Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae and Flocculants
2.2. Harvesting Experiments
2.3. Kinetics of Flocculation and Sedimentation
2.4. Statistical Analysis
3. Results and Discussion
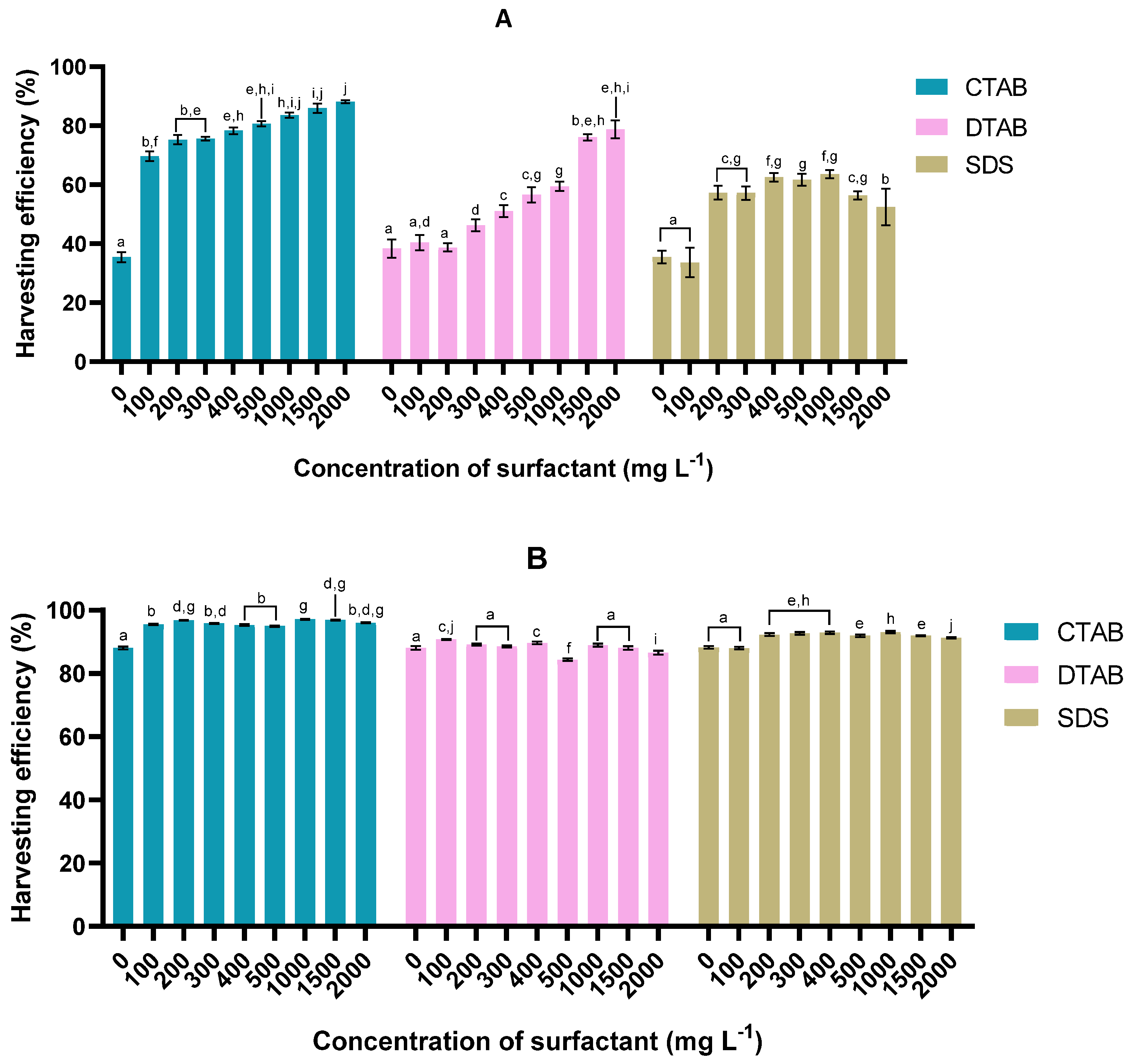
3.1. Harvesting Efficiency
3.1.1. Microalga Tetraselmis sp. 75LG
3.1.2. Microalga Tetraselmis sp. 46NLG
3.1.3. Tetraselmis sp. harvesting performance
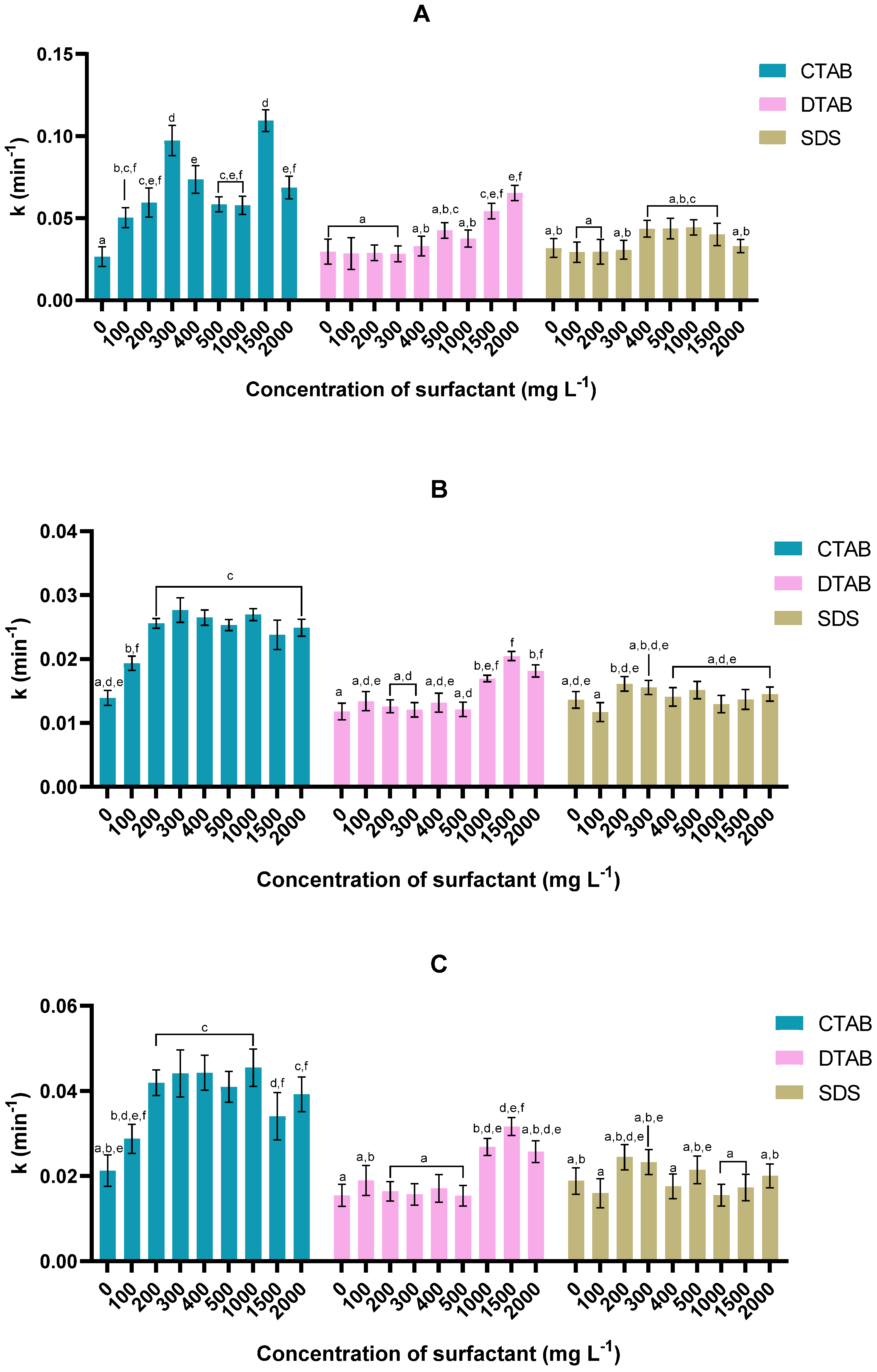
3.2. Harvesting Kinetics
3.2.1. Microalga Tetraselmis sp. 75LG
3.2.2. Microalga Tetraselmis sp. 46NLG
3.3. Future Work
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taghavijeloudar, M.; Park, J.; Hashemi, S.; Han, M. The effects of surfactants (sodium dodecyl sulfate, triton X-100 and cetyl trimethyl ammonium bromide) on the dewaterability of microalgae biomass using pressure filtration. Bioresour. Technol. 2019, 273, 565–572. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development—Advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Dammak, M.; Hlima, H.B.; Tounsi, L.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effect of heavy metals mixture on the growth and physiology of Tetraselmis sp.: Applications to lipid production and bioremediation. Bioresour. Technol. 2022, 360, 127584. [Google Scholar] [CrossRef]
- Dammak, M.; Hlima, H.B.; Elleuch, F.; Pichon, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Flow cytometry assay to evaluate lipid production by the marine microalga Tetraselmis sp. using a two stage process. Renew. Energy 2021, 177, 280–289. [Google Scholar] [CrossRef]
- Kumar, S.D.; Ro, K.-M.; Santhanam, P.; Dhanalakshmi, B.; Latha, S.; Kim, M.-K. Initial population density plays a vital role to enhance biodiesel productivity of Tetraselmis sp. under reciprocal nitrogen concentration. Bioresour. Technol. Rep. 2018, 3, 15–21. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current perspective on wastewater treatment using photobioreactor for Tetraselmis sp.: An emerging and foreseeable sustainable approach. Environ. Sci. Pollut. Res. 2022, 29, 61905–61937. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.L.; Jamaluddin, H.; Zain, N.A.M.; Idris, A. Biodiesel production via lipase catalysed transesterification of microalgae lipids from Tetraselmis sp. Renew. Energy 2014, 68, 1–5. [Google Scholar] [CrossRef]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M.M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Carneiro, M.; Pôjo, V.; Malcata, F.; Otero, A. Lipid accumulation in selected Tetraselmis strains. J. Appl. Phycol. 2019, 31, 2845–2853. [Google Scholar] [CrossRef]
