve allografts to generate porcine xenografts for implantation into rats. The xenograft processed in this manner was compared against a hollow nerve guidance conduit, a chemically decellularized only porcine xenograft, and an autologous graft, showing a recovery similar to an autograft and significantly better than the nerve conduit or chemically treated only xenograft in a 15 mm gap [33].
4.2.2. Fat Grafting
As mentioned previously, adipose-derived stem cells are a frequently employed SC-like cell currently being investigated for delivery in biomaterial conduits and hydrogels as an alternative to SCs, showing benefits comparable to an autograft in several metrics [83,96,99,100]. In addition to this use, autologous fat grafting has also been explored in nerve repair, employed in a manner similar to that seen in several cosmetic procedures; autologous fat is harvested, minced, centrifuged, and injected around a nerve repair. This has been shown to improve remyelination, fiber density, and axon count, as well as provide benefits to pinprick sensation, motor sensory recovery, SC migration, and inflammation [209,210,211]. These effects have been attributed to neurotrophic secretions of ADSCs, as well as potential proangiogenic and immunomodulatory signals that may help to decrease neuropathic pain and suppress neuroinflammation [212]. Though not a “graft” in the sense of serving as a connection between nerve stumps, this likely represents another approach that may be applied synergistically for clinical benefits in nerve repair.
5. Commercial Availability
At this time, several nerve conduits have been cleared by the FDA for use in peripheral nerve repair, with most being allowed through a 510 (k) pathway by proving substantial equivalency to an existing device [213]. A summary of the characteristics of these devices can be found in Table 2. Of these, the vast majority are simple, hollow tubular designs or wraps to encircle a repair site, though recently, options incorporating additional internal structures have been introduced. These include Nerbridge (Toyobo Co. Ltd., Osaka, Japan), which hosts an inner collagen matrix, and Neuragen 3D (Integra LifeSciences Co., Princeton, NJ, USA), with a collagen/chondroitin-6-sulfate inner hydrogel matrix [214]. Another structural characteristic of a recently approved wrap (NerveTape, BioCircuit Technologies, Atlanta, GA, USA) is the use of microhooks of nitinol, which allow for repair without the use of sutures to secure the nerve ends or attach the wrap to the repaired nerve, per the manufacturer’s website.

Table 2.
Commercially available nerve conduits and wraps.
The majority of available NCs are composed of porcine- or bovine-derived collagen, often from the small intestinal submucosa, though pericardium and skin have also served as collagen sources. Other natural materials employed in commercially available NCs include calcium alginate/hyaluronic acid and chitosan. Among synthetic materials, PVA, PLCL, and PGA conduits have been approved for use [213,214,215].
Only one acellular nerve allograft is commercially available for use in peripheral nerve repair at this time, the Avance Nerve Graft (Axogen, Inc., Alachua, FL, USA), which is composed of decellularized human cadaveric nerve.
6. Comparative Studies
Given the heterogeneity involved in injury characteristics, surgical techniques, and institutional approaches to nerve repair, high-quality comparative studies are difficult to carry out and frequently reach inconsistent conclusions. For instance, a recent systematic review and meta-analysis of in vivo preclinical models found autografts to be superior to ANAs in seven of eight assessed outcomes [216]. In humans, a recent systematic review reported being unable to draw any conclusions regarding comparisons of ANAs, NCs, and autografts due to very low certainty evidence [217]. A systematic review by Lans et al. [218] evaluating currently employed NCs, ANAs, and autografts found that ANAs and autografts were superior in restoring meaningful recovery compared to NCs, though not significantly different from one another. The authors also performed a cost analysis, finding that the total cost of allograft usage was less than autografts in inpatient settings, but comparable in outpatient settings [218]. Their results added further support to conclusions detailed by Mauch et al. in a systematic review performed 3 years prior [219]. Though limited to digital nerve repair exclusively, evaluating the three methods in addition to primary repair showed autografts and allografts to perform similarly in return of sensation, whereas NC repairs more often resulted in poor sensory recovery, as well as a higher rate of complications [219]. Taken together, these findings could support the further adoption of ANA use not because of a clear benefit but rather noninferiority, particularly when consideration is given to the increased cost, prolonged operating time, incapacitation of a donor nerve, and increased risk for neuroma formation necessitated by autologous grafting.
7. Further Study and Synergistic Applications
Though ANAs have shown promise in comparison to autografts and NCs thus far, several unforeseen circumstances have arisen that will require further exploration and potential refinement. These include cases in which a definitive cause of allograft failure cannot be found, poor recovery in repairs of longer lengths or larger diameters, and other generally abnormal performances of ANAs [220,221]. Examples of these abnormal performances include isolated reports of the reabsorption of ANAs postoperatively without noticeable recovery [222], a report of “regenerated cable” formation with only minimal axonal regeneration that improved upon revision and autograft [223], and abnormal neuroma formation [221]. As such, continual outcome reporting and analysis will be vital to improve methods of ANA application or refine more specific indications for their use if necessary.
Future applications of biomaterials appear promising provided the benefits seen in early in vitro and in vivo studies hold true as they are translated to clinical practice. Relatively few NCs have progressed to clinical trials [224] relative to the immense amount of NCs that have been proposed and developed. A major contributor to this especially high hurdle is the fact that for the majority of the time the applications mentioned in this review have existed, autografts have remained superior in almost every metric, aside from morbidity associated with a donor site. However, with conduits, grafts, and other surgical adjuncts nearing the efficacy of the gold-standard autograft in some in vivo models, it is likely that the near-century study of complex neural repair mechanisms, material properties, and tissue processing may soon yield repairs rivaling and potentially surpassing those of autografts. This will be a welcome shift away from an intervention that causes some degree of patient harm by design and delivers relatively poor postoperative outcomes relative to many interventions in the wider fields of surgery and medicine.
It is not difficult to imagine a future nerve repair that leverages aspects of each intervention mentioned here, such as that illustrated in Figure 2. Though most have been developed as controlled, separate variables, this does not preclude their use synergistically except in select cases. For example, a hypothetical nerve deficit of critical length could be bridged with an affordable and safe acellular xenograft, with ends flanked by bioprinted fascicles meeting the exact specifications needed for a near perfectly aligned repair. This graft could be pre-seeded with Schwann or Schwann-like cells with the ability to replicate, migrate, and myelinate with temporal precision through the controlled delivery of drugs and/or growth factors, and be encased by a wrap of conductive or piezoelectric polymers. In addition to this, electrical stimulation could be applied intraoperatively or in the immediate postoperative period by a biodegradable, implantable nerve stimulator. Each of these interventions have shown promise in respective in vivo models, with many nearing human implementation.
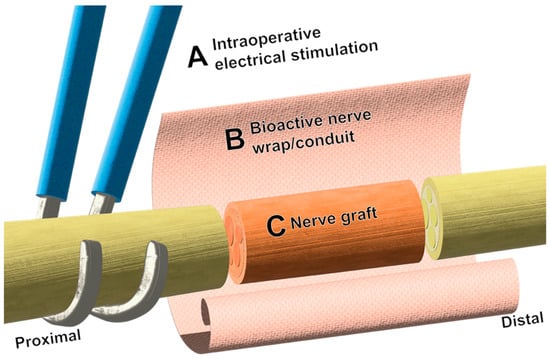
Figure 2.
