Screening and Selection of a New Medium and Culture Conditions for Diosgenin Production via Microbial Biocatalysis of SYt1
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Preparation of Inoculum
2.3. Biotransformation of DZW to Diosgenin by Liquid Fermentation
2.4. Purification of Liquid Fermentation Production
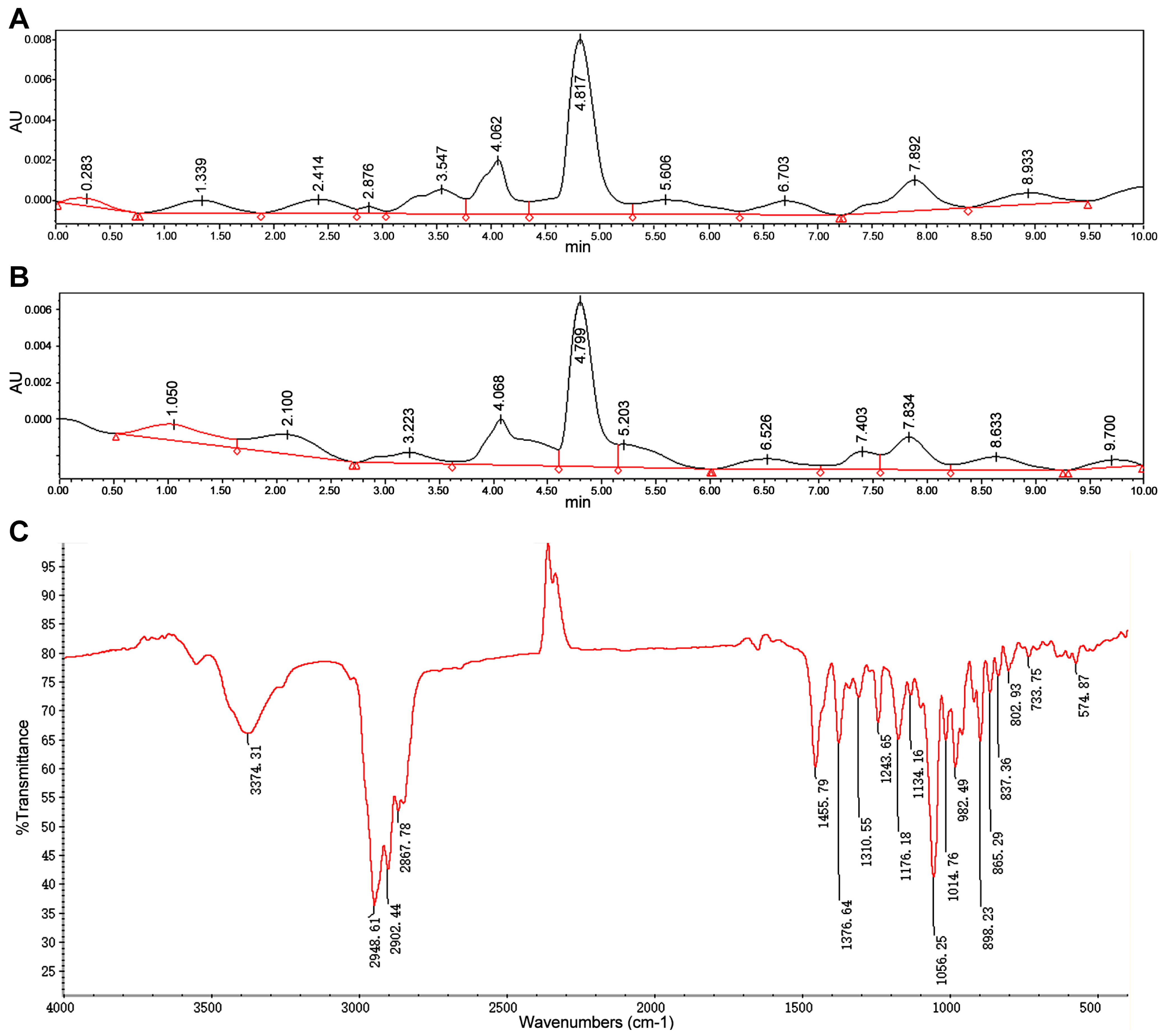
2.5. Determination of Diosgenin by Thin-Layer Chromatography
2.6. Determination of Diosgenin by HPLC
2.7. Determination of Diosgenin by Fourier-Transform Infrared
2.8. Nuclear Magnetic Resonance Method
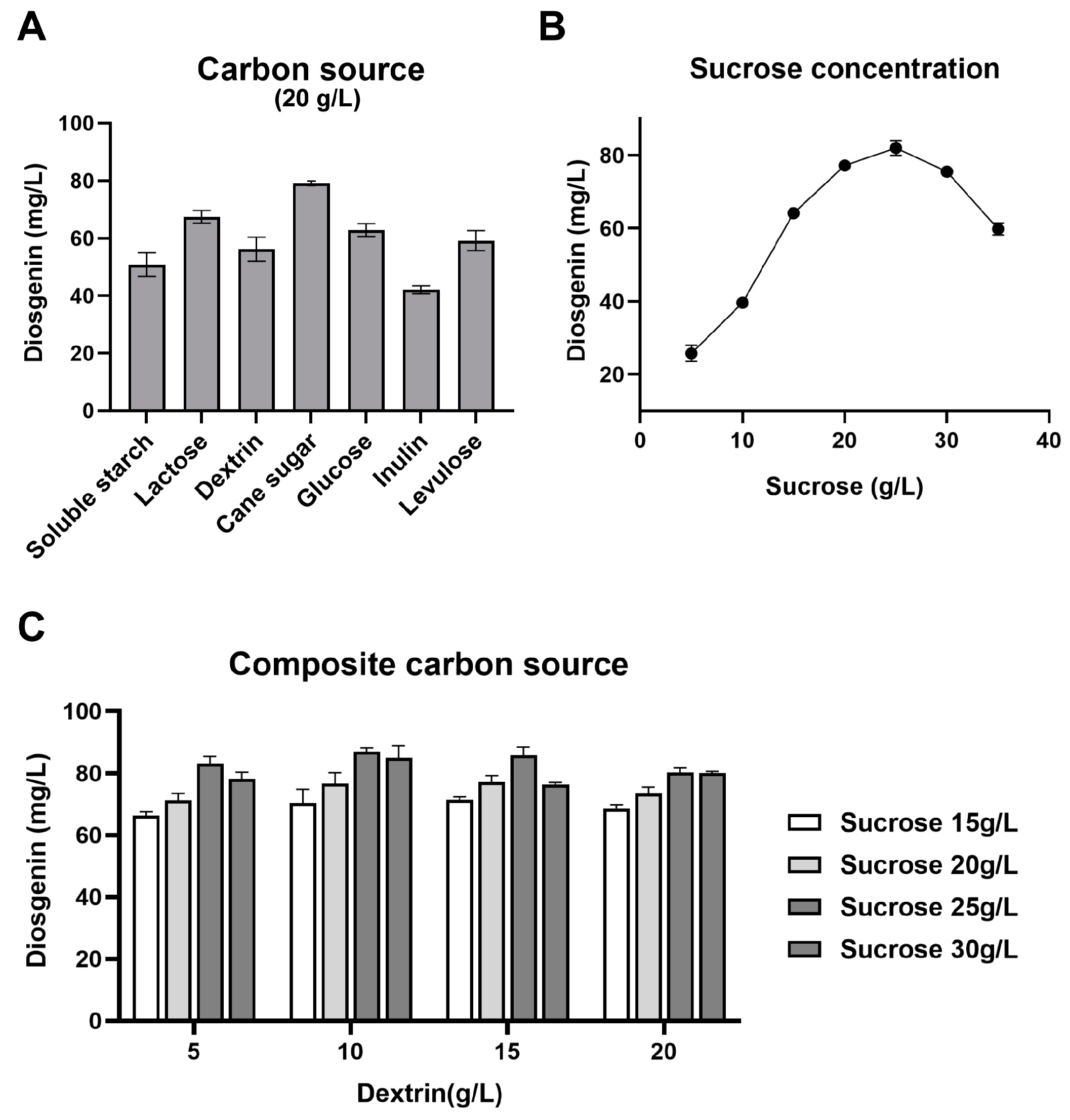
2.9. Effect of Carbon Source on Diosgenin Production
2.10. Effect of Nitrogen Source on Diosgenin Productio
2.11. Effect of Surfactant on Diosgenin Production
2.12. Selection of Significant Factors by the Plackett–Burman Design
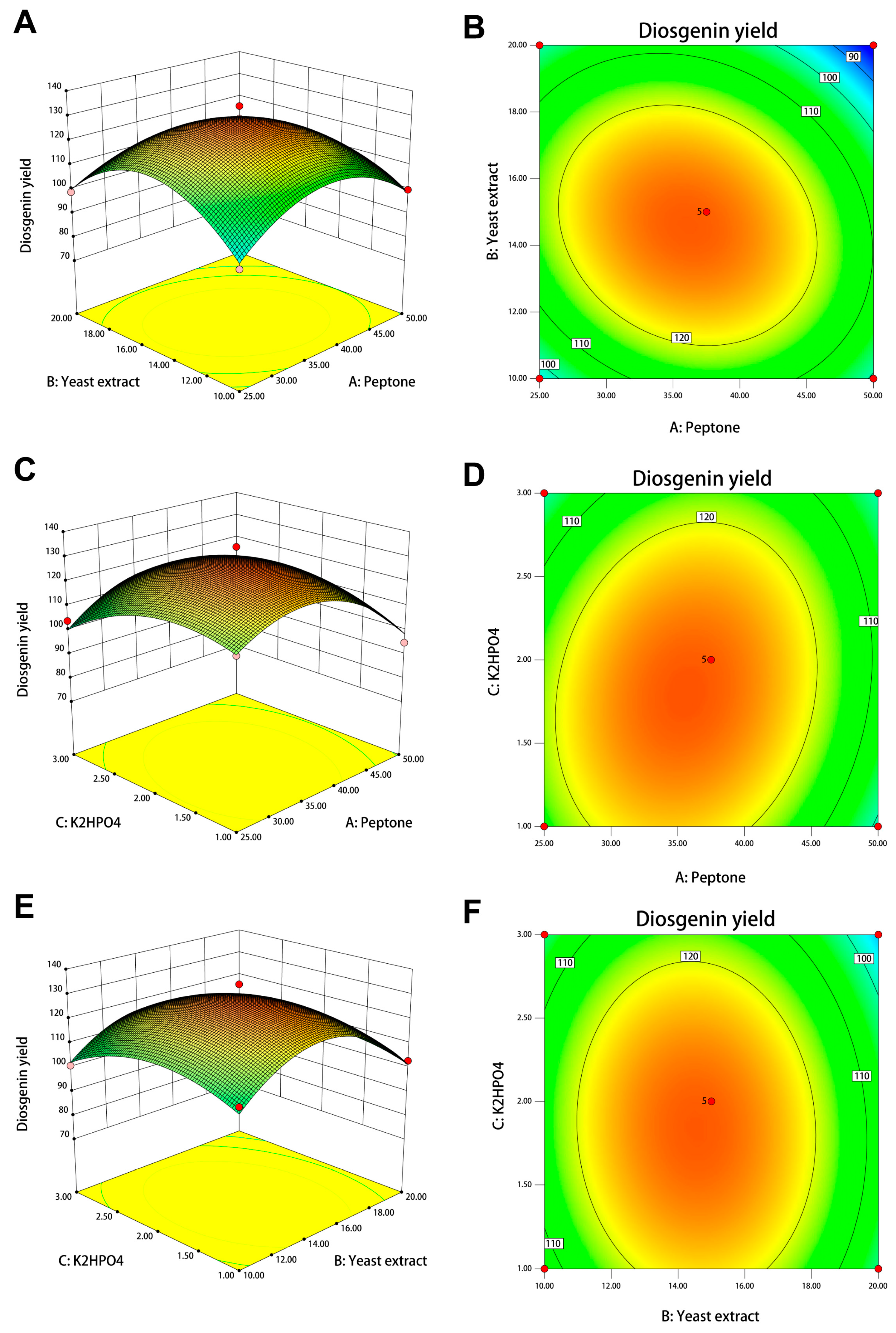
2.13. Bioprocess Optimization by Box–Behnken Design
2.14. Verification Experiments
2.15. Statistical Analysis
3. Results
3.1. Identifcation of Diosgenin Product in Fermentation Broth
3.2. Selection of Optimal Carbon Source for Diosgenin Production
3.3. Selection of Optimal Nitrogen Source for Diosgenin Production
3.4. Selection of Optimal Liquid Fermentation Medium Components for Diosgenin Production
3.5. Selection of Optimal Liquid Fermentation Conditions for Diosgenin Production
3.6. Screening the Main Effect Factors on Diosgenin Production by Plackett–Burman Design
3.7. Optimization of Fermentation Conditions for Diosgenin Production by Box–Behnken Design
3.8. Experimental Validation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, G.; Qin, Y.; Huang, W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo. Phytomedicine 2011, 18, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Silvestre, S. Diosgenin: Recent Highlights on Pharmacology and Analytical Methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef] [PubMed]