- Taghavijeloudar, M.; Kebria, D.Y.; Yaqoubnejad, P. Simultaneous harvesting and extracellular polymeric substances extrusion of microalgae using surfactant: Promoting surfactant-assisted flocculation through pH adjustment. Bioresour. Technol. 2021, 319, 124224. [Google Scholar] [CrossRef] [PubMed]
- Coward, T.; Lee, J.G.; Caldwell, G.S. Harvesting microalgae by CTAB-aided foam flotation increases lipid recovery and improves fatty acid methyl ester characteristics. Biomass Bioenergy 2014, 67, 354–362. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent progress in flocculation, dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Taghavijeloudar, M.; Yaqoubnejad, P.; Ahangar, A.K.; Rezania, S. A rapid, efficient and eco-friendly approach for simultaneous biomass harvesting and bioproducts extraction from microalgae: Dual flocculation between cationic surfactants and bio-polymer. Sci. Total Environ. 2023, 854, 158717. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Rehman, M.S.U.; Han, J.-I. Use of chitosan acid solutions to improve separation efficiency for harvesting of the microalga Chlorella vulgaris. J. Chem. Eng. 2013, 226, 238–242. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S. Microalgae harvesting techniques: A review. J. Environ. Manage. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Danquah, M.K.; Gladman, B.; Moheimani, N.; Forde, G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009, 151, 73–78. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, C.; Negi, S.; Shukla, P. Microalgae harvesting techniques: Updates and recent technological interventions. Crit. Rev. Biotechnol. 2023, 43, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Diao, Y.; Gong, X.; Xu, D.; Duan, P.; Wang, S.; Guo, Y. From culture, harvest to pretreatment of microalgae and its high-value utilization. Algal Res. 2024, 78, 103405. [Google Scholar] [CrossRef]
- McGrath, S.J.; Laamanen, C.A.; Senhorinho, G.N.; Scott, J.A. Microalgal harvesting for biofuels–Options and associated operational costs. Algal Res. 2024, 77, 103343. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Kiong, T.S.; Jathar, L.; Ghazali, N.N.N.; Ramesh, S.; Awasarmol, U.; Ong, H.C. Perspectives on cultivation and harvesting technologies of microalgae, towards environmental sustainability and life cycle analysis. Chemosphere 2024, 353, 141540. [Google Scholar] [CrossRef] [PubMed]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of microalgae by flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]