Schematic representation of potential synergistic approaches to nerve repair. (A) Intraoperative electrical stimulation is applied proximal to the nerve repair site, commonly by hook electrodes. (B) Bioactive wraps and conduits can be fashioned around joined nerve stumps for the delivery of therapeutics such as drugs and neurotrophic factors and have shown promise in preclinical models. Wraps and conduits can also provide protection and topographic guidance cues to regenerating axons. (C) At this time, the use of acellular nerve allografts serves as a suitable method to avoid the donor morbidity associated with autografts.
8. Conclusions
Efforts to improve nerve healing are multidisciplinary, drawing input from medical experts, biomedical engineers, and material chemists, and novel approaches leverage a wide variety of advancements in countless fields and specialties. Many interventions to supplement traditional end-to-end repairs have been shown to improve in vivo healing with immediate clinical relevance. These have been made possible by advancements in the safety of biomaterials, the properties instilled upon them in their fabrication, and the functionalization of many natural and synthetic polymers. Among these, the local delivery of bioactive agents that would otherwise produce harsh or unknown side effects has created new opportunities for improving the complex pathways of neural regeneration. Additionally, the electrical stimulation of nerves in the perioperative period has been shown to be beneficial in clinical trials, and the incorporation of safe biomaterials to supplement its delivery may further increase its efficacy and translation into clinical practice. Finally, acellular nerve allografts have allowed for results approaching those of autografts, and with further refinement, may allow for the widespread and cost-effective adoption of an intervention that eliminates the morbidity of harvesting a donor nerve.
Returning to the paper quoted in the introduction of this review, the remainder of the paragraph published in 1944 deserves consideration in this light:
“An unbiased survey of existing methods of nerve repair… shows plainly that no one of them is sufficiently superior to the others to deserve a monopoly of attention. In times of urgency such as these, the weighing of one method against another had therefore better give way to a concerted effort to extract the best features from all available methods and combine them to the best practical advantage”[1]
No one intervention discussed in this review has eliminated the need for improvement allowed by the others. In time, the synergy allowed by their combination may revolutionize the treatment of peripheral nerve injuries, and ideally, be supplanted by future, unforeseen innovations.
Author Contributions
Conceptualization, G.H.B., J.R.C. and K.T.; writing—original draft preparation, J.R.C., C.M.M. and K.T.; writing—review and editing, J.R.C., K.T., J.P.S., K.F. and G.H.B.; visualization, J.R.C.; supervision, G.H.B.; project administration, G.H.B. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Borschel is a consultant for AxoGen, Inc. The remaining authors have no disclosures to note.
References
- Weiss, P. The technology of nerve regeneration: A review. Sutureless tubulation and related methods of nerve repair. J. Neurosurg. 1944, 1, 400–450. [Google Scholar] [CrossRef]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Neurotrophic factor expression in denervated motor and sensory Schwann cells: Relevance to specificity of peripheral nerve regeneration. Exp. Neurol. 2014, 254, 99–108. [Google Scholar] [CrossRef]
- Bolívar, S.; Navarro, X.; Udina, E. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells 2020, 9, 2131. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.E.D.; Toni, F.; Orazi, V.D.; Ortensi, A.; Tata, A.M. Therapeutic approaches enhancing peripheral nerve regeneration. ABB 2013, 4, 53–60. [Google Scholar] [CrossRef]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef]
- Seckel, B.R. Enhancement of peripheral nerve regeneration. Muscle Nerve 1990, 13, 785–800. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Belkas, J.S.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 2004, 26, 151–160. [Google Scholar] [CrossRef]
- Stenberg, L.; Stößel, M.; Ronchi, G.; Geuna, S.; Yin, Y.; Mommert, S.; Mårtensson, L.; Metzen, J.; Grothe, C.; Dahlin, L.B.; et al. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 2017, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Wilmshurst, J.M.; Ouvrier, R.A.; Ryan, M.M. Peripheral nerve disease secondary to systemic conditions in children. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419866367. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. A history of biomaterials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–34. [Google Scholar]
- Zhang, X.; Williams, D. Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gluck, T. Ueber neuroplastik auf dem wege der transplantation. Arch. Klin. Chir. 1880, 25, 6. [Google Scholar]
- Büngner, O.V. Uber die Degenerations-und Regenerationsvorgange am Nerven nach verletzungen. Beiträge Pathol. Anat. Allg. Pathol. 1891, 10, 321. [Google Scholar]
- Kirk, E.G.; Lewis, D. Fascial tubulization in the repair of nerve defects. J. Am. Med. Assoc. 1915, LXV, 486–492. [Google Scholar] [CrossRef]
- Kraus, H.; Reisner, H. Behandlungsergenisse von Verletungen pripherer Nerven mit besonderer Berücksichtigung der Schussverletzungen del Jahre 1919, 1927, und 1934. Arch. Klin. Chir. 1940, 199, 318–336. [Google Scholar]
- Payr, E. Beitrage zur Technik der Blutgesfass und Nervennaht nebst Mittheilungen die Verwendung eines Resorbierharen Metalles in der Chirurgie. Arch. Klin. Chir. 1900, 62, 67–71. [Google Scholar]
- Lotheissen, G. Zur Technik der Nerven-und Sehnennaht. Arch. Klin. Chir. 1901, 64, 310–313. [Google Scholar]
- Auerbach, S. Galalith zur Tubulisation der Nerven nach Neurolysen und Nervennahten. Munch. Med. Wochenschr. 1915, 62, 1457–1458. [Google Scholar]
