Reverse Engineering Orthognathic Surgery and Orthodontics in Individuals with Cleft Lip and/or Palate: A Case Report
Abstract
1. Introduction
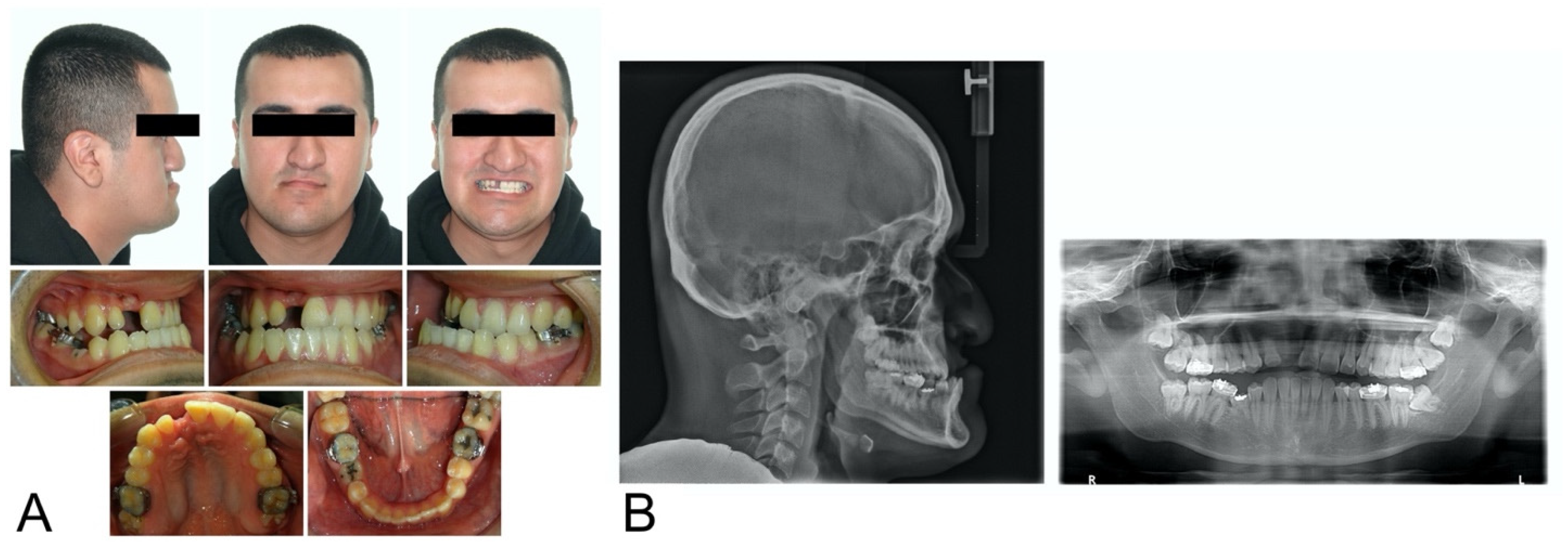
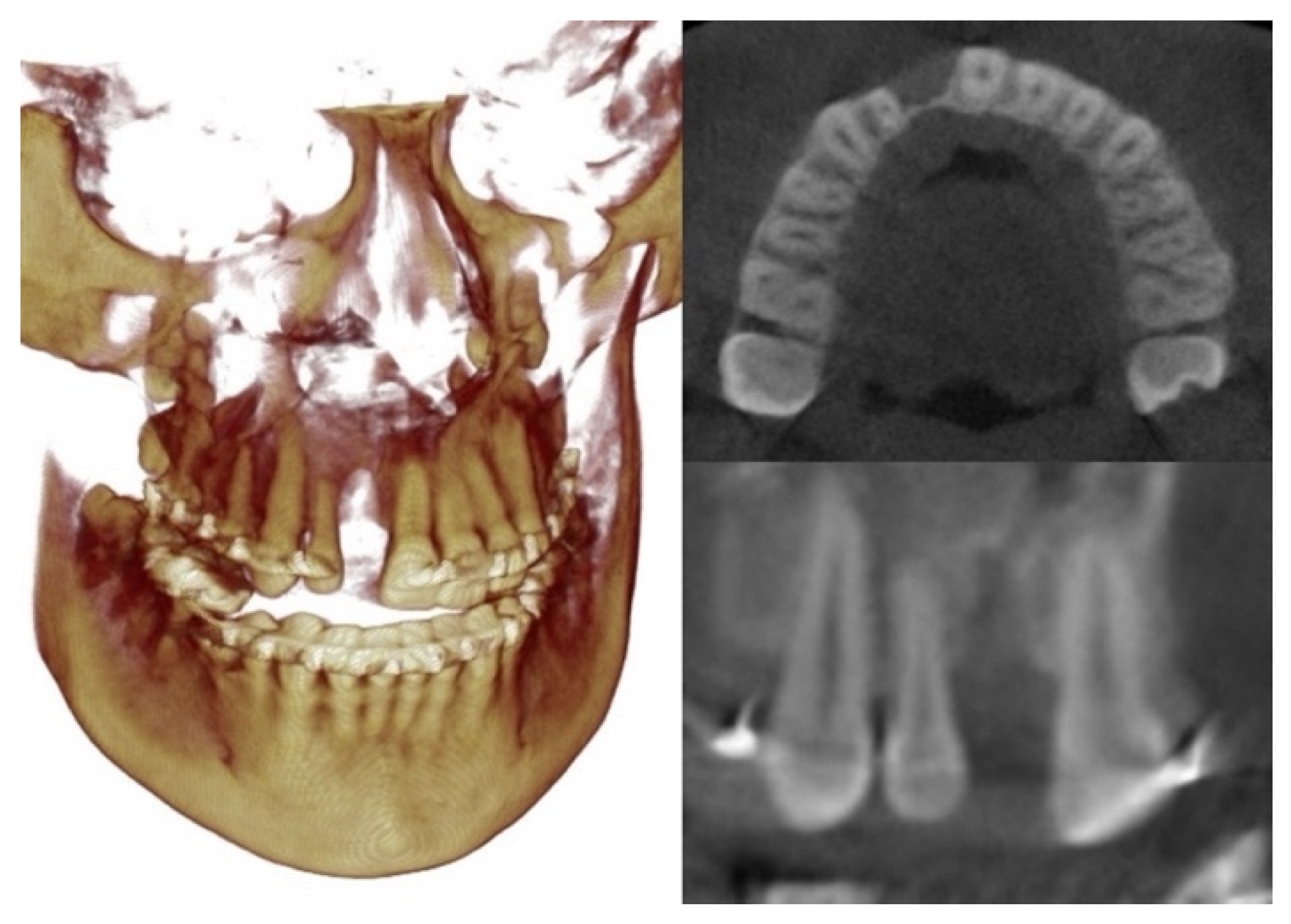
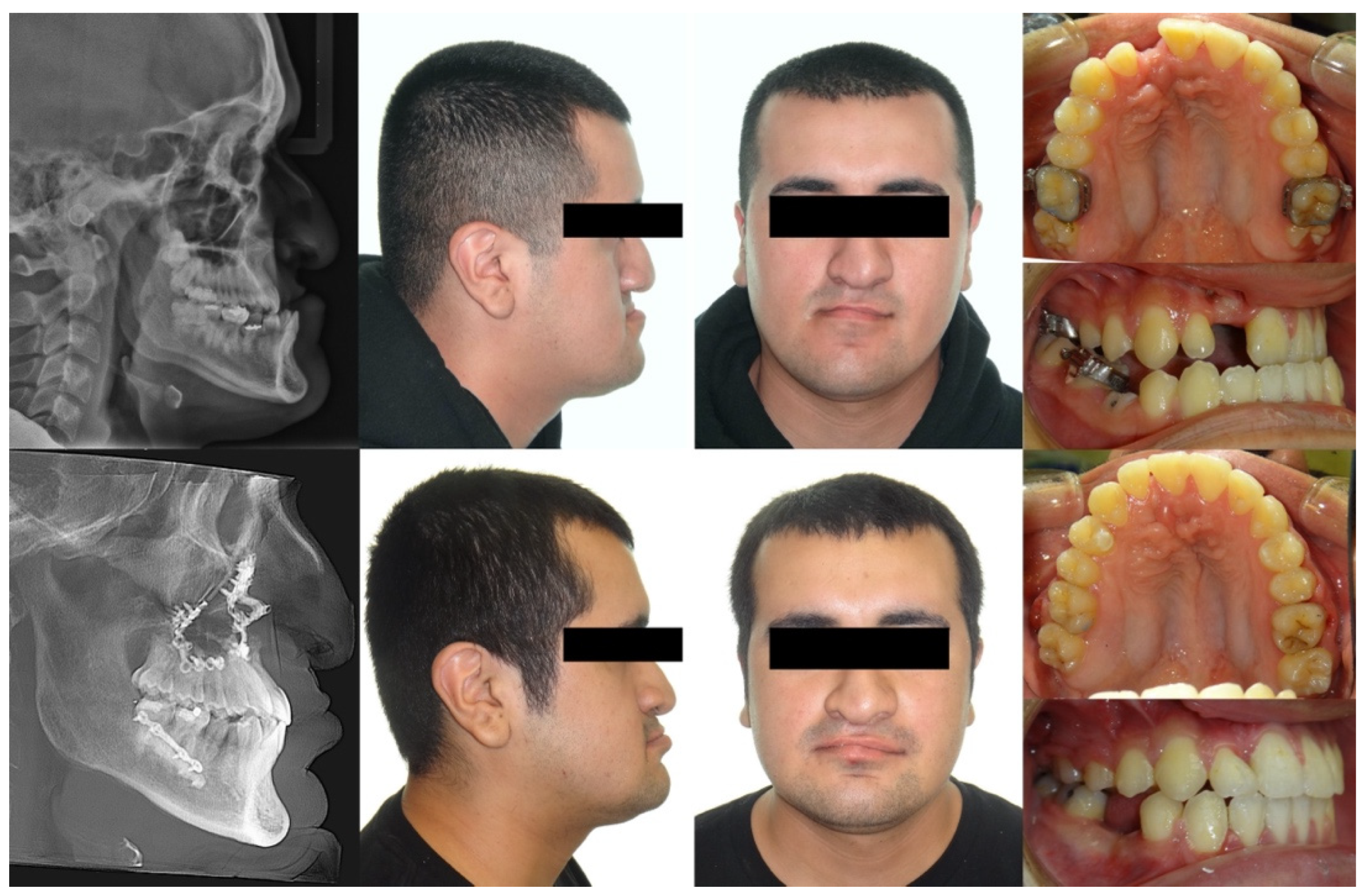
2. Case Description
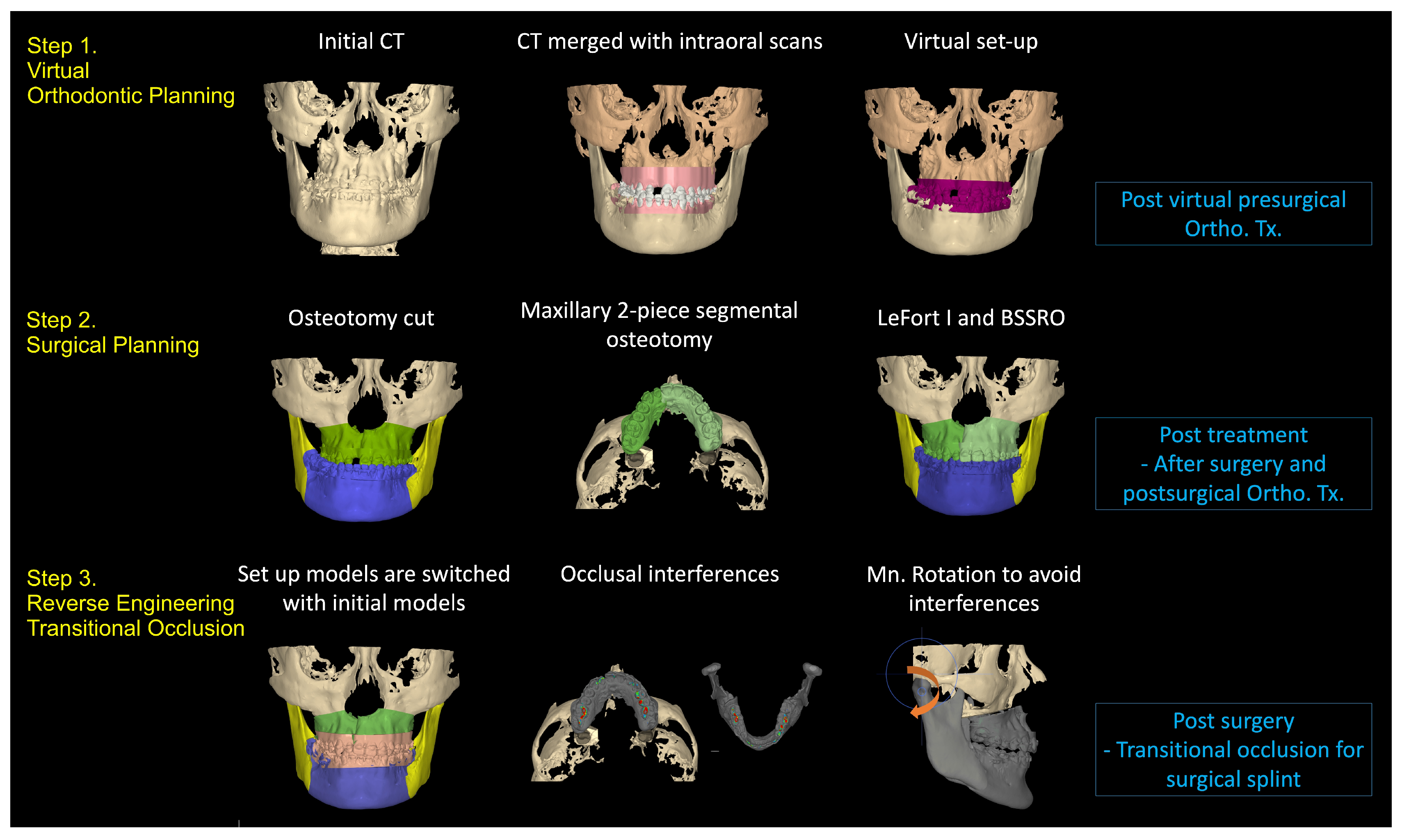
3. Virtual Treatment Planning
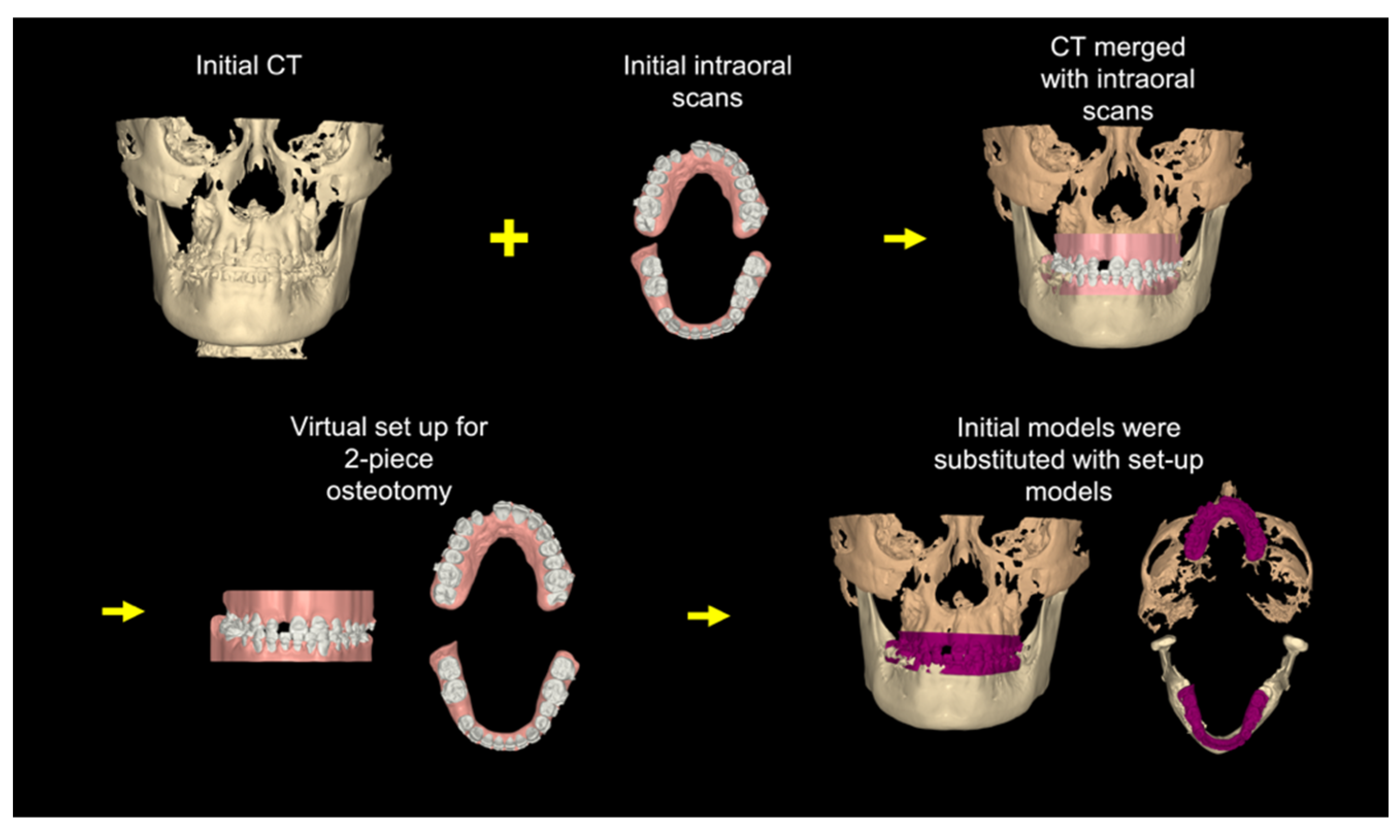
3.1. Step 1: Virtual Orthodontic Setup
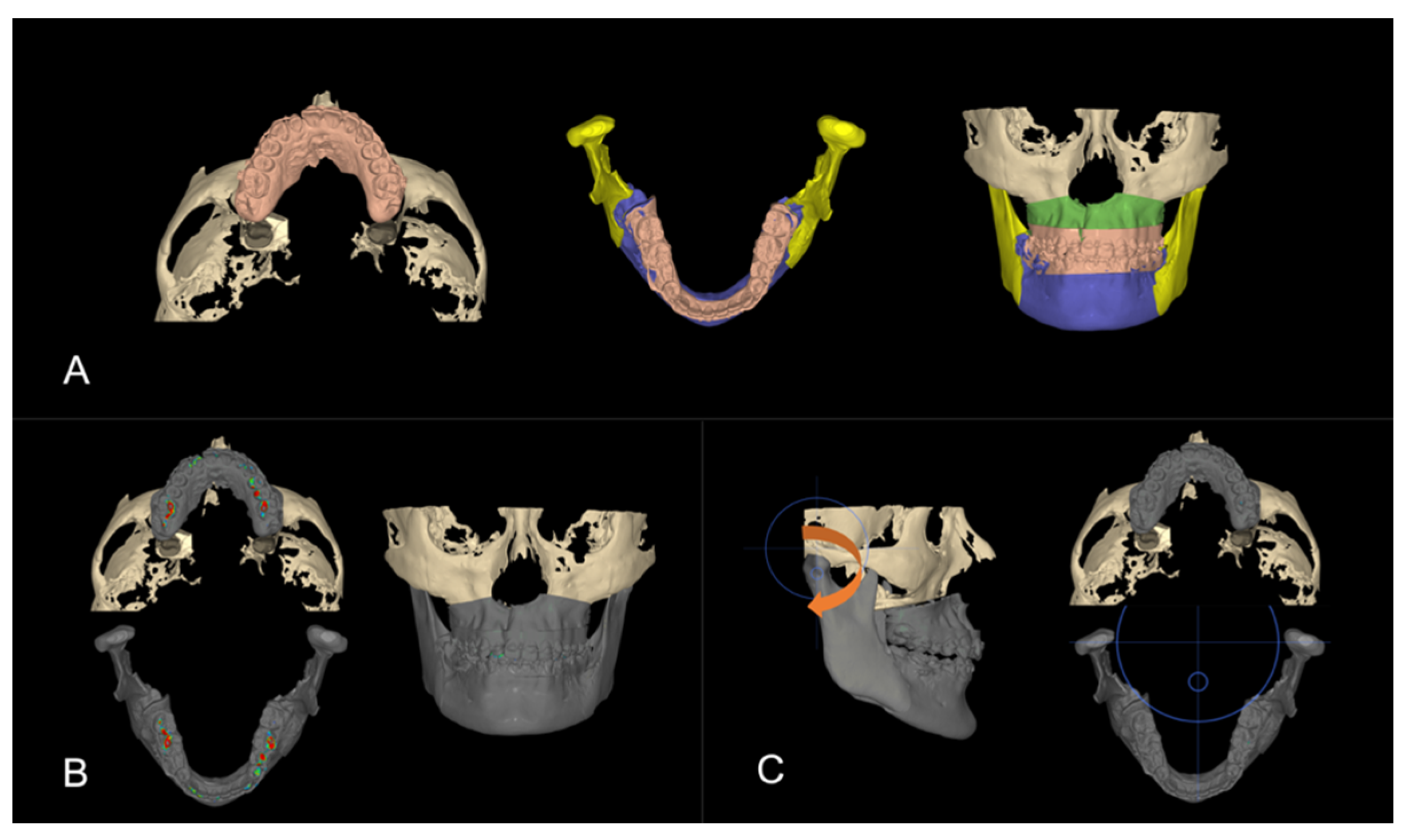
3.2. Step 2: Virtual Surgical Repositioning
3.3. Step 3: Reverse Engineering the Transitional Occlusion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, S.E.; Mai, C.T.; Canfield, M.A.; Rickard, R.; Wang, Y.; Meyer, R.E.; Anderson, P.; Mason, C.A.; Collins, J.S.; Kirby, R.S.; et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Normando, A.D.; da Silva Filho, O.G.; Capelozza Filho, L. Influence of surgery on maxillary growth in cleft lip and/or palate patients. J. Craniomaxillofac. Surg. 1992, 20, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Losee, J.E. The impact of cleft lip and palate repair on maxillofacial growth. Int. J. Oral Sci. 2015, 7, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Sarver, D.M.; Sadowsky, P.L.; Bradley, E. Combined rapid maxillary expansion and protraction facemask in the treatment of Class III malocclusions in growing children: A prospective long-term study. Semin. Orthod. 1997, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Terumi Ozawa, O.; Daniela Salzedas, C.; Beatriz Oliveira, L.; Sathler, R.; Baessa, G.; Garib, D. Efficacy of Rapid Maxillary Expansion Associated With Maxillary Protraction in Patients With Unilateral Complete Cleft Lip and Palate. Cleft Palate Craniofac. J. 2020, 57, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Tindlund, R.S. Skeletal response to maxillary protraction in patients with cleft lip and palate before age 10 years. Cleft Palate Craniofac. J. 1994, 31, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Tindlund, R.S. Orthopaedic protraction of the midface in the deciduous dentition. Results covering 3 years out of treatment. J. Craniomaxillofac. Surg. 1989, 17 (Suppl. 1), 17–19. [Google Scholar] [CrossRef] [PubMed]