- Liagre, B.; Vergne-Salle, P.; Corbiere, C.; Charissoux, J.L.; Beneytout, J.L. Diosgenin, a plant steroid, induces apoptosis in human rheumatoid arthritis synoviocytes with cyclooxygenase-2 overexpression. Arthritis Res. Ther. 2004, 6, R373–R383. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Wang, Z.; Ju, Y.; Wong, R.N.; Wu, Q.Y. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother. Pharmacol. 2005, 55, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Son, I.S.; Kim, J.H.; Sohn, H.Y.; Son, K.H.; Kim, J.S.; Kwon, C.S. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci. Biotechnol. Biochem. 2007, 71, 3063–3071. [Google Scholar] [CrossRef]
- Ghosh, S.; More, P.; Derle, A.; Patil, A.B.; Markad, P.; Asok, A.; Kumbhar, N.; Shaikh, M.L.; Ramanamurthy, B.; Shinde, V.S.; et al. Diosgenin from Dioscorea bulbifera: Novel hit for treatment of type II diabetes mellitus with inhibitory activity against α-amylase and α-glucosidase. PLoS ONE 2014, 9, e106039. [Google Scholar] [CrossRef]
- Fernández-Herrera, M.A.; Sandoval-Ramírez, J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar-Sánchez, M.L. Probing the selective antitumor activity of 22-oxo-26-selenocyanocholestane derivatives. Eur. J. Med. Chem. 2014, 74, 451–460. [Google Scholar] [CrossRef]
- Huang, C.H.; Ku, C.Y.; Jan, T.R. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med. 2009, 75, 1300–1305. [Google Scholar] [CrossRef]
- Kato, A.; Miura, T.; Fukunaga, T. Effects of steroidal glycosides on blood glucose in normal and diabetic mice. Biol. Pharm. Bull. 1995, 18, 167–168. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Zhang, X.; Bing, B.; Zhang, L.; Wang, H.; Zhao, W. Anti-tumour and immunomodulating activities of diosgenin, a naturally occurring steroidal saponin. Nat. Prod. Res. 2012, 26, 2243–2246. [Google Scholar] [CrossRef]