- Weiss, P.; Taylor, A.C. Guides for nerve regeneration across gaps. J. Neurosurg. 1946, 3, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tong, Z.; Luo, L.; Zhao, Y.; Chen, F.; Li, Y.; Huselstein, C.; Ye, Q.; Ye, Q.; Chen, Y. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021, 6, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Burks, S.S.; Diaz, A.; Haggerty, A.E.; Oliva, N.d.l.; Midha, R.; Levi, A.D. Schwann cell delivery via a novel 3D collagen matrix conduit improves outcomes in critical length nerve gap repairs. J. Neurosurg. 2021, 135, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.-q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, A.; Faroni, A.; Minogue, B.M.; Downes, S.; Terenghi, G.; Reid, A.J. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng. Part A 2015, 21, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, H.; Zhu, T.; Liu, Y.; Pan, H.; Fan, C.; Zhao, X.; Lu, W.W. Bioinspired Multichannel Nerve Guidance Conduit Based on Shape Memory Nanofibers for Potential Application in Peripheral Nerve Repair. ACS Nano 2020, 14, 12579–12595. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Hata, K.I.; Kagami, H.; Okada, K.; Ito, Y.; Narita, Y.; Hirata, H.; Sekiya, I.; Otsuka, T.; Ueda, M. Recovery process of sciatic nerve defect with novel bioabsorbable collagen tubes packed with collagen filaments in dogs. J. Biomed. Mater. Res. Part A 2010, 92, 859–868. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, H.; Liu, H.; Niu, Y.; Yan, R.; Hu, M. A comparative study of acellular nerve xenografts and allografts in repairing rat facial nerve defects. Mol. Med. Rep. 2015, 12, 6330–6336. [Google Scholar] [CrossRef][Green Version]
- Wei, S.; Hu, Q.; Ma, J.; Dai, X.; Sun, Y.; Han, G.; Meng, H.; Xu, W.; Zhang, L.; Ma, X.; et al. Acellular nerve xenografts based on supercritical extraction technology for repairing long-distance sciatic nerve defects in rats. Bioact. Mater. 2022, 18, 300–320. [Google Scholar] [CrossRef]
- Daeschler, S.C.; So, K.J.W.; Feinberg, K.; Manoraj, M.; Cheung, J.; Zhang, J.; Mirmoeini, K.; Santerre, J.P.; Gordon, T.; Borschel, G.H. A functional tacrolimus-releasing nerve wrap for enhancing nerve regeneration following surgical nerve repair. Neural Regen. Res. 2025, 20, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Hsu, S.H.; Yen, H.J.; Chang, H.; Hsu, S.K. Effects of unidirectional permeability in asymmetric poly (dl-lactic acid-co-glycolic acid) conduits on peripheral nerve regeneration: An in vitro and in vivo study. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to Peripheral Nerve Repair: Generations of Biomaterial Conduits Yielding to Replacing Autologous Nerve Grafts in Craniomaxillofacial Surgery. Biomed. Res. Int. 2016, 2016, 3856262. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.R.; Hansson, H.-A. Regeneration of peripheral nerve through a preformed tissue space. Preliminary observations on the reorganization of regenerating nerve fibres and perineurium. Brain Res. 1979, 178, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Dahlin, L.B.; Danielsen, N.; Gelberman, R.H.; Longo, F.M.; Powell, H.C.; Varon, S. Nerve regeneration in silicone chambers: Influence of gap length and of distal stump components. Exp. Neurol. 1982, 76, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Dahlin, L.B.; Danielsen, N. Ulnar nerve repair by the silicone chamber technique. Case report. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1991, 25, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Merle, M.; Dellon, A.L.; Campbell, J.N.; Chang, P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef]
- Konofaos, P.; Ver Halen, J.P. Nerve repair by means of tubulization: Past, present, future. J. Reconstr. Microsurg. 2013, 29, 149–164. [Google Scholar] [CrossRef]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral nerve conduit: Materials and structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef]
- Harrison, R.G. The cultivation of tissues in extraneous media as a method of morpho-genetic study. Anat. Rec. 1912, 6, 181–194. [Google Scholar] [CrossRef]
- Zhou, F.; Yuan, L.; Huang, H.; Chen, H. Phenomenon of “contact guidance “on the surface with nano-micro-groove-like pattern and cell physiological effects. Chin. Sci. Bull. 2009, 54, 3200–3205. [Google Scholar] [CrossRef]
- Mobasseri, S.; Terenghi, G.; Downes, S. Micro-structural geometry of thin films intended for the inner lumen of nerve conduits affects nerve repair. J. Mater. Sci. Mater. Med. 2013, 24, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Arul, M.R.; Rudraiah, S.; Kalajzic, I.; Kumbar, S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: Small molecule drug delivery. J. Control. Release 2019, 296, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Rudraiah, S.; Arul, M.R.; Bartley, J.M.; Baker, J.T.; Yu, X.; Kumbar, S.G. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. Bioact. Mater. 2021, 6, 2881–2893. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. Part A 2007, 83A, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yan, S.; Liu, Y.; Liu, J.; Li, R.; Zhao, L.; Liu, B. Conductive and alignment-optimized porous fiber conduits with electrical stimulation for peripheral nerve regeneration. Mater. Today Bio. 2024, 26, 101064. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.M.; Laughter, M.R.; Lee, D.J.; Lee, Y.M.; Freed, C.R.; Park, D. A nerve guidance conduit with topographical and biochemical cues: Potential application using human neural stem cells. Nanoscale Res. Lett. 2015, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.; Oka, M.; Shima, M.; Taniguchi, A.; Akagi, M. Bridging a 30-mm nerve defect using collagen filaments. J. Biomed. Mater. Res. Part A 2003, 67, 467–474. [Google Scholar] [CrossRef]
- Spivey, E.C.; Khaing, Z.Z.; Shear, J.B.; Schmidt, C.E. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials 2012, 33, 4264–4276. [Google Scholar] [CrossRef]
- Aebischer, P.; Guénard, V.; Valentini, R.F. The morphology of regenerating peripheral nerves is modulated by the surface microgeometry of polymeric guidance channels. Brain Res. 1990, 531, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Drzeniek, N.M.; Mazzocchi, A.; Schlickeiser, S.; Forsythe, S.D.; Moll, G.; Geißler, S.; Reinke, P.; Gossen, M.; Gorantla, V.S.; Volk, H.-D.; et al. Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication 2021, 13, 045002. [Google Scholar] [CrossRef]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects. Adv. Sci. 2022, 9, 2103875. [Google Scholar] [CrossRef]
- Apablaza, J.A.; Lezcano, M.F.; Lopez Marquez, A.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration—A Scoping Review. Polymers 2021, 13, 2563. [Google Scholar] [CrossRef]
- Stanec, S.; Stanec, Z. Reconstruction of upper-extremity peripheral-nerve injuries with ePTFE conduits. J. Reconstr. Microsurg. 1998, 14, 227–232. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Dellon, A.L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast. Reconstr. Surg. 1990, 85, 419–424. [Google Scholar] [CrossRef]
- Weber, R.A.; Breidenbach, W.C.; Brown, R.E.; Jabaley, M.E.; Mass, D.P. A Randomized Prospective Study of Polyglycolic Acid Conduits for Digital Nerve Reconstruction in Humans. Plast. Reconstr. Surg. 2000, 106, 1036–1045. [Google Scholar] [CrossRef]