- Liou, E.J.; Tsai, W.C. A new protocol for maxillary protraction in cleft patients: Repetitive weekly protocol of alternate rapid maxillary expansions and constrictions. Cleft Palate Craniofac. J. 2005, 42, 121–127. [Google Scholar] [CrossRef]
- Meazzini, M.C.; Zappia, L.B.; Tortora, C.; Autelitano, L.; Tintinelli, R. Short- and Long-Term Effects of Late Maxillary Advancement With the Liou-Alt-RAMEC Protocol in Unilateral Cleft Lip and Palate. Cleft Palate Craniofac. J. 2019, 56, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lane, C.; Azeredo, F.; Landsberger, M.; Kapadia, H.; Sheller, B.; Yen, S.L. Clinical effectiveness of late maxillary protraction in cleft lip and palate: A methods paper. Orthod. Craniofac. Res. 2017, 20 (Suppl. 1), 129–133. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Lane, C.J.; Yen, S.L. Late maxillary protraction in patients with unilateral cleft lip and palate: A retrospective study. Cleft Palate Craniofac. J. 2014, 51, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. Systematic Review and Meta-Analysis of the Index of Orthognathic Functional Treatment Need for Detecting Subjects with Great Need for Orthognathic Surgery. Cleft Palate Craniofac. J. 2023, 10556656231216833, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Daskalogiannakis, J.; Mehta, M. The need for orthognathic surgery in patients with repaired complete unilateral and complete bilateral cleft lip and palate. Cleft Palate Craniofac. J. 2009, 46, 498–502. [Google Scholar] [CrossRef]
- Good, P.M.; Mulliken, J.B.; Padwa, B.L. Frequency of Le Fort I osteotomy after repaired cleft lip and palate or cleft palate. Cleft Palate Craniofac. J. 2007, 44, 396–401. [Google Scholar] [CrossRef]