- Machado, C.A.; Esteves, A.F.; Pires, J.C. Optimization of Microalgal Harvesting with Inorganic and Organic Flocculants Using Factorial Design of Experiments. Processes 2022, 10, 1124. [Google Scholar] [CrossRef]
- Pahariya, R.; Chauhan, A.; Ranjan, A.; Basniwal, R.K.; Upadhyay, S.; Thakur, S.; Jindal, T. A Critical Review on the Efficacy and Mechanism of Nanoparticle-Based Flocculants for Biodiesel Feedstock Production from Microalgae. Bioenerg. Res. 2023, 17, 1065–1079. [Google Scholar] [CrossRef]
- Qin, L.; Alam, M.A.; Feng, P.; Zhu, S.; Wang, Z. Advancements in the application of surfactants in microalgal production, harvesting and processing: A review. J. Environ. Chem. Eng. 2022, 10, 107504. [Google Scholar] [CrossRef]
- Huang, W.-C.; Kim, J.-D. Cationic surfactant-based method for simultaneous harvesting and cell disruption of a microalgal biomass. Bioresour. Technol. 2013, 149, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Ulloa, G.; Coutens, C.; Sánchez, M.; Sineiro, J.; Fábregas, J.; Deive, F.; Rodríguez, A.; Núñez, M. On the double role of surfactants as microalga cell lysis agents and antioxidants extractants. Green Chem. 2012, 14, 1044–1051. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.S.; Eustance, E.; Rittmann, B.E. Promoting Synechocystis sp. PCC 6803 harvesting by cationic surfactants: Alkyl-chain length and dose control for the release of extracellular polymeric substances and biomass aggregation. ACS Sustain. Chem. Eng. 2018, 7, 2127–2133. [Google Scholar] [CrossRef]
- Arora, J.; Ranjan, A.; Chauhan, A.; Biswas, R.; Rajput, V.D.; Sushkova, S.; Mandzhieva, S.; Minkina, T.; Jindal, T. Surfactant pollution, an emerging threat to ecosystem: Approaches for effective bacterial degradation. J. Appl. Microbiol. 2022, 133, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Bergero, M.F.; Lucchesi, G.I. Degradation of cationic surfactants using Pseudomonas putida A ATCC 12633 immobilized in calcium alginate beads. Biodegradation 2013, 24, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Ontiveros-Valencia, A.; Ilhan, Z.E.; Zhou, Y.; Miranda, E.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E. Enhancing biodegradation of C16-alkyl quaternary ammonium compounds using an oxygen-based membrane biofilm reactor. Water Res. 2017, 123, 825–833. [Google Scholar] [CrossRef]