- Wan, T.; Wang, Y.-L.; Zhang, F.-S.; Zhang, X.-M.; Zhang, Y.-C.; Jiang, H.-R.; Zhang, M.; Zhang, P.-X. The Porous Structure of Peripheral Nerve Guidance Conduits: Features, Fabrication, and Implications for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 14132. [Google Scholar] [CrossRef]
- Zou, S.; Wang, X.; Fan, S.; Yao, X.; Zhang, Y.; Shao, H. Electrospun regenerated Antheraea pernyi silk fibroin scaffolds with improved pore size, mechanical properties and cytocompatibility using mesh collectors. J. Mater. Chem. B 2021, 9, 5514–5527. [Google Scholar] [CrossRef]
- Yu, J.; Lin, Y.; Wang, G.; Song, J.; Hayat, U.; Liu, C.; Raza, A.; Huang, X.; Lin, H.; Wang, J.-Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater. 2022, 140, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tringale, K.R.; Woller, S.A.; You, S.; Johnson, S.; Shen, H.; Schimelman, J.; Whitney, M.; Steinauer, J.; Xu, W.; et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today 2018, 21, 951–959. [Google Scholar] [CrossRef]
- Wang, W.Y.; Kent, R.N.; Huang, S.A.; Jarman, E.H.; Shikanov, E.H.; Davidson, C.D.; Hiraki, H.L.; Lin, D.; Wall, M.A.; Matera, D.L.; et al. Direct comparison of angiogenesis in natural and synthetic biomaterials reveals that matrix porosity regulates endothelial cell invasion speed and sprout diameter. Acta Biomater. 2021, 135, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Wang, H.; Zhao, Y.; Chai, R. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Appl. Mater. Today 2022, 27, 101431. [Google Scholar] [CrossRef]
- Kim, D.H.; Connolly, S.E.; Zhao, S.; Beuerman, R.W.; Voorhies, R.M.; Kline, D.G. Comparison of macropore, semipermeable, and nonpermeable collagen conduits in nerve repair. J. Reconstr. Microsurg. 1993, 9, 415–420. [Google Scholar] [CrossRef]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices Evid. Res. 2014, 7, 405–424. [Google Scholar]
- Casal, D.; Casimiro, M.H.; Ferreira, L.M.; Leal, J.P.; Rodrigues, G.; Lopes, R.; Moura, D.L.; Gonçalves, L.; Lago, J.B.; Pais, D.; et al. Review of Piezoelectrical Materials Potentially Useful for Peripheral Nerve Repair. Biomedicines 2023, 11, 3195. [Google Scholar] [CrossRef]
- Bianchini, M.; Micera, S.; Redolfi Riva, E. Recent Advances in Polymeric Drug Delivery Systems for Peripheral Nerve Regeneration. Pharmaceutics 2023, 15, 640. [Google Scholar] [CrossRef]
- Sanchez Rezza, A.; Kulahci, Y.; Gorantla, V.S.; Zor, F.; Drzeniek, N.M. Implantable Biomaterials for Peripheral Nerve Regeneration–Technology Trends and Translational Tribulations. Front. Bioeng. Biotechnol. 2022, 10, 863969. [Google Scholar] [CrossRef]
- Maksoud, F.J.; Velázquez de la Paz, M.F.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef] [PubMed]
- Alarcón Apablaza, J.; Lezcano, M.F.; Godoy Sánchez, K.; Oporto, G.H.; Dias, F.J. Optimal Morphometric Characteristics of a Tubular Polymeric Scaffold to Promote Peripheral Nerve Regeneration: A Scoping Review. Polymers 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Krarup, C.; Shefner, J.; Li, S.-T.; Madison, R.D. A collagen-based nerve guide conduit for peripheral nerve repair: An electrophysiological study of nerve regeneration in rodents and nonhuman primates. J. Comp. Neurol. 1991, 306, 685–696. [Google Scholar] [CrossRef]
- Freier, T.; Montenegro, R.; Shan Koh, H.; Shoichet, M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef]
- Pillai, M.M.; Sathishkumar, G.; Houshyar, S.; Senthilkumar, R.; Quigley, A.; Shanthakumari, S.; Padhye, R.; Bhattacharyya, A. Nanocomposite-Coated Silk-Based Artificial Conduits: The Influence of Structures on Regeneration of the Peripheral Nerve. ACS Appl. Bio Mater. 2020, 3, 4454–4464. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, M.-H.; Liao, S.-Y.; Wu, H.-C.; Kuan, C.-H.; Sun, J.-S.; Wang, T.-W. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl. Mater. Interfaces 2017, 9, 37623–37636. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Costa, J.B.; Costa, L.; Silva-Correia, J.; Moay, Z.K.; Ng, K.W.; Reis, R.L.; Oliveira, J.M. Enhanced performance of chitosan/keratin membranes with potential application in peripheral nerve repair. Biomater. Sci. 2019, 7, 5451–5466. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Bao, Y.; Yan, X.; Yin, Y.; Li, Y.; Wang, X.; Huang, Z.; Xu, P. Preparation and characterization of injectable chitosan–hyaluronic acid hydrogels for nerve growth factor sustained release. J. Bioact. Compat. Polym. 2017, 32, 146–162. [Google Scholar] [CrossRef]
- Sun, D. Sacrificial gelatin of PAM-Alginate-BC hydrogel tube with tunable diameter as nerve conduit. J. Biomater. Sci. Polym. Ed. 2023, 34, 1398–1407. [Google Scholar] [CrossRef]
- Chato-Astrain, J.; Campos, F.; Roda, O.; Miralles, E.; Durand-Herrera, D.; Sáez-Moreno, J.A.; García-García, S.; Alaminos, M.; Campos, A.; Carriel, V. In vivo Evaluation of Nanostructured Fibrin-Agarose Hydrogels with Mesenchymal Stem Cells for Peripheral Nerve Repair. Front. Cell. Neurosci. 2018, 12, 501. [Google Scholar] [CrossRef]
- Chiono, V.; Tonda-Turo, C.; Ciardelli, G. Chapter 9 Artificial Scaffolds for Peripheral Nerve Reconstruction. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2009; Volume 87, pp. 173–198. [Google Scholar]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, A.; Bunge, R.; Duncan, I.; Wood, P.; Bray, G. Rat Schwann-Cells, Cultured Invitro, Can Ensheath Axons Regenerating In Mouse Nerves. In Neurology; Lippincott-Raven Publisher: Philadelphia, PA, USA, 1979; Volume 29, p. 589. [Google Scholar]
- Duncan, I.; Aguayo, A.; Bunge, R.; Wood, P. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J. Neurol. Sci. 1981, 49, 241–252. [Google Scholar] [CrossRef]
- Evans, G.R.D.; Brandt, K.; Katz, S.; Chauvin, P.; Otto, L.; Bogle, M.; Wang, B.; Meszlenyi, R.K.; Lu, L.; Mikos, A.G.; et al. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23, 841–848. [Google Scholar] [CrossRef]