- DeLuke, D.M.; Marchand, A.; Robles, E.C.; Fox, P. Facial growth and the need for orthognathic surgery after cleft palate repair: Literature review and report of 28 cases. J. Oral Maxillofac. Surg. 1997, 55, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Nakano, M.; Yoshizaki, K.; Yasunaga, A.; Haruyama, N.; Takahashi, I. A Longitudinal Study of the Presence of Dental Anomalies in the Primary and Permanent Dentitions of Cleft Lip and/or Palate Patients. Cleft Palate Craniofac. J. 2017, 54, 309–320. [Google Scholar] [CrossRef]
- Al Jamal, G.A.; Hazza’a, A.M.; Rawashdeh, M.A. Prevalence of dental anomalies in a population of cleft lip and palate patients. Cleft Palate Craniofac. J. 2010, 47, 413–420. [Google Scholar] [CrossRef]
- Tannure, P.N.; Oliveira, C.A.; Maia, L.C.; Vieira, A.R.; Granjeiro, J.M.; Costa, M.e.C. Prevalence of dental anomalies in nonsyndromic individuals with cleft lip and palate: A systematic review and meta-analysis. Cleft Palate Craniofac. J. 2012, 49, 194–200. [Google Scholar] [CrossRef]
- da Silva Filho, O.G.; Ramos, A.L.; Abdo, R.C. The influence of unilateral cleft lip and palate on maxillary dental arch morphology. Angle Orthod. 1992, 62, 283–290. [Google Scholar]
- Zheng, J.; Kuang, W.; Yuan, S.; He, H.; Yuan, W. Three-dimensional Analysis of Maxillary Morphology in Infants with Unilateral Cleft Lip and Palate. Cleft Palate Craniofac. J. 2024, 10556656241228903, online ahead of print. [Google Scholar] [CrossRef]
- Baek, S.H.; Ahn, H.W.; Kwon, Y.H.; Choi, J.Y. Surgery-first approach in skeletal class III malocclusion treated with 2-jaw surgery: Evaluation of surgical movement and postoperative orthodontic treatment. J. Craniofac. Surg. 2010, 21, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Kang, S.H.; Lee, J.Y.; Kim, M.K.; Kim, J.H. Surgery-first approach using a three-dimensional virtual setup and surgical simulation for skeletal Class III correction. Korean J. Orthod. 2014, 44, 330–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.H.; Park, Y.C.; Yu, H.S.; Kim, M.K.; Kang, S.H.; Choi, Y.J. Accuracy of 3-Dimensional Virtual Surgical Simulation Combined With Digital Teeth Alignment: A Pilot Study. J. Oral Maxillofac. Surg. 2017, 75, 2441.e1–2441.e13. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Sugawara, J.; Kawamura, H.; Nanda, R. “Surgery first” skeletal Class III correction using the Skeletal Anchorage System. J. Clin. Orthod. 2009, 43, 97–105. [Google Scholar] [PubMed]