- Pabón, A.; Escobar, G.; Vargas, E.; Cruz, V.; Notario, R.; Blair, S.; Echeverri, F. Diosgenone synthesis, anti-malarial activity and QSAR of analogues of this natural product. Molecules 2013, 18, 3356–3378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Liu, Z.; Bao, J.; Hong, Y. Pathways for the steroidal saponins conversion to diosgenin during acid hydrolysis of Dioscorea zingiberensis CH Wright. Chem. Eng. Res. Des. 2011, 89, 2620–2625. [Google Scholar]
- Xiang, H.; Zhang, T.; Pang, X.; Wei, Y.; Liu, H.; Zhang, Y.; Ma, B.; Yu, L. Isolation of endophytic fungi from Dioscorea zingiberensis C. H. Wright and application for diosgenin production by solid-state fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 5519–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Huang, W.; Ni, J.R.; Liu, W.; Li, H. Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl. Microbiol. Biotechnol. 2010, 85, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods 2021, 10, 2547. [Google Scholar] [CrossRef]
- Białkowska, A.M.; Jędrzejczak-Krzepkowska, M.; Gromek, E.; Krysiak, J.; Sikora, B.; Kalinowska, H.; Kubik, C.; Schütt, F.; Turkiewicz, M. Effects of genetic modifications and fermentation conditions on 2,3-butanediol production by alkaliphilic Bacillus subtilis. Appl. Microbiol. Biotechnol. 2016, 100, 2663–2676. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Xia, H.; Huang, Z.; Zhu, Y.; Xu, L.; Liu, Y.; Li, J.; Du, G.; Lv, X.; et al. Engineered Bacillus subtilis for the de novo production of 2′-fucosyllactose. Microb. Cell Factories 2022, 21, 110. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, W.; Liu, C. Isolation, identification and characterization of novel Bacillus subtilis. J. Vet. Med. Sci. 2018, 80, 427–433. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, J.; Jin, Z.; Bai, Y. Analysis of the action pattern of sequential α-amylases from B. stearothermophilus and B. amyloliquefaciens on highly concentrated soluble starch. Carbohydr. Polym. 2023, 320, 121190. [Google Scholar] [CrossRef]