- Takeya, H.; Itai, S.; Kimura, H.; Kurashina, Y.; Amemiya, T.; Nagoshi, N.; Iwamoto, T.; Sato, K.; Shibata, S.; Matsumoto, M.; et al. Schwann cell-encapsulated chitosan-collagen hydrogel nerve conduit promotes peripheral nerve regeneration in rodent sciatic nerve defect models. Sci. Rep. 2023, 13, 11932. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell. Neurosci. 2022, 16, 929593. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Krichevsky, A.; Sumarto, A.; Jaffurs, D.; Wirth, G.A.; Paydar, K.; Evans, G.R.D. Peripheral Nerve Injuries: An International Survey of Current Treatments and Future Perspectives. J. Reconstr. Microsurg. 2009, 25, 339–344. [Google Scholar] [CrossRef]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells 2020, 9, 2497. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.; Lee, D.Y.; Kim, Y.D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell–based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Ladak, A.; Olson, J.; Tredget, E.E.; Gordon, T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp. Neurol. 2011, 228, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, S.; Feng, G.-Y.; Zhang, L.-P.; Zhao, D.-M.; Sun, Y.; Liu, Q.; Huang, F. Neural Stem Cells Enhance Nerve Regeneration after Sciatic Nerve Injury in Rats. Mol. Neurobiol. 2012, 46, 265–274. [Google Scholar] [CrossRef]
- di Summa, P.G.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Kingham, P.J.; Terenghi, G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 2011, 181, 278–291. [Google Scholar] [CrossRef]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef]
- Gordon, T. The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus FOC 2009, 26, E3. [Google Scholar] [CrossRef]
- Alastra, G.; Aloe, L.; Baldassarro, V.A.; Calzà, L.; Cescatti, M.; Duskey, J.T.; Focarete, M.L.; Giacomini, D.; Giardino, L.; Giraldi, V.; et al. Nerve Growth Factor Biodelivery: A Limiting Step in Moving Toward Extensive Clinical Application? Front. Neurosci. 2021, 15, 695592. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhang, F.-S.; Qin, M.-Y.; Jiang, H.-R.; Zhang, M.; Qu, Y.; Wang, Y.-L.; Zhang, P.-X. Growth factors: Bioactive macromolecular drugs for peripheral nerve injury treatment—Molecular mechanisms and delivery platforms. Biomed. Pharmacother. 2024, 170, 116024. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Schwartz, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and Safety of Recombinant Human Nerve Growth Factor in Patients with Diabetic PolyneuropathyA Randomized Controlled Trial. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef]
- McArthur, J.; Yiannoutsos, C.; Simpson, D.; Adornato, B.; Singer, E.; Hollander, H.; Marra, C.; Rubin, M.; Cohen, B.; Tucker, T. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. Neurology 2000, 54, 1080–1088. [Google Scholar] [CrossRef]
- Hamrah, P.; Massaro-Giordano, M.; Schanzlin, D.; Holland, E.; Berdy, G.; Goisis, G.; Pasedis, G.; Mantelli, F. Phase IV Multicenter, Prospective, Open-Label Clinical Trial of Cenegermin (rhNGF) for Stage 1 Neurotrophic Keratopathy (DEFENDO). Ophthalmol. Ther. 2024, 13, 553–570. [Google Scholar] [CrossRef]
- Fu, K.; Klibanov, A.M.; Langer, R. Protein stability in controlled-release systems. Nat. Biotechnol. 2000, 18, 24–25. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Doolabh, V.B.; Novak, C.B.; Trulock, E.P. Clinical outcome following nerve allograft transplantation. Plast. Reconstr. Surg. 2001, 107, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Lyons, W.E.; George, E.B.; Dawson, T.M.; Steiner, J.P.; Snyder, S.H. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc. Natl. Acad. Sci. USA 1994, 91, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Daeschler, S.C.; Feinberg, K.; Harhaus, L.; Kneser, U.; Gordon, T.; Borschel, G.H. Advancing Nerve Regeneration: Translational Perspectives of Tacrolimus (FK506). Int. J. Mol. Sci. 2023, 24, 12771. [Google Scholar] [CrossRef]
- Xiao, B.; Feturi, F.; Su, A.-J.A.; Van der Merwe, Y.; Barnett, J.M.; Jabbari, K.; Khatter, N.J.; Li, B.; Katzel, E.B.; Venkataramanan, R.; et al. Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 847. [Google Scholar] [CrossRef]
- Seixas, S.F.; Forte, G.C.; Magnus, G.A.; Stanham, V.; Mattiello, R.; Silva, J.B. Effect of Tacrolimus and Cyclosporine Immunosuppressants on Peripheral Nerve Regeneration: Systematic Review and Meta-analysis. Rev. Bras. Ortop. 2022, 57, 207–213. [Google Scholar]
- Naesens, M.; Kuypers, D.R.J.; Sarwal, M. Calcineurin Inhibitor Nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef]
- Zuo, K.J.; Saffari, T.M.; Chan, K.; Shin, A.Y.; Borschel, G.H. Systemic and Local FK506 (Tacrolimus) and its Application in Peripheral Nerve Surgery. J. Hand Surg. 2020, 45, 759–765. [Google Scholar] [CrossRef]
- Bolandghamat, S.; Behnam-Rassouli, M. Recent Findings on the Effects of Pharmacological Agents on the Nerve Regeneration after Peripheral Nerve Injury. Curr. Neuropharmacol. 2020, 18, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Komatsu, D.E.; Gurevich, M.; Hurst, L.C. Emerging Strategies on Adjuvant Therapies for Nerve Recovery. J. Hand. Surg. Am. 2018, 43, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Zhu, C.; Yin, J.-B.; Zhang, T.; Lu, Y.-C.; Ren, J.; Li, Y.-Q. Slow-releasing rapamycin-coated bionic peripheral nerve scaffold promotes the regeneration of rat sciatic nerve after injury. Life Sci. 2015, 122, 92–99. [Google Scholar] [CrossRef]
- Degrugillier, L.; Prautsch, K.M.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Schären, S.; Madduri, S. Systematic Investigation and Comparison of US FDA-Approved Immunosuppressive Drugs FK506, Cyclosporine and Rapamycin for Neuromuscular Regeneration following Chronic Nerve Compression Injury. Regen. Med. 2021, 16, 989–1003. [Google Scholar] [CrossRef]
- Rahim, M.; Hadi, H.; Keyvan, A. Effect of local administration of cyclosporine A on peripheral nerve regeneration in a rat sciatic nerve transection model. Chin. J. Traumatol. 2014, 17, 12–18. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Cao, X.-c.; Luo, D.-q.; Lian, K.-j. Methylprednisolone microsphere sustained-release membrane inhibits scar formation at the site of peripheral nerve lesion. Neural Regen. Res. 2016, 11, 835–841. [Google Scholar] [CrossRef]
- Mohammadi, R.; Azad-Tirgan, M.; Amini, K. Dexamethasone topically accelerates peripheral nerve repair and target organ reinnervation: A transected sciatic nerve model in rat. Injury 2013, 44, 565–569. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Vaez, A.; Esmaeilpour, F.; Ehterami, A.; Sahrapeyma, H.; Samadian, H.; Hamidieh, A.-A.; Ghorbani, S.; Goodarzi, A.; et al. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose 2018, 25, 1229–1238. [Google Scholar] [CrossRef]
- Wu, F.; Jiao, C.; Yang, Y.; Liu, F.; Sun, Z. Nerve conduit based on HAP/PDLLA/PRGD for peripheral nerve regeneration with sustained release of valproic acid. Cell Biol. Int. 2021, 45, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, Q.; Zhao, X.; Song, J.; Cheng, Y.; Fang, Z.; Ouyang, Y.; Yuan, W.-E.; Fan, C. 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J. Pineal Res. 2018, 65, e12516. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, C.; Hai, B.; Ma, T.; Zhang, W.; Tan, J.; Fu, X.; Wang, H.; Xu, Y.; Song, C. Chitosan conduits filled with simvastatin/Pluronic F-127 hydrogel promote peripheral nerve regeneration in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 787–799. [Google Scholar] [CrossRef]