- Uribe, F.; Janakiraman, N.; Shafer, D.; Nanda, R. Three-dimensional cone-beam computed tomography-based virtual treatment planning and fabrication of a surgical splint for asymmetric patients: Surgery first approach. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Ebker, T.; Korn, P.; Heiland, M.; Bumann, A. Comprehensive virtual orthognathic planning concept in surgery-first patients. Br. J. Oral Maxillofac. Surg. 2022, 60, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Andrews, W.A. AP relationship of the maxillary central incisors to the forehead in adult white females. Angle Orthod. 2008, 78, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. Orthodontic considerations in restorative management of hypodontia patients with endosseous implants. J. Oral Implantol. 2012, 38, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Duś-Ilnicka, I. Current Concepts and Challenges in the Treatment of Cleft Lip and Palate Patients-A Comprehensive Review. J. Pers. Med. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Vyas, T.; Gupta, P.; Kumar, S.; Gupta, R.; Gupta, T.; Singh, H.P. Cleft of lip and palate: A review. J. Family Med. Prim. Care 2020, 9, 2621–2625. [Google Scholar] [CrossRef]
- Zaroni, F.M.; Sales, P.H.D.H.; Maffìa, F.; Scariot, R. Complications of orthognathic surgery in patients with cleft lip and palate: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101795. [Google Scholar] [CrossRef] [PubMed]
- Feu, D.; de Oliveira, B.H.; Palomares, N.B.; Celeste, R.K.; Miguel, J.A.M. Oral health-related quality of life changes in patients with severe Class III malocclusion treated with the 2-jaw surgery-first approach. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Choi, J.Y.; Yang, I.H.; Baek, S.H. Patient’s Satisfaction in Skeletal Class III Cases Treated With Two-Jaw Surgery Using Orthognathic Quality of Life Questionnaire: Conventional Three-Stage Method Versus Surgery-First Approach. J. Craniofac. Surg. 2015, 26, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, Z.; Zang, J.; Wang, X. Surgery-first/early-orthognathic approach may yield poorer postoperative stability than conventional orthodontics-first approach: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Soverina, D.; Gasparini, G.; Pelo, S.; Doneddu, P.; Todaro, M.; Boniello, R.; Azzuni, C.; Grippaudo, C.; Saponaro, G.; D’Amato, G.; et al. Skeletal stability in orthognathic surgery with the surgery first approach: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 930–940. [Google Scholar] [CrossRef]
- Mah, D.H.; Kim, S.G.; Oh, J.S.; You, J.S.; Jung, S.Y.; Kim, W.G.; Yu, K.H. Comparative study of postoperative stability between conventional orthognathic surgery and a surgery-first orthognathic approach after bilateral sagittal split ramus osteotomy for skeletal class III correction. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.I.; Hwang, D.S.; Kim, K.B.; Park, S.B. Effect of occlusal vertical dimension changes on postsurgical skeletal changes in a surgery-first approach for skeletal Class III deformities. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 612–619. [Google Scholar] [CrossRef]
- Liou, E.J.; Chen, P.H.; Wang, Y.C.; Yu, C.C.; Huang, C.S.; Chen, Y.R. Surgery-first accelerated orthognathic surgery: Orthodontic guidelines and setup for model surgery. J. Oral Maxillofac. Surg. 2011, 69, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Lu, X.; Urata, M.M.; Hammoudeh, J.A.; Yen, S.L.-K. Precise Prediction of Transitional Occlusion Using Reverse Engineering in Surgery-first Orthognathic Surgery Case. FACE 2024, 5, 197–204. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, S.G. Redefining precision and efficiency in orthognathic surgery through virtual surgical planning and 3D printing: A narrative review. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 42. [Google Scholar] [CrossRef]
