Emerging Medical Technologies and Their Use in Bionic Repair and Human Augmentation
Abstract
1. Introduction
2. Smart Neuroprosthetic Devices: Restoring Sensory and Motor Functions Following Injury and Limb Loss
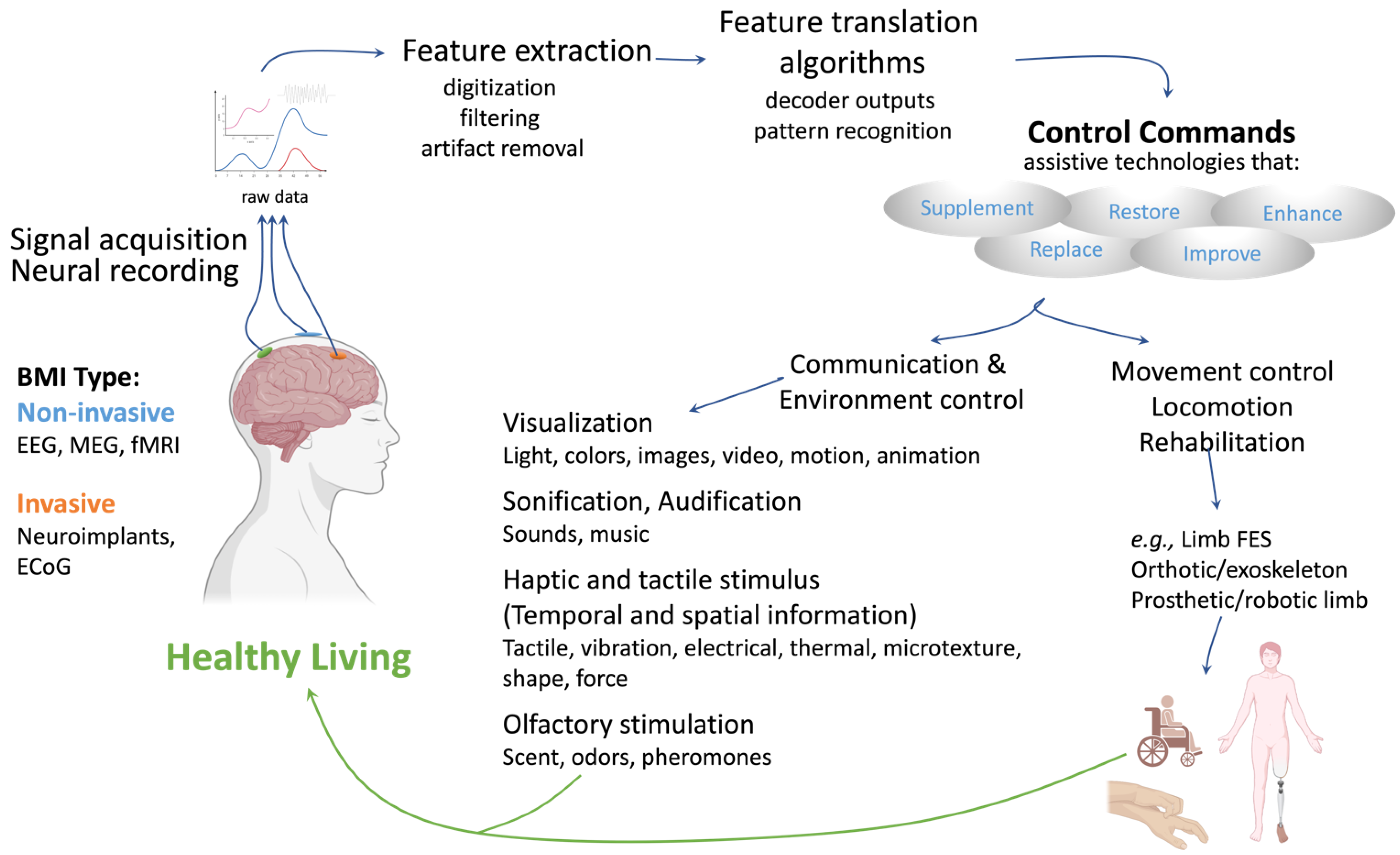
2.1. The Brain–Machine Interface
2.2. Prosthetic Limbs and Peripheral Neural Interfaces
2.3. Exoskeletons and Exosuits

2.4. Electronic Skin
2.5. Visual Prostheses

3. Augmented Human Performance
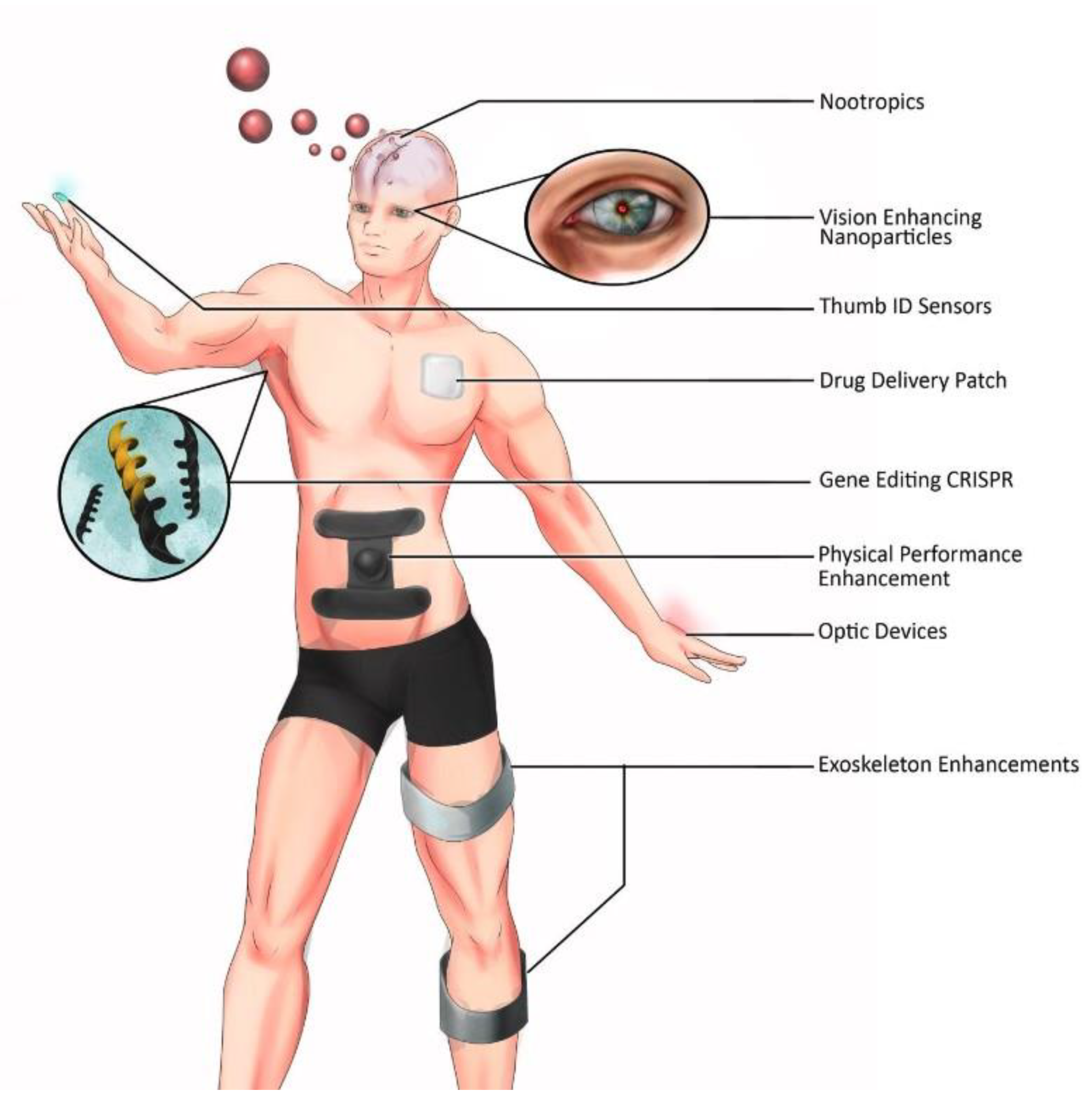
3.1. Biohacking and Prosthetics for Human Enhancement
3.2. Genetic Engineering Technology
4. The Ethical Challenges of Augmented Human Performance
- Autonomy: Free individuals in a forthcoming enhanced, transhuman, and posthuman society, containing social norms and anticipated experiences of community pressure.
- Identity: Technologically-altered or alterable human nature, its dignity, normality, with choices of elective enhancements and elective disability (and disadvantage).
