Using the Probability Density Function-Based Channel-Combination Bloch–Siegert Method Realizes Permittivity Imaging at 3T
Abstract
1. Introduction
2. Theory and Methods
2.1. BSS Method
2.2. PDF-Based Channel-Combination BSS Method
2.3. Helmholtz-Based EPT
2.4. Open-Ended Coaxial Probe Method
2.5. Accuracy Evaluation of Imaging Results
2.6. MRI Experiments
2.6.1. Phantom Experiment
2.6.2. In Vivo Rat Experiment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, K.R.; Schwan, H.P. Dielectric properties of tissues. In CRC Handbook of Biological Effects of Electromagnetic Fields; CRC Press: Boca Raton, FL, USA, 2019; pp. 27–96. [Google Scholar]
- Di Biasio, A.; Cametti, C. Effect of shape on the dielectric properties of biological cell suspensions. Bioelectrochemistry 2007, 71, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, B.; Xu, J.; Yu, J. Dielectric properties of human glioma and surrounding tissue. Int. J. Hyperth. 2009, 8, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Peyman, A. Dielectric properties of biological tissues; variation with age. In Conn’s Handbook of Models for Human Aging; Elsevier: Amsterdam, The Netherlands, 2018; pp. 939–952. [Google Scholar]
- Costa, E.L.; Gonzalez Lima, R.; Amato, M.B. Electrical impedance tomography. In Yearbook of Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 394–404. [Google Scholar]
- Franchineau, G.; Jonkman, A.H.; Piquilloud, L.; Yoshida, T.; Costa, E.; Rozé, H.; Camporota, L.; Piraino, T.; Spinelli, E.; Combes, A. Electrical impedance tomography to monitor hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2024, 209, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Songsangvorn, N.; Xu, Y.; Lu, C.; Rotstein, O.; Brochard, L.; Slutsky, A.S.; Burns, K.E.; Zhang, H. Electrical impedance tomography-guided positive end-expiratory pressure titration in ARDS: A systematic review and meta-analysis. Intensive Care Med. 2024, 50, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nawaz, M.; Khan, S.; Xia, P.; Irfan, M.; Wong, E.C.; Chan, R.; Cao, P. Cross modality generative learning framework for anatomical transitive Magnetic Resonance Imaging (MRI) from Electrical Impedance Tomography (EIT) image. Comput. Med. Imaging Graph. 