- Bengtsson, M.; Wall, G.; Greiff, L.; Rasmusson, L. Treatment outcome in orthognathic surgery-A prospective randomized blinded case-controlled comparison of planning accuracy in computer-assisted two- and three-dimensional planning techniques (part II). J. Craniomaxillofac. Surg. 2017, 45, 1419–1424. [Google Scholar] [CrossRef]
- Barone, M.; De Stefani, A.; Baciliero, U.; Bruno, G.; Gracco, A. The Accuracy of Jaws Repositioning in Bimaxillary Orthognathic Surgery with Traditional Surgical Planning Compared to Digital Surgical Planning in Skeletal Class III Patients: A Retrospective Observational Study. J. Clin. Med. 2020, 9, 1840. [Google Scholar] [CrossRef]
- Lin, H.H.; Denadai, R.; Sato, N.; Hung, Y.T.; Pai, B.C.J.; Lo, L.J. Avoiding Inferior Alveolar Nerve Injury during Osseous Genioplasty: A Guide for the Safe Zone by Three-Dimensional Virtual Imaging. Plast. Reconstr. Surg. 2020, 146, 847–858. [Google Scholar] [CrossRef]
- Xie, K.; Wang, L.; Chen, K.; Hu, X.; Wu, G. Application of 3D-printed Surgical Cutting Guide in Anterior Mandibular Body Ostectomy. J. Craniofac. Surg. 2023, 34, 656–657. [Google Scholar] [CrossRef]
- Alkhayer, A.; Piffkó, J.; Lippold, C.; Segatto, E. Accuracy of virtual planning in orthognathic surgery: A systematic review. Head Face Med. 2020, 16, 34. [Google Scholar] [CrossRef]
- Alkaabi, S.; Maningky, M.; Helder, M.N.; Alsabri, G. Virtual and traditional surgical planning in orthognathic surgery—Systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1184–1191. [Google Scholar] [CrossRef]
- Sabiq, F.; Cherukupalli, A.; Khalil, M.; Tran, L.K.; Kwon, J.J.Y.; Milner, T.; Durham, J.S.; Prisman, E. Evaluating the benefit of virtual surgical planning on bony union rates in head and neck reconstructive surgery. Head Neck 2024, 46, 1322–1330. [Google Scholar] [CrossRef]
- Wrzosek, M.K.; Peacock, Z.S.; Laviv, A.; Goldwaser, B.R.; Ortiz, R.; Resnick, C.M.; Troulis, M.J.; Kaban, L.B. Comparison of time required for traditional versus virtual orthognathic surgery treatment planning. Int. J. Oral Maxillofac. Surg. 2016, 45, 1065–1069. [Google Scholar] [CrossRef]