- Sayanagi, J.; Tanaka, H.; Ebara, M.; Okada, K.; Oka, K.; Murase, T.; Yoshikawa, H. Combination of Electrospun Nanofiber Sheet Incorporating Methylcobalamin and PGA-Collagen Tube for Treatment of a Sciatic Nerve Defect in a Rat Model. J. Bone Jt. Surg. 2020, 102, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Funnell, J.L.; Balouch, B.; Gilbert, R.J. Magnetic Composite Biomaterials for Neural Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Calatayud, M.P.; Poggetti, A.; Corbianco, S.; Novelli, M.; Paoli, M.; Battistini, P.; Castagna, M.; Dente, L.; Parchi, P.; et al. Magnetic Nanoparticles for Efficient Delivery of Growth Factors: Stimulation of Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2017, 6, 1601429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shan, K.; Song, J.; Liu, J.; Rajendran, S.; Pugazhendhi, A.; Jacob, J.A.; Chen, B. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019, 220, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Xu, L.; Cui, X.; Wang, E.; Jiang, F.; Li, J.; Ouyang, H.; Yin, T.; Feng, H.; Luo, D.; et al. A responsive cascade drug delivery scaffold adapted to the therapeutic time window for peripheral nerve injury repair. Mater. Horiz. 2024, 11, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.C.; Errante, E.L.; Smartz, T.; Ray, W.Z.; Levi, A.D.; Burks, S.S. Clinical applications of electrical stimulation for peripheral nerve injury: A systematic review. Front. Neurosci. 2023, 17, 1162851. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Plunkett, A.; Vijjeswarapu, A.M.; Hackworth, R.; Dhanjal, S.; Turan, A.; Cohen, S.P.; Eisenach, J.C.; Griffith, S.; Hanling, S.; et al. Percutaneous Neuromodulation of the Brachial Plexus and Sciatic Nerve for the Treatment of Acute Pain Following Surgery: Secondary Outcomes from a Multicenter, Randomized, Controlled Pilot Study. Neuromodulation 2023, 26, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Luckey, A.M.; McLeod, S.L.; Robertson, I.H.; To, W.T.; Vanneste, S. Greater Occipital Nerve Stimulation Boosts Associative Memory in Older Individuals: A Randomized Trial. Neurorehabil Neural. Repair. 2020, 34, 1020–1029. [Google Scholar] [CrossRef]
- Hoffman, H. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust. J. Exp. Biol. Med. Sci. 1952, 30, 541–566. [Google Scholar] [CrossRef]
- Nix, W.A.; Hopf, H. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983, 272, 21–25. [Google Scholar] [CrossRef]
- Pockett, S.; Gavin, R. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci. Lett. 1985, 59, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.M.; Xu, Y.; Geng, D.F.; Yan, T.B. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: A meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2010, 89, 10–15. [Google Scholar] [CrossRef]
- Piccinini, G.; Cuccagna, C.; Caliandro, P.; Coraci, D.; Germanotta, M.; Pecchioli, C.; Padua, L. Efficacy of electrical stimulation of denervated muscle: A multicenter, double-blind, randomized clinical trial. Muscle Nerve 2020, 61, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.B. A clinical pilot study to assess functional return following continuous muscle stimulation after nerve injury and repair in the upper extremity using a completely implantable electrical system. Microsurgery 1996, 17, 597–605. [Google Scholar] [CrossRef]
- Barber, B.; Seikaly, H.; Ming Chan, K.; Beaudry, R.; Rychlik, S.; Olson, J.; Curran, M.; Dziegielewski, P.; Biron, V.; Harris, J.; et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: A double-blinded, randomized controlled trial. J. Otolaryngol.-Head Neck Surg. 2018, 47, 7. [Google Scholar] [CrossRef]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: A randomized controlled trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Power, H.A.; Morhart, M.J.; Olson, J.L.; Chan, K.M. Postsurgical Electrical Stimulation Enhances Recovery Following Surgery for Severe Cubital Tunnel Syndrome: A Double-Blind Randomized Controlled Trial. Neurosurgery 2020, 86, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef]
- Franz, C.K.; Rutishauser, U.; Rafuse, V.F. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain 2008, 131, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Schwartz, G.; Meador, W.; Sabatier, M.J.; Mulligan, A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007, 67, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp. Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef]
- Aglah, C.; Gordon, T.; Posse de Chaves, E.I. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology 2008, 55, 8–17. [Google Scholar] [CrossRef]
- Gordon, T.; Udina, E.; Verge, V.M.; de Chaves, E.I. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor. Control. 2009, 13, 412–441. [Google Scholar] [CrossRef]
- Wan, L.; Xia, R.; Ding, W. Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neurotrophic factor on Schwann cell polarization. J. Neurosci. Res. 2010, 88, 2578–2587. [Google Scholar] [CrossRef]
- Koppes, A.N.; Nordberg, A.L.; Paolillo, G.M.; Goodsell, N.M.; Darwish, H.A.; Zhang, L.; Thompson, D.M. Electrical stimulation of schwann cells promotes sustained increases in neurite outgrowth. Tissue Eng. Part A 2014, 20, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ye, Z.; Hu, X.; Lu, L.; Luo, Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 2010, 58, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Marzo, S.J.; Jones, K.J.; Foecking, E.M. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp. Neurol. 2010, 223, 183–191. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Tam, S.L.; Gordon, T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol. Neurobiol. 2004, 24, 379–402. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief Electrical Stimulation Promotes the Speed and Accuracy of Motor Axonal Regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiu, X.; Wang, P.; Han, Y.; Chang, W.; Zhao, J. Intraoperative electrical stimulation promotes the short-term recovery of patients with cubital tunnel syndrome after surgery. J. Orthop. Surg. Res. 2023, 18, 270. [Google Scholar] [CrossRef]
- Maeng, W.-Y.; Tseng, W.-L.; Li, S.; Koo, J.; Hsueh, Y.-Y. Electroceuticals for peripheral nerve regeneration. Biofabrication 2022, 14, 042002. [Google Scholar] [CrossRef]