- Futures: Children’s welfare, parents’ preferences but with obligations to their children’s future living in society.
- Community: Government and society’s responsibilities to diverse groups; integration, exclusion, and fears of extinction of minorities.
5. Continued Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bio-Implants—Global Market Outlook (2017–2026): Expected to Grow at a CAGR of 10.1% ResearchAndMarkets.com. 2018. Available online: https://www.businesswire.com/news/home/20181109005315/en/Bio-Implants---Global-Market-Outlook-2017-2026-Expected-to-Grow-at-a-CAGR-of-10.1---ResearchAndMarkets.com (accessed on 6 April 2024).
- Aging, N.I. Why Population Aging Matters: A Global Perspective; National Institute on Aging, National Institutes of Health: Bethesda, MD, USA, 2017.
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015; U.S. Census Bureau: Suitland, MD, USA, 2016.
- Zhang, H.; Liu, Y.; Zhou, K.; Wei, W.; Liu, Y. Restoring Sensorimotor Function Through Neuromodulation After Spinal Cord Injury: Progress and Remaining Challenges. Front. Neurosci. 2021, 15, 749465. [Google Scholar] [CrossRef] [PubMed]
- Armour, B.S.; Courtney-Long, E.A.; Fox, M.H.; Fredine, H.; Cahill, A. Prevalence and Causes of Paralysis—United States, 2013. Am. J. Public Health 2016, 106, 1855–1857. [Google Scholar] [CrossRef]
- Rao, R.P.N. Brain-Computer Interfacing: An Introduction; Google-Books-ID: LIHBAgAAQBAJ; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Wolpaw, J.R. Brain–computer interfaces. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 110, pp. 67–74. [Google Scholar]
- Rao, R.P. Towards Neural Co-Processors for the Brain: Combining Decoding and Encoding in Brain-Computer Interfaces. Curr. Opin. Neurobiol. 2019, 55, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Vansteensel, M.J.; Pels, E.G.M.; Bleichner, M.G.; Branco, M.P.; Denison, T.; Freudenburg, Z.V.; Gosselaar, P.; Leinders, S.; Ottens, T.H.; Van Den Boom, M.A.; et al. Fully Implanted Brain-Computer Interface in a Locked-In Patient with ALS. N. Engl. J. Med. 2016, 375, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Anumanchipalli, G.K.; Chartier, J.; Chang, E.F. Speech synthesis from neural decoding of spoken sentences. Nature 2019, 568, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Eun, K.J.; Chung, C.K.; Kim, J.S.; Chou, N.; Kim, S. Electrocorticogram (ECoG): Engineering approaches and clinical challegenes for translational medicine. Adv. Mater. Healthc. 2024, 9, 2301692. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Millán, J.D.R.; Ramsey, N.F. Brain-computer interfaces: Definitions and principles. Handb. Clin. Neurol. 2020, 168, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Bernabei, J.M.; Kini, L.G.; Ashourvan, A.; Boccanfuso, J.; Archer, R.; Oechsel, K.; Das, S.R.; Stein, J.M.; Lucas, T.H.; et al. High interictal connectivity within the resection zone is associated with favorable post-surgical outcomes in focal epilepsy. NeuroImage Clin. 2019, 23, 101908. [Google Scholar] [CrossRef] [PubMed]
- Rolston, J.D.; Ouyang, D.; Englot, D.J.; Wang, D.D.; Change, E.F. National trends and complication rates for invasive extraoperative electrocorticography in the USA. J. Clin. Neurophysiol. 2015, 22, 823–827. [Google Scholar] [CrossRef]
- Lebedev, M.A.; Nicolelis, M.A.L. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol. Rev. 2017, 97, 767–837. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain-computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, K.S. fNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef]
- Luu, S.; Chau, T. Decoding subjective preference from single-trial near-infrared spectroscopy signals. J. Neural Eng. 2009, 6, 016003. [Google Scholar] [CrossRef] [PubMed]
- Fazli, S.; Mehnert, J.; Steinbrink, J.; Curio, G.; Villringer, A.; Müller, K.R.; Blankertz, B. Enhanced performance by a hybrid NIRS-EEG brain computer interface. NeuroImage 2012, 59, 519–529. [Google Scholar] [CrossRef]