- El-Bendary, M.A.; Afifi, S.S.; Moharam, M.E.; Abo Elsoud, M.M.; Gawdat, N.A. Optimization of Bacillus subtilis growth parameters for biosynthesis of silver nanoparticles by using response surface methodology. Prep. Biochem. Biotechnol. 2023, 53, 183–194. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, Y.; Yan, Y.; Du, S.; Pan, F.; Li, S.; Xu, H.; Luo, Z. Metabolic Engineering of Bacillus amyloliquefaciens to Efficiently Synthesize L-Ornithine From Inulin. Front. Bioeng. Biotechnol. 2022, 10, 905110. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, X.; Zhang, W.; Tian, X.; Ping, W.; Ge, J. Enhanced β-mannanase production by Bacillus licheniformis by optimizing carbon source and feeding regimes. Prep. Biochem. Biotechnol. 2022, 52, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Li, Q.; Liu, H.M.; Yu, N.; Xie, T.J.; Yang, M.Y.; Shen, P.; Chen, X.D. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl. Microbiol. Biotechnol. 2010, 85, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Chikindas, M.L. Recent Advances in the Physiology of Spore Formation for Bacillus Probiotic Production. Probiotics Antimicrob. Proteins 2019, 11, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Poust, S.; Hagen, A.; Katz, L.; Keasling, J.D. Narrowing the gap between the promise and reality of polyketide synthases as a synthetic biology platform. Curr. Opin. Biotechnol. 2014, 30, 32–39. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Dong, Z.Y.; Kan, Y.; Xiao, M.; Kang, Y.Q.; Li, W.J. Bacillus tepidiphilus sp. nov., isolated from tepid spring. Arch. Microbiol. 2020, 202, 2367–2371. [Google Scholar] [CrossRef]
- Guérin, A.; Rønning, H.T.; Dargaignaratz, C.; Clavel, T.; Broussolle, V.; Mahillon, J.; Granum, P.E.; Nguyen-The, C. Cereulide production by Bacillus weihenstephanensis strains during growth at different pH values and temperatures. Food Microbiol. 2017, 65, 130–135. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, S.; Bai, B.; Fan, S. Isolation and screening for limonin-producing endophytic bacteria from Citrus maxima (Burm.) Merr. cv. Shatian Yu. Biotechnol. Appl. Biochem. 2019, 66, 192–201. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Lu, Y.; Wu, N.; Chen, J.; Ji, Z.; Zhan, Y.; Ma, X.; Chen, J.; Cai, D.; et al. Metabolic engineering of Bacillus amyloliquefaciens for efficient production of α-glucosidase inhibitor1-deoxynojirimycin. Synth. Syst. Biotechnol. 2023, 8, 378–385. [Google Scholar] [CrossRef]
- Ling, H.L.; Rahmat, Z.; Bakar, F.D.A.; Murad, A.M.A.; Illias, R.M. Secretome analysis of alkaliphilic bacterium Bacillus lehensis G1 in response to pH changes. Microbiol. Res. 2018, 215, 46–54. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, L. Optimization of Xylanase production from Penicillium sp.WX-Z1 by a two-step statistical strategy: Plackett-Burman and Box-Behnken experimental design. Int. J. Mol. Sci. 2012, 13, 10630–10646. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Li, Y.L.; Xu, S.C.; Liu, Y.; Wang, X.; Tang, J.W. Improvement of xylanase production by Aspergillus niger XY-1 using response surface methodology for optimizing the medium composition. J. Zhejiang Univ. Sci. B 2008, 9, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Pectin extraction from citron peel: Optimization by Box-Behnken response surface design. Food Sci. Biotechnol. 2018, 27, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Moosavi-Nasab, M.; Behzadnia, A.; Rezaei, M. Enhanced biosurfactant production with low-quality date syrup by Lactobacillus rhamnosus using a fed-batch fermentation. Food Sci. Biotechnol. 2018, 27, 1137–1144. [Google Scholar] [CrossRef]
- Sun, T.; Yan, P.; Zhan, N.; Zhang, L.; Chen, Z.; Zhang, A.; Shan, A. The optimization of fermentation conditions for Pichia pastoris GS115 producing recombinant xylanase. Eng. Life Sci. 2020, 20, 216–228. [Google Scholar] [CrossRef]





| Symbol | Factor | Actual Experimentation Value | |
|---|---|---|---|
| Low (−1) | High (1) | ||
| A | Yeast extraction (g/L) | 15 | 25 |
| B | Peptone (g/L) | 10 | 20 |
| C | Virtual item1 | - | - |
| D | Sucrose (g/L) | 5 | 15 |
| E | Dextrin (g/L) | 10 | 20 |
| F | Virtual item2 | - | - |
| G | Dioscorea rhizome powder (g/L) | 3 | 8 |
| H | Fermentation period (day) | 4 | 6 |
| J | Virtual item3 | - | - |
| K | Inorganic salt concentration (g/L) | 1 | 3 |
| L | Inoculum age (h) | 18 | 30 |
| Variables | Symbol | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Peptone (g/L) | A | 25 | 37.5 | 50 |
| Yeast extract (g/L) | B | 10 | 15 | 20 |
| Inorganic salt (g/L) | C | 1 | 2 | 3 |
| Sum of | Mean | F | p-Value | ||
|---|---|---|---|---|---|
| Source | Squares | df | Square | Value | Prob > F |
| Model | 298.33 | 8 | 37.29 | 9.87 | 0.043 |
| Yeast infusion powder (g/L) | 85.33 | 1 | 85.33 | 22.59 | 0.0177 |
| Peptone (g/L) | 108 | 1 | 108 | 28.59 | 0.0128 |
| Sucrose (g/L) | 8.33 | 1 | 8.33 | 2.21 | 0.2342 |
| Dextrin (g/L) | 1.33 | 1 | 1.33 | 0.35 | 0.5943 |
| Inducer (g/L) | 0.33 | 1 | 0.33 | 0.088 | 0.7858 |
| Time (d) | 40.33 | 1 | 40.33 | 10.68 | 0.0469 |
| Inorganic salt (g/L) | 33.33 | 1 | 33.33 | 8.82 | 0.059 |
| Inoculum age (h) | 21.33 | 1 | 21.33 | 5.65 | 0.0979 |
| Residual | 11.33 | 3 | 3.78 | ||
| Cor Total | 309.67 | 11 |
| Sum of | Mean | F | p-Value | ||
|---|---|---|---|---|---|
| Source | Squares | df | Square | Value | Prob > F |
| Model | 3840.714706 | 9 | 426.7461 | 34.31617 | <0.0001 |