- Huang, J.; Lu, L.; Zhang, J.; Hu, X.; Zhang, Y.; Liang, W.; Wu, S.; Luo, Z. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. PLoS ONE 2012, 7, e39526. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, B.; Liu, S.; Chen, W.; Zhang, Y.; Wang, C.; Mo, X.; Che, J.; Ouyang, Y.; Yuan, W.; et al. Polymerizing Pyrrole Coated Poly (l-lactic acid-co-ε-caprolactone) (PLCL) Conductive Nanofibrous Conduit Combined with Electric Stimulation for Long-Range Peripheral Nerve Regeneration. Front. Mol. Neurosci. 2016, 9, 117. [Google Scholar] [CrossRef]
- Moroder, P.; Runge, M.B.; Wang, H.; Ruesink, T.; Lu, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J. Material properties and electrical stimulation regimens of polycaprolactone fumarate–polypyrrole scaffolds as potential conductive nerve conduits. Acta Biomater. 2011, 7, 944–953. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Y.; Chen, F.; Xiao, A.; Du, Q.; Dong, Q.; Ke, M.; Liang, X.; Zhou, Q.; Chen, Y. Conductive Hydroxyethyl Cellulose/Soy Protein Isolate/Polyaniline Conduits for Enhancing Peripheral Nerve Regeneration via Electrical Stimulation. Front. Bioeng. Biotechnol. 2020, 8, 709. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrical Stimulation of Nerve Cells Using Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. Tissue Eng. Part A 2009, 15, 3605–3619. [Google Scholar] [CrossRef]
- Abidian, M.R.; Daneshvar, E.D.; Egeland, B.M.; Kipke, D.R.; Cederna, P.S.; Urbanchek, M.G. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv. Healthc. Mater. 2012, 1, 762–767. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef] [PubMed]
- Shlapakova, L.E.; Surmeneva, M.A.; Kholkin, A.L.; Surmenev, R.A. Revealing an important role of piezoelectric polymers in nervous-tissue regeneration: A review. Mater. Today Bio 2024, 25, 100950. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, Y.; Deng, J.; Saif Ur Rahman, M.; Sun, C.; Xu, S. Physical Stimulation Combined with Biomaterials Promotes Peripheral Nerve Injury Repair. Bioengineering 2022, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, Y.; Song, J.; Xu, Y.; Yuan, W.-E.; Fan, C.; Zheng, X. Mechano-Informed Biomimetic Polymer Scaffolds by Incorporating Self-Powered Zinc Oxide Nanogenerators Enhance Motor Recovery and Neural Function. Small 2020, 16, 2000796. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, T.; Yuan, T.; Du, L.; Lai, C.; Wu, Q.; Zhao, Y.; Sun, F.; Gu, L.; Wang, T.; et al. Physiologically Self-Regulated, Fully Implantable, Battery-Free System for Peripheral Nerve Restoration. Adv. Mater. 2021, 33, 2104175. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, H.; Faghihi, A.; Azizzadeh Delshad, A.; Bahadori, M.H.; Mohamadi, J.; Roozbehi, A. Repair of peripheral nerve defects using a polyvinylidene fluoride channel containing nerve growth factor and collagen gel in adult rats. Cell J. 2011, 13, 137–142. [Google Scholar] [PubMed]
- Biazar, E.; Keshel, S.H. Gelatin-Modified Nanofibrous PHBV Tube as Artificial Nerve Graft for Rat Sciatic Nerve Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 330–336. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, X.; Wang, H.; Qiu, W.; Li, L.; Li, F.; Shan, Y.; Zhao, Z.; Li, Z.; Guo, J.; et al. Engineering a wirelessly self-powered and electroconductive scaffold to promote peripheral nerve regeneration. Nano Energy 2023, 107, 108145. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Guo, H.; D’Andrea, D.; Zhao, J.; Xu, Y.; Qiao, Z.; Janes, L.E.; Murthy, N.K.; Li, R.; Xie, Z.; Song, Z.; et al. Advanced Materials in Wireless, Implantable Electrical Stimulators that Offer Rapid Rates of Bioresorption for Peripheral Axon Regeneration. Adv. Funct. Mater. 2021, 31, 2102724. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; MacEwan, M.R.; Kang, S.-K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.-Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Hsueh, Y.-Y.; Koo, J.; Yang, Q.; Avila, R.; Hu, B.; Xie, Z.; Lee, G.; Ning, Z.; Liu, C.; et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nat. Commun. 2020, 11, 5990. [Google Scholar] [CrossRef]
- Philipeaux, J.; Vulpian, A. Note sur des essais de greffe d’un troncon du nerf lingual entre les deux bouts du nerf hypoglosse, apres excision d’un segment de ce dernier nerf. Arch. Physiol. Norm. Pathol. 1870, 3, 618–620. [Google Scholar]
- Dellon, E.S.; Dellon, A.L. The first nerve graft, Vulpian, and the nineteenth century neural regeneration controversy. J. Hand. Surg. Am. 1993, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Sherren, J. Some points in the surgery of the peripheral nerves. Edinb. Med. J. 1906, 20, 297. [Google Scholar]
- Little, K.M.; Zomorodi, A.R.; Selznick, L.A.; Friedman, A.H. An eclectic history of peripheral nerve surgery. Neurosurg. Clin. N. Am. 2004, 15, 109–123. [Google Scholar] [CrossRef]
- Beris, A.; Gkiatas, I.; Gelalis, I.; Papadopoulos, D.; Kostas-Agnantis, I. Current concepts in peripheral nerve surgery. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef]
- Pan, D.; Mackinnon, S.E.; Wood, M.D. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle Nerve 2020, 61, 726–739. [Google Scholar] [CrossRef]
- Ducic, I.; Yoon, J.; Buncke, G. Chronic postoperative complications and donor site morbidity after sural nerve autograft harvest or biopsy. Microsurgery 2020, 40, 710–716. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O’Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2017, 3, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; El-Hawary, H.; Efanov, J.I.; Xu, L. Peripheral Nerve Healing: So Near and Yet So Far. Semin. Plast. Surg. 2021, 35, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Isaacs, J. Management of “Long” Nerve Gaps. J. Hand Surg. Glob. Online 2024. [Google Scholar] [CrossRef]
- Thomson, S.E.; Ng, N.Y.; Riehle, M.O.; Kingham, P.J.; Dahlin, L.B.; Wiberg, M.; Hart, A.M. Bioengineered nerve conduits and wraps for peripheral nerve repair of the upper limb. Cochrane Database Syst. Rev. 2022, 12, Cd012574. [Google Scholar] [CrossRef] [PubMed]
- Albert, E. Einige operationen an nerven. Wien Med. Presse 1885, 26, 1285–1288. [Google Scholar]
- Boyd, K.U.; Nimigan, A.S.; Mackinnon, S.E. Nerve reconstruction in the hand and upper extremity. Clin. Plast. Surg. 2011, 38, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Hudson, A.R. Clinical application of peripheral nerve transplantation. Plast. Reconstr. Surg. 1992, 90, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Azouz, S.M.; Lucas, H.D.; Mahabir, R.C.; Noland, S.S. A survey of the prevalence and practice patterns of human acellular nerve allograft use. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1803. [Google Scholar] [CrossRef]
- Johnson, P.; Wood, M.; Moore, A.; Mackinnon, S. Tissue engineered constructs for peripheral nerve surgery. Eur. Surg. 2013, 45, 122–135. [Google Scholar] [CrossRef]