- Seo, J.Y.; Praveenkumar, R.; Kim, B.; Seo, J.-C.; Park, J.-Y.; Na, J.-G.; Jeon, S.G.; Park, S.B.; Lee, K.; Oh, Y.-K. Downstream integration of microalgae harvesting and cell disruption by means of cationic surfactant-decorated Fe3O4 nanoparticles. Green Chem. 2016, 18, 3981–3989. [Google Scholar] [CrossRef]
- Darley, W.M.; Volcani, B. Role of silicon in diatom metabolism: A silicon requirement for deoxyribonucleic acid synthesis in the diatom Cylindrotheca fusiformis Reimann and Lewin. Exp. Cell Res. 1969, 58, 334–342. [Google Scholar] [CrossRef]
- Salgado, E.M.; Esteves, A.F.; Gonçalves, A.L.; Pires, J.C. Microalgal cultures for the remediation of wastewaters with different nitrogen to phosphorus ratios: Process modelling using artificial neural networks. Environ. Res. 2023, 231, 116076. [Google Scholar] [CrossRef]
- Gerde, J.A.; Yao, L.; Lio, J.; Wen, Z.; Wang, T. Microalgae flocculation: Impact of flocculant type, algae species and cell concentration. Algal Res. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Process Biochem. 2011, 46, 1927–1933. [Google Scholar] [CrossRef]
- Gani, P.; Mohamed Sunar, N.; Matias-Peralta, H.; Abdul Latiff, A.A. Effect of pH and alum dosage on the efficiency of microalgae harvesting via flocculation technique. Int. J. Green Energy 2017, 14, 395–399. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Van Den Hende, S.; Vervaeren, H.; Coward, T.; Skill, S.C. Harvesting of microalgae within a biorefinery approach: A review of the developments and case studies from pilot-plants. Algal Res. 2015, 11, 248–262. [Google Scholar] [CrossRef]
- Mirzaei Nia, H.; Sarvi, M.N.; Bagherpour, R. Development of a kinetical investigation method using adsorption kinetic models for selection and optimization of flocculation process. Sep. Sci. Technol. 2021, 56, 1830–1841. [Google Scholar] [CrossRef]
- Krishnan, A.; Devasya, R.; Hu, Y.; Bassi, A. Fundamental investigation of bio-surfactants-assisted harvesting strategy for microalgae. Biomass Bioenergy 2022, 158, 106364. [Google Scholar] [CrossRef]


| Harvesting Method | Advantages | Disadvantages |
|---|---|---|
| Flocculation |
|
|
| Sedimentation |
|
|
| Flotation |
|
|
| Filtration |
|
|
| Centrifugation |
|
|
| Concentration (mg L−1) | Modified Gompertz Model | ||||
|---|---|---|---|---|---|
| Tetraselmis sp. 75LG | Tetraselmis sp. 46NLG | ||||
| k (min−1) | λ (min) | k (min−1) | λ (min) | ||
| CTAB | 0 | 0.021 ± 0.009 | 20 ± 10 | 0.026 ± 0.006 | 7 ± 9 |