- Donaldson, C.D.; Manisali, M.; Naini, F.B. Three-dimensional virtual surgical planning (3D-VSP) in orthognathic surgery: Advantages, disadvantages and pitfalls. J. Orthod. 2021, 48, 52–63. [Google Scholar] [CrossRef]
- Baan, F.; de Waard, O.; Bruggink, R.; Xi, T.; Ongkosuwito, E.M.; Maal, T.J.J. Virtual setup in orthodontics: Planning and evaluation. Clin. Oral Investig. 2020, 24, 2385–2393. [Google Scholar] [CrossRef]
- Roy, A.A.; Rtshiladze, M.A.; Stevens, K.; Phillips, J. Orthognathic Surgery for Patients with Cleft Lip and Palate. Clin. Plast. Surg. 2019, 46, 157–171. [Google Scholar] [CrossRef]
- Posnick, J.C.; Ricalde, P. Cleft-orthognathic surgery. Clin. Plast. Surg. 2004, 31, 315–330. [Google Scholar] [CrossRef]
- Kalmar, C.L.; Xu, W.; Zimmerman, C.E.; Vu, G.H.; Humphries, L.S.; Swanson, J.W.; Bartlett, S.P.; Taylor, J.A. Trends in Utilization of Virtual Surgical Planning in Pediatric Craniofacial Surgery. J. Craniofac. Surg. 2020, 31, 1900–1905. [Google Scholar] [CrossRef]
- Sozzi, D.; Filippi, A.; Canzi, G.; De Ponti, E.; Bozzetti, A.; Novelli, G. Surgical Navigation in Mandibular Reconstruction: Accuracy Evaluation of an Innovative Protocol. J. Clin. Med. 2022, 11, 2060. [Google Scholar] [CrossRef]
- Velarde, K.; Cafino, R.; Isla, A.; Ty, K.M.; Palmer, X.L.; Potter, L.; Nadorra, L.; Pueblos, L.V.; Velasco, L.C. Virtual surgical planning in craniomaxillofacial surgery: A structured review. Comput. Assist. Surg. 2023, 28, 2271160. [Google Scholar] [CrossRef]




| Measurement | Normal | Pretreatment |
|---|---|---|
| Skeletal | ||
| SNA (°) | 82.0 ± 3.5 | 78 |
| SNB (°) | 80.9 ±3.4 | 87.7 |
| ANB (°) | 1.6 ± 1.5 | −9.7 |
| A-Na Perpendicular (mm) | 1.1 ± 2.7 | 2.4 |
| Pog-Na Perpendicular (mm) | −0.3 ± 3.8 | 30.5 |
| Co-A (mm) | 99.8 ± 6.0 | 79.8 |
| Co-Gn (mm) | 134.3 ± 6.8 | 132.6 |
| Co-Gn—Co-A (mm) | 34.5 ± 4.0 | 52.8 |
| Wits (mm) | −1 ± 1.0 | −17.4 |
| SN-MP (°) | 33.0 ± 6.0 | 27.1 |
| Dental | ||
| U1-SN (°) | 103.1 ± 5.5 | 108.3 |