2023, 108, 102272. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Woo, E.J. Electrical tissue property imaging at low frequency using MREIT. IEEE Trans. Biomed. Eng. 2014, 61, 1390–1399. [Google Scholar] [PubMed]
- Wang, H.; Song, Y. Stability of the isotropic conductivity reconstruction using magnetic resonance electrical impedance tomography (MREIT). Inverse Probl. 2024, 40, 075005. [Google Scholar] [CrossRef]
- Katscher, U.; Voigt, T.; Findeklee, C.; Vernickel, P.; Nehrke, K.; Doessel, O. Determination of electric conductivity and local SAR via B1 mapping. IEEE Trans. Med. Imaging 2009, 28, 1365–1374. [Google Scholar] [CrossRef]
- Insko, E.; Bolinger, L. Mapping of the radiofrequency field. J. Magn. Reson. Ser. A 1993, 103, 82–85. [Google Scholar] [CrossRef]
- Pohmann, R.; Scheffler, K. A theoretical and experimental comparison of different techniques for B1 mapping at very high fields. NMR Biomed. 2013, 26, 265–275. [Google Scholar] [CrossRef]
- van Lier, A.L.; Brunner, D.O.; Pruessmann, K.P.; Klomp, D.W.; Luijten, P.R.; Lagendijk, J.J.; van den Berg, C.A. B1(+) phase mapping at 7 T and its application for in vivo electrical conductivity mapping. Magn. Reson. Med. 2012, 67, 552–561. [Google Scholar] [CrossRef]
- Yarnykh, V.L. Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2007, 57, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.V. Rapid B1 mapping using orthogonal, equal-amplitude radio-frequency pulses. Magn. Reson. Med. 2012, 67, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Nehrke, K.; Börnert, P. DREAM—A novel approach for robust, ultrafast, multislice B1 mapping. Magn. Reson. Med. 2012, 68, 1517–1526. [Google Scholar] [CrossRef]