| 100 | 0.020 ± 0.009 | 0 ± 22 | 0.05 ± 0.006 | 5 ± 3 | |
| 200 | 0.058 ± 0.005 | 7 ± 2 | 0.059 ± 0.009 | 0 ± 4 | |
| 300 | 0.042 ± 0.004 | 0 ± 4 | 0.097 ± 0.009 | 8 ± 1 | |
| 400 | 0.078 ± 0.008 | 0 ± 2 | 0.074 ± 0.008 | 5 ± 2 | |
| 500 | 0.07 ± 0.01 | 0 ± 4 | 0.058 ± 0.005 | 0 ± 2 | |
| 1000 | 0.14 ± 0.04 | 0 ± 6 | 0.058 ± 0.006 | 0 ± 3 | |
| 1500 | 0.16 ± 0.06 | 0 ± 7 | 0.109 ± 0.007 | 14 ± 1 | |
| 2000 | 0.16 ± 0.04 | 0 ± 5 | 0.069 ± 0.007 | 5 ± 2 | |
| DTAB | 0 | 0.021 ± 0.006 | 15 ± 9 | 0.030 ± 0.008 | 20 ± 8 |
| 100 | 0.022 ± 0.009 | 0 ± 18 | 0.028 ± 0.009 | 10 ± 11 | |
| 200 | 0.023 ± 0.006 | 5 ± 11 | 0.029 ± 0.005 | 13 ± 5 | |
| 300 | 0.025 ± 0.005 | 0 ± 9 | 0.028 ± 0.005 | 15 ± 5 | |
| 400 | 0.026 ± 0.005 | 17 ± 6 | 0.033 ± 0.006 | 18 ± 4 | |
| 500 | 0.026 ± 0.004 | 15 ± 5 | 0.042 ± 0.005 | 20 ± 2 | |
| 1000 | 0.023 ± 0.004 | 11 ± 7 | 0.038 ± 0.005 | 0 ± 6 | |
| 1500 | 0.084 ± 0.006 | 13 ± 1 | 0.054 ± 0.005 | 0 ± 3 | |
| 2000 | 0.091 ± 0.009 | 9 ± 1 | 0.065 ± 0.005 | 11 ± 1 | |
| SDS | 0 | 0.029 ± 0.004 | 2 ± 7 | 0.032 ± 0.006 | 20 ± 6 |
| 100 | 0.020 ± 0.005 | 0 ± 12 | 0.029 ± 0.006 | 20 ± 6 | |
| 200 | 0.023 ± 0.007 | 0 ± 15 | 0.030 ± 0.007 | 0 ± 11 | |
| 300 | 0.021 ± 0.008 | 0 ± 18 | 0.031 ± 0.006 | 5 ± 7 | |
| 400 | 0.020 ± 0.008 | 15 ± 13 | 0.044 ± 0.005 | 20 ± 2 | |
| 500 | 0.02 ± 0.01 | 18 ± 19 | 0.044 ± 0.006 | 20 ± 3 | |
| 1000 | 0.02 ± 0.01 | 20 ± 18 | 0.044 ± 0.005 | 20 ± 2 | |
| 1500 | 0.026 ± 0.007 | 20 ± 9 | 0.040 ± 0.007 | 20 ± 4 | |
| 2000 | 0.02 ± 0.01 | 20 ± 15 | 0.033 ± 0.004 | 14 ± 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, C.; Pôjo, V.; Tavares, T.; Pires, J.C.M.; Malcata, F.X. Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling. Bioengineering 2024, 11, 722. https://doi.org/10.3390/bioengineering11070722
Maia C, Pôjo V, Tavares T, Pires JCM, Malcata FX. Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling. Bioengineering. 2024; 11(7):722. https://doi.org/10.3390/bioengineering11070722
Chicago/Turabian StyleMaia, Carolina, Vânia Pôjo, Tânia Tavares, José C. M. Pires, and Francisco Xavier Malcata. 2024. "Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling" Bioengineering 11, no. 7: 722. https://doi.org/10.3390/bioengineering11070722
APA StyleMaia, C., Pôjo, V., Tavares, T., Pires, J. C. M., & Malcata, F. X. (2024). Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling. Bioengineering, 11(7), 722. https://doi.org/10.3390/bioengineering11070722