- Rinker, B.D.; Ingari, J.V.; Greenberg, J.A.; Thayer, W.P.; Safa, B.; Buncke, G.M. Outcomes of short-gap sensory nerve injuries reconstructed with processed nerve allografts from a multicenter registry study. J. Reconstr. Microsurg. 2015, 31, 384–390. [Google Scholar] [PubMed]
- Isaacs, J.; Browne, T. Overcoming short gaps in peripheral nerve repair: Conduits and human acellular nerve allograft. Hand 2014, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Koob, J.W.; Liu, D.Z.; Tong, A.Y.; Hunter, D.A.; Parsadanian, A.; Mackinnon, S.E.; Myckatyn, T.M. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp. Neurol. 2007, 207, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Flores, A.; Geronimo-Olvera, C.; Girardi, K.; Necuñir-Ibarra, D.; Patel, S.K.; Bons, J.; Wright, M.C.; Geschwind, D.; Hoke, A.; Gomez-Sanchez, J.A.; et al. Senescent Schwann cells induced by aging and chronic denervation impair axonal regeneration following peripheral nerve injury. EMBO Mol. Med. 2023, 15, e17907. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.R.; Gordon, T. Role of chronic schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery 2009, 65, A105–A114. [Google Scholar] [CrossRef] [PubMed]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Feger, M.A.; Mallu, S.; Patel, G.; Debkowska, M.; Yager, D.; Ernst, B.; Chilukuri, S.; Moser, M.; Kurtz, C. Side-to-side supercharging nerve allograft enhances neurotrophic potential. Muscle Nerve 2020, 61, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Patel, G.; Mallu, S.; Ugwu-Oju, O.; Desai, A.; Borschel, G.; David, D.; Protzuk, O.; Shah, S.; Semus, R. Effect of Reverse End-to-Side (Supercharging) Neurotization in Long Processed Acellular Nerve Allograft in a Rat Model. J. Hand. Surg. Am. 2019, 44, 419.e1–419.e10. [Google Scholar] [CrossRef]
- Isaacs, J. Reverse End-to-Side (Supercharging) Nerve Transfer: Conceptualization, Validation, and Translation. Hand 2022, 17, 1017–1023. [Google Scholar] [CrossRef]
- Golshadi, M.; Claffey, E.F.; Grenier, J.K.; Miller, A.; Willand, M.; Edwards, M.G.; Moore, T.P.; Sledziona, M.; Gordon, T.; Borschel, G.H.; et al. Delay modulates the immune response to nerve repair. NPJ Regen. Med. 2023, 8, 12. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Zeugolis, D.I. Decellularized xenografts in regenerative medicine: From processing to clinical application. Xenotransplantation 2021, 28, e12683. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Zhu, G.; Lou, W. Regeneration of facial nerve defects with xenogeneic acellular nerve grafts in a rat model. Head Neck 2014, 36, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Bolívar, S.; Nieto-Nicolau, N.; Fariñas, O.; López-Chicón, P.; Navarro, X.; Udina, E. A novel decellularized nerve graft for repairing peripheral nerve long gap injury in the rat. Cell Tissue Res. 2022, 390, 355–366. [Google Scholar] [CrossRef]
- Kilic, A.; Ojo, B.; Rajfer, R.A.; Konopka, G.; Hagg, D.; Jang, E.; Akelina, Y.; Mao, J.J.; Rosenwasser, M.P.; Tang, P. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery 2013, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, U.; Kostakoglu, N.; Turan, A.; Çevik, B.; Çayli, S.; Demir, O.; Elmas, C. The effect of autologous fat graft with different surgical repair methods on nerve regeneration in a rat sciatic nerve defect model. Plast. Reconstr. Surg. 2015, 136, 1181–1191. [Google Scholar] [CrossRef]
- Bloancă, V.; Ceauşu, A.R.; Jitariu, A.A.; Barmayoun, A.; Moş, R.; Crăiniceanu, Z.; Bratu, T. Adipose tissue graft improves early but not late stages of nerve regeneration. In Vivo 2017, 31, 649–655. [Google Scholar]
- Dehdashtian, A.; Bratley, J.V.; Svientek, S.R.; Kung, T.A.; Awan, T.M.; Cederna, P.S.; Kemp, S.W. Autologous fat grafting for nerve regeneration and neuropathic pain: Current state from bench-to-bedside. Regen. Med. 2020, 15, 2209–2228. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Rhodes, D.I.; O’Brien, C.M.; Rodda, A.E.; Cameron, N.R. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef]
- Crook, B.S.; Cullen, M.M.; Pidgeon, T.S. The Role of Tissue Engineering and Three-Dimensional–Filled Conduits in Bridging Nerve Gaps: A Review of Recent Advancements. J. Hand Surg. Glob. Online 2024. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int. J. Mol. Sci. 2023, 24, 9170. [Google Scholar] [CrossRef] [PubMed]
- Broeren, B.O.; Hundepool, C.A.; Kumas, A.H.; Duraku, L.S.; Walbeehm, E.T.; Hooijmans, C.R.; Power, D.M.; Zuidam, J.M.; De Jong, T. The effectiveness of acellular nerve allografts compared to autografts in animal models: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0279324. [Google Scholar] [CrossRef]
- Frostadottir, D.; Chemnitz, A.; Johansson OT, L.J.; Holst, J.; Dahlin, L.B. Evaluation of Processed Nerve Allograft in Peripheral Nerve Surgery: A Systematic Review and Critical Appraisal. Plast. Reconstr. Surg.-Glob. Open 2023, 11, e5088. [Google Scholar] [CrossRef] [PubMed]
- Lans, J.; Eberlin, K.R.; Evans, P.J.; Mercer, D.; Greenberg, J.A.; Styron, J.F. A Systematic Review and Meta-Analysis of Nerve Gap Repair: Comparative Effectiveness of Allografts, Autografts, and Conduits. Plast. Reconstr. Surg. 2023, 151, 814e–827e. [Google Scholar] [CrossRef] [PubMed]
- Mauch, J.T.; Bae, A.; Shubinets, V.; Lin, I.C. A Systematic Review of Sensory Outcomes of Digital Nerve Gap Reconstruction With Autograft, Allograft, and Conduit. Ann. Plast. Surg. 2019, 82, S247–S255. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.; Schneider, J.M.; Pohl, U.; Power, D.M. Failed Acellular Nerve Allografts: A Critical Review. Ann. Plast. Surg. 2022, 89, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.R.; Wood, M.D.; Hunter, D.A.; Mackinnon, S.E. Acellular Nerve Allografts in Major Peripheral Nerve Repairs: An Analysis of Cases Presenting with Limited Recovery. HAND 2023, 18, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Nietosvaara, Y.; Grahn, P.; Sommarhem, A. Failed peripheral nerve reconstruction with processed nerve allografts in three patients. J. Hand Surg. 2019, 44, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, Y.A.; Almeida, V.W.; Levi, A.D. Limitations of nerve repair of segmental defects using acellular conduits: Case report. J. Neurosurg. 2013, 119, 733–738. [Google Scholar] [CrossRef]
- Chrząszcz, P.; Derbisz, K.; Suszyński, K.; Miodoński, J.; Trybulski, R.; Lewin-Kowalik, J.; Marcol, W. Application of peripheral nerve conduits in clinical practice: A literature review. Neurol. Neurochir. Pol. 2018, 52, 427–435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).