- Jung, K.J.; Mandija, S.; Kim, J.H.; Ryu, K.; Jung, S.; Cui, C.; Kim, S.Y.; Park, M.; van den Berg, C.A.; Kim, D.H. Improving phase-based conductivity reconstruction by means of deep learning–based denoising of phase data for 3T MRI. Magn. Reson. Med. 2021, 86, 2084–2094. [Google Scholar] [CrossRef]
- Soullié, P.; Missoffe, A.; Ambarki, K.; Felblinger, J.; Odille, F. MR electrical properties imaging using a generalized image-based method. Magn. Reson. Med. 2021, 85, 762–776. [Google Scholar] [CrossRef]
- Balidemaj, E.; van Lier, A.L.; Crezee, H.; Nederveen, A.J.; Stalpers, L.J.; Van Den Berg, C.A. Feasibility of electric property tomography of pelvic tumors at 3T. Magn. Reson. Med. 2015, 73, 1505–1513. [Google Scholar] [CrossRef]
- Meerbothe, T.; Meliado, E.; Stijnman, P.; van den Berg, C.; Mandija, S. A database for MR-based electrical properties tomography with in silico brain data—ADEPT. Magn. Reson. Med. 2024, 91, 1190–1199. [Google Scholar] [CrossRef]
- Hampe, N.; Katscher, U.; Van den Berg, C.A.; Tha, K.K.; Mandija, S. Investigating the challenges and generalizability of deep learning brain conductivity mapping. Phys. Med. Biol. 2020, 65, 135001. [Google Scholar] [CrossRef]
- Mori, N.; Tsuchiya, K.; Sheth, D.; Mugikura, S.; Takase, K.; Katscher, U.; Abe, H. Diagnostic value of electric properties tomography (EPT) for differentiating benign from malignant breast lesions: Comparison with standard dynamic contrast-enhanced MRI. Eur. Radiol. 2019, 29, 1778–1786. [Google Scholar] [CrossRef]
- Mandija, S.; Sbrizzi, A.; Katscher, U.; Luijten, P.R.; van den Berg, C.A. Error analysis of helmholtz-based MR-electrical properties tomography. Magn. Reson. Med. 2018, 80, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, J.; Guo, L.; Li, M.; Tesiram, Y.; Chen, H.; Liu, F.; Xin, X.; Crozier, S. MR-based electrical property tomography using a modified finite difference scheme. Phys. Med. Biol. 2018, 63, 145013. [Google Scholar] [CrossRef] [PubMed]
- Bulumulla, S.; Lee, S.; Yeo, D. Conductivity and permittivity imaging at 3.0 T. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2012, 41, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Michel, E.; Hernandez, D.; Cho, M.H.; Lee, S.Y. Denoising of B 1+ field maps for noise-robust image reconstruction in electrical properties tomography. Med. Phys. 2014, 41, 102304. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jin, J.; Liu, C.; Liu, F.; Crozier, S. An efficient integral-based method for three-dimensional MR-EPT and the calculation of the RF-coil-induced Bz field. IEEE Trans. Biomed. Eng. 2017, 65, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, S.; van den Berg, C.A.; Sbrizzi, A.; Kok, H.P.; Stalpers, L.J.; Lagendijk, J.J.; Crezee, H.; van Lier, A.L. Accuracy and precision of electrical permittivity mapping at 3T: The impact of three mapping techniques. Magn. Reson. Med. 2019, 81, 3628–3642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tadanki, S.; Jankiewicz, M.; Grissom, W.A. Highly-accelerated Bloch-Siegert mapping using joint autocalibrated parallel image reconstruction. Magn. Reson. Med. 2014, 71, 1470–1477. [Google Scholar] [CrossRef]
- Wiggins, G.C.; Polimeni, J.R.; Potthast, A.; Schmitt, M.; Alagappan, V.; Wald, L.L. 96-Channel receive-only head coil for 3 Tesla: Design optimization and evaluation. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 62, 754–762. [Google Scholar] [CrossRef]
- Uğurbil, K.; Auerbach, E.; Moeller, S.; Grant, A.; Wu, X.; Van de Moortele, P.F.; Olman, C.; DelaBarre, L.; Schillak, S.; Radder, J. Brain imaging with improved acceleration and SNR at 7 Tesla obtained with 64-channel receive array. Magn. Reson. Med. 2019, 82, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef]
- Gao, Y.; Han, J.; Zhu, Y.; Wang, J.; Wei, X.; Xin, X. Channel-combination method for phase-based |B1+| mapping techniques. Magn. Reson. Imaging 2020, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stuchly, M.A.; Stuchly, S.S. Coaxial line reflection methods for measuring dielectric properties of biological substances at radio and microwave frequencies-a review. IEEE Trans. Instrum. Meas. 1980, 29, 176–183. [Google Scholar] [CrossRef]
- Marsland, T.; Evans, S. Dielectric measurements with an open-ended coaxial probe. IEE Proc. H Microw. Antennas Propag. 1987, 134, 341–349. [Google Scholar] [CrossRef]
- He, Z.; Lefebvre, P.M.; Soullié, P.; Doguet, M.; Ambarki, K.; Chen, B.; Odille, F. Phantom evaluation of electrical conductivity mapping by MRI: Comparison to vector network analyzer measurements and spatial resolution assessment. Magn. Reson. Med. 2024, 91, 2374–2390. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, Y. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271. [Google Scholar] [CrossRef]
- Brown, R.W.; Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]

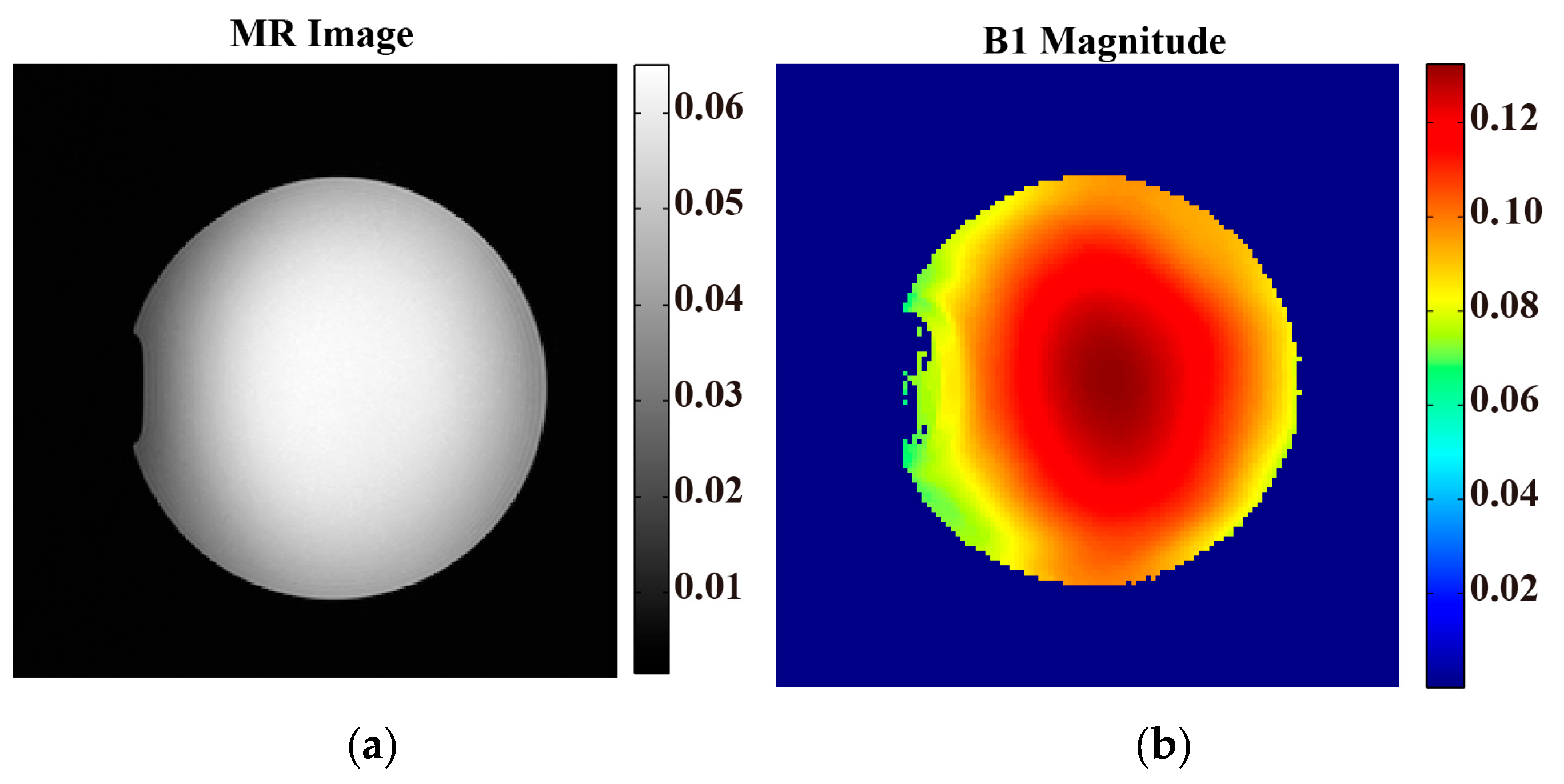

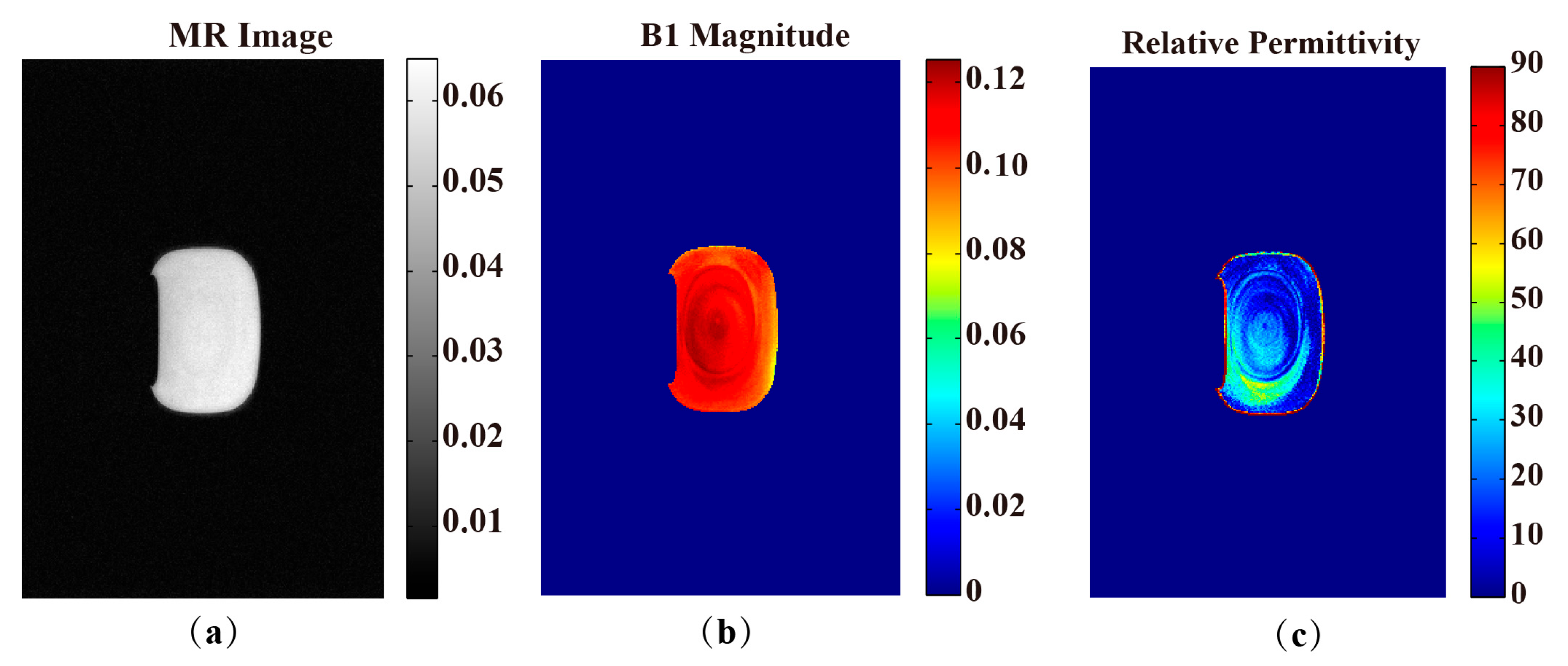

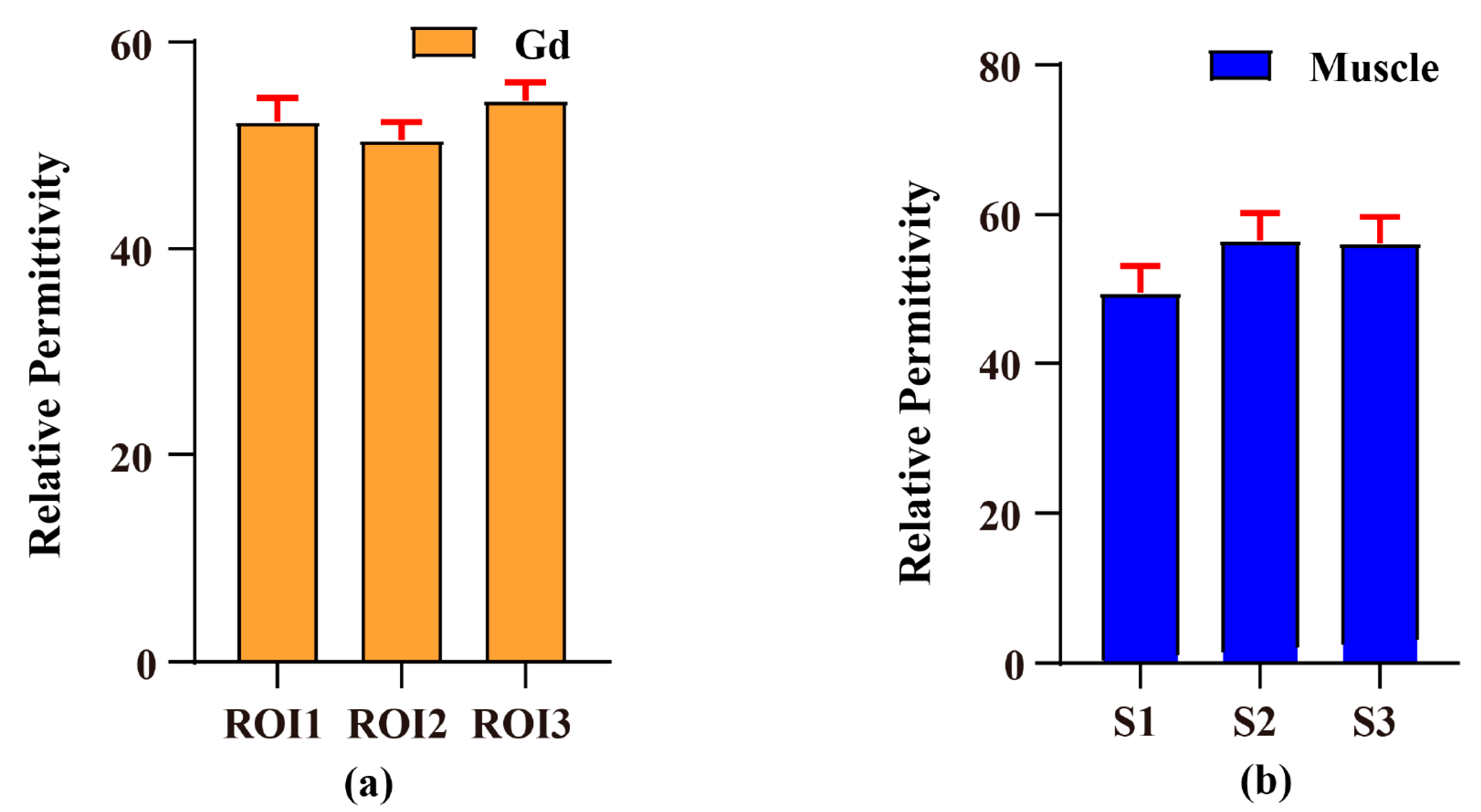

| Method/Subjects | PDF-Based BSS Combination EPT | Open-Ended Coaxial Probe | Relative Error (%) |
|---|---|---|---|
| Gd phantom | 52.413 ± 1.919 | 78.86 | 33.53 |
| Rat leg muscles | 54.053 ± 4.027 | 49.04 | 10.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, Y.; Xin, S.X. Using the Probability Density Function-Based Channel-Combination Bloch–Siegert Method Realizes Permittivity Imaging at 3T. Bioengineering 2024, 11, 699. https://doi.org/10.3390/bioengineering11070699
Wang J, Gao Y, Xin SX. Using the Probability Density Function-Based Channel-Combination Bloch–Siegert Method Realizes Permittivity Imaging at 3T. Bioengineering. 2024; 11(7):699. https://doi.org/10.3390/bioengineering11070699
Chicago/Turabian StyleWang, Jiajia, Yunyu Gao, and Sherman Xuegang Xin. 2024. "Using the Probability Density Function-Based Channel-Combination Bloch–Siegert Method Realizes Permittivity Imaging at 3T" Bioengineering 11, no. 7: 699. https://doi.org/10.3390/bioengineering11070699
APA StyleWang, J., Gao, Y., & Xin, S. X. (2024). Using the Probability Density Function-Based Channel-Combination Bloch–Siegert Method Realizes Permittivity Imaging at 3T. Bioengineering, 11(7), 699. https://doi.org/10.3390/bioengineering11070699