- Huang, D.; Lin, P.; Fei, D.Y.; Chen, X.; Bai, O. Decoding human motor activity from EEG single trials for a discrete two-dimensional cursor control. J. Neural Eng. 2009, 6, 046005. [Google Scholar] [CrossRef] [PubMed]
- Agashe, H.A.; Paek, A.Y.; Zhang, Y.; Contreras-Vidal, J.L. Global cortical activity predicts shape of hand during grasping. Front. Neurosci. 2015, 9, 130471. [Google Scholar] [CrossRef]
- Bhagat, N.A.; Venkatakrishnan, A.; Abibullaev, B.; Artz, E.J.; Yozbatiran, N.; Blank, A.A.; French, J.; Karmonik, C.; Grossman, R.G.; O’Malley, M.K.; et al. Design and Optimization of an EEG-Based Brain Machine Interface (BMI) to an Upper-Limb Exoskeleton for Stroke Survivors. Front. Neurosci. 2016, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.S.; Müller, K.R.; Lee, S.W. A lower limb exoskeleton control system based on steady state visual evoked potentials. J. Neural Eng. 2015, 12, 056009. [Google Scholar] [CrossRef]
- Gordleeva, S.; Lobov, S.; Grigorev, N.; Savosenkov, A.; Shamshin, M.; Lukoyanov, M.; Khoruzhko, M.; Kazantsev, V. Real-Time EEG–EMG Human–Machine Interface-Based Control System for a Lower-Limb Exoskeleton. IEEE Access 2020, 8, 84070–84081. [Google Scholar] [CrossRef]
- Gant, K.; Guerra, S.; Zimmerman, L.; Parks, B.A.; Prins, N.W.; Prasad, A. EEG-controlled functional electrical stimulation for hand opening and closing in chronic complete cervical spinal cord injury. Biomed. Phys. Eng. Express 2018, 4, 065005. [Google Scholar] [CrossRef]
- Salazar-Gomez, A.F.; DelPreto, J.; Gil, S.; Guenther, F.H.; Rus, D. Correcting robot mistakes in real time using EEG signals. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; 2017; pp. 6570–6577. [Google Scholar]
- Bi, L.; Xin’an, F.; Liu, Y. EEG-Based Brain-Controlled Mobile Robots: A Survey. IEEE Trans. Hum.-Mach. Syst. 2013, 43, 161–176. [Google Scholar] [CrossRef]
- LaFleur, K.; Cassady, K.; Doud, A.; Shades, K.; Rogin, E.; He, B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain-computer interface. J. Neural Eng. 2013, 10, 046003. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N. Communication in locked-in state after brainstem stroke: A brain-computer-interface approach. Ann. Transl. Med. 2015, 3, S29. [Google Scholar] [CrossRef] [PubMed]
- Holz, E.M.; Botrel, L.; Kaufmann, T.; Kübler, A. Long-term independent brain-computer interface home use improves quality of life of a patient in the locked-in state: A case study. Arch. Phys. Med. Rehabil. 2015, 96, S16–S26. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.W.; Ryan, D.B.; Hauser, C.K. Noninvasive brain-computer interface enables communication after brainstem stroke. Sci. Transl. Med. 2014, 6, 257re7. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Gao, X.; Hong, B.; Gao, S. Frequency and Phase Mixed Coding in SSVEP-Based Brain–Computer Interface. IEEE Trans. Biomed. Eng. 2011, 58, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Violante, I.R.; Alania, K.; Cassarà, A.M.; Neufeld, E.; Acerbo, E.; Carron, B.; Williamson, A.; Kurtin, D.L.; Rhodes, E.; Hampshire, A.; et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat. Neurosci. 2023, 26, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.-J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Yatsuda, K.; Yu, W.; Gomez-Tames, J. Population-level insights into temporal interference for focused deep brain neuromodulation. Front. Hum. Neurosci. 2024, 18, 1308549. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; van der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef]
- Bouton, C.E.; Shaikhouni, A.; Annetta, N.V.; Bockbrader, M.A.; Friedenberg, D.A.; Nielson, D.M.; Sharma, G.; Sederberg, P.B.; Glenn, B.C.; Mysiw, W.J.; et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016, 533, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.B.; Willett, F.R.; Young, D.R.; Memberg, W.D.; Murphy, B.A.; Miller, J.P.; Walter, B.L.; Sweet, J.A.; Hoyen, H.A.; Keith, M.W.; et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: A proof-of-concept demonstration. Lancet 2017, 389, 1821–1830. [Google Scholar] [CrossRef]