| L1-MP (°) | 95.0 ± 7.0 | 83.2 |
| Soft tissue | ||
| Upper lip to E-line (mm) | −4.0 ± 2.0 | −14.7 |
| Lower lip to E-line (mm) | −2.0 ± 2.0 | −5.3 |
| Option 1. Orthodontic Space Closure | Option 2. Surgical Space Closure | ||
|---|---|---|---|
| Orthognathic surgery (surgery first) | Orthognathic surgery (surgery first) | ||
| Maxilla | LeFort I osteotomy to advance the maxilla | Maxilla | LeFort I osteotomy to advance the maxilla |
| 2-piece segmental osteotomy to close the gap | |||
| Mandible | Sagittal split ramus osteotomy to setback the mandible and correct the facial asymmetry | Mandible | Sagittal split ramus osteotomy to setback the mandible and correct the facial asymmetry |
| Post-surgical orthodontics | Post-surgical orthodontics | ||
| Space closure | Decompensation | ||
| Decompensation | Finishing and Detailing | ||
| Finishing and Detailing | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.; Urata, M.M.; Hammoudeh, J.A.; Yamashita, D.-D.; Yen, S.L.-K. Reverse Engineering Orthognathic Surgery and Orthodontics in Individuals with Cleft Lip and/or Palate: A Case Report. Bioengineering 2024, 11, 771. https://doi.org/10.3390/bioengineering11080771
Ko J, Urata MM, Hammoudeh JA, Yamashita D-D, Yen SL-K. Reverse Engineering Orthognathic Surgery and Orthodontics in Individuals with Cleft Lip and/or Palate: A Case Report. Bioengineering. 2024; 11(8):771. https://doi.org/10.3390/bioengineering11080771
Chicago/Turabian StyleKo, Jaemin, Mark M. Urata, Jeffrey A. Hammoudeh, Dennis-Duke Yamashita, and Stephen L.-K. Yen. 2024. "Reverse Engineering Orthognathic Surgery and Orthodontics in Individuals with Cleft Lip and/or Palate: A Case Report" Bioengineering 11, no. 8: 771. https://doi.org/10.3390/bioengineering11080771
APA StyleKo, J., Urata, M. M., Hammoudeh, J. A., Yamashita, D.-D., & Yen, S. L.-K. (2024). Reverse Engineering Orthognathic Surgery and Orthodontics in Individuals with Cleft Lip and/or Palate: A Case Report. Bioengineering, 11(8), 771. https://doi.org/10.3390/bioengineering11080771