| A | 136.125 | 1 | 136.125 | 10.9463 | 0.0130 |
| B | 105.125 | 1 | 105.125 | 8.453475 | 0.0227 |
| C | 84.5 | 1 | 84.5 | 6.794945 | 0.0351 |
| AB | 121 | 1 | 121 | 9.73004 | 0.0169 |
| AC | 56.25 | 1 | 56.25 | 4.523262 | 0.0710 |
| BC | 6.25 | 1 | 6.25 | 0.502585 | 0.5013 |
| A^2 | 1125.568421 | 1 | 1125.568 | 90.51096 | <0.0001 |
| B^2 | 1496.094737 | 1 | 1496.095 | 120.3063 | <0.0001 |
| C^2 | 388.0421053 | 1 | 388.0421 | 31.20385 | 0.0008 |
| Residual | 87.05 | 7 | 12.43571 | ||
| Lack of Fit | 56.25 | 3 | 18.75 | 2.435065 | 0.2049 |
| Pure Error | 30.8 | 4 | 7.7 | ||
| Cor Total | 3927.764706 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhao, Y.; Mou, F.; Yang, Z.; Li, N.; Cheng, M.; Xie, H.; Qin, B.; Tang, Y. Screening and Selection of a New Medium and Culture Conditions for Diosgenin Production via Microbial Biocatalysis of SYt1. Bioengineering 2024, 11, 1098. https://doi.org/10.3390/bioengineering11111098
Han S, Zhao Y, Mou F, Yang Z, Li N, Cheng M, Xie H, Qin B, Tang Y. Screening and Selection of a New Medium and Culture Conditions for Diosgenin Production via Microbial Biocatalysis of SYt1. Bioengineering. 2024; 11(11):1098. https://doi.org/10.3390/bioengineering11111098
Chicago/Turabian StyleHan, Shiyao, Yiyu Zhao, Fangyuan Mou, Zhen Yang, Ningxiao Li, Mengqi Cheng, Heshaungyi Xie, Baofu Qin, and Young Tang. 2024. "Screening and Selection of a New Medium and Culture Conditions for Diosgenin Production via Microbial Biocatalysis of SYt1" Bioengineering 11, no. 11: 1098. https://doi.org/10.3390/bioengineering11111098
APA StyleHan, S., Zhao, Y., Mou, F., Yang, Z., Li, N., Cheng, M., Xie, H., Qin, B., & Tang, Y. (2024). Screening and Selection of a New Medium and Culture Conditions for Diosgenin Production via Microbial Biocatalysis of SYt1. Bioengineering, 11(11), 1098. https://doi.org/10.3390/bioengineering11111098