- Flesher, S.N.; Collinger, J.L.; Foldes, S.T.; Weiss, J.M.; Downey, J.E.; Tyler-Kabara, E.C.; Bensmaia, S.J.; Schwartz, A.B.; Boninger, M.L.; Gaunt, R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016, 8, 361ra141. [Google Scholar] [CrossRef] [PubMed]
- Willett, F.R.; Avansino, D.T.; Hochberg, L.R.; Henderson, J.M.; Shenoy, K.V. High-performance brain-to-text communication via handwriting. Nature 2021, 593, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S.; et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mortiz, C.; field-Fote, E.C.; Tefertiller, C.; van Nes, I.; Trumbower, R.; Kalsi-Ryan, S.; Purcell, M.; Janssen, T.W.J.; Krassioukov, A.; Morse, L.R.; et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: A safety and efficacy trial. Nat. Med. 2024, 30, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Littlejohn, K.T.; Liu, J.R.; Moses, D.A.; Chang, E.F. The speech neuroprosthesis. Nat. Rev. 2024, 25, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Willett, F.R.; Kunz, E.M.; Fan, C.; Avansino, D.T.; Wilson, G.H.; Choi, E.Y.; Kamdar, F.; Glasser, M.F.; Hochberg, L.R.; Dricmann, S.; et al. A high-performance speech neuroprosthesis. Nature 2023, 620, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Won, S.M.; Song, E.; Reeder, J.T.; Rogers, J.A. Emerging Modalities and Implantable Technologies for Neuromodulation. Cell 2020, 181, 115–135. [Google Scholar] [CrossRef]
- Nurmikko, A. Challenges for Large-Scale Cortical Interfaces. Neuron 2020, 108, 259–269. [Google Scholar] [CrossRef]
- Musk, E.; Neuralink. An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, K.; Khan, A.A.; Singh, A.P.; Stern, T.M.; Ng, Y.; Tadić, A.; Orel, P.; LaReau, C.; Pouzzner, D.; Nishimura, K.; et al. 1032 The Argo: A high channel count recording system for neural recording in vivo. J. Neural Eng. 2021, 18, 015002. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.; Hanna, M.E.; Wu, Y.W.; Kollo, M.; Racz, R.; Angle, M.R.; Müller, J.; Brackbill, N.; Wray, W.; Franke, F.; et al. 1035 Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 2020, 6, eaay2789. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Neely, R.M.; Shen, K.; Singhal, U.; Alon, E.; Rabaey, J.M.; Carmena, J.M.; Maharbiz, M.M. Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron 2016, 91, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.; Karimi, Y.; Wang, Q.; Garikapati, S.; Montlouis, W.; Stanacevic, M.; Thakor, N.; Etienne-Cummings, R. The Microbead: A Highly Miniaturized Wirelessly Powered Implantable Neural Stimulating System. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Cavuto, M.L.; Feng, P.; Leene, L.B.; Maslik, M.; Mazza, F.; Savolainen, O.; Szostak, K.M.; Bouganis, C.S.; Ekanayake, J.; et al. Towards a Distributed, Chronically-Implantable Neural Interface. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 719–724. [Google Scholar] [CrossRef]
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Sellers, K.K.; Leonard, M.K.; Gwilliams, L.; Xu, D.; Dougherty, M.E.; Kharazla, V.; Metzger, S.L.; Welkenhuysen, M.; Dutta, B.; et al. High-density single-unit human cortical recordings using the Neuropizels probe. Neuron 2022, 110, 2409–2421.e3. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, B.; Munoz, W.; Kfir, Y.; Toung, M.J.; Meszena, D.; Jamali, M.; Caprara, I.; Hardstone, R.; Khanna, A.; Mustroph, M.L.; et al. Modified Neuropixels probes for recording human neurophysiology in the operating room. Nat. Protoc. 2023, 18, 2927–2953. [Google Scholar] [CrossRef] [PubMed]
- Cash, S.S.; Hochberg, L.R. The emergence of single neurons in clinical neurology. Neuron 2015, 86, 79–91. [Google Scholar] [CrossRef]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef]
- Kozak, L.J.; Owings, M.F. Ambulatory and Inpatient Procedures in the United States, 1995; Vital and Health Statistics. Series 13, Data from the National Health Survey; National Institutes of Health: Bethesda, MD, USA, 1998; pp. 1–116.
- Becher, S.; Smith, M.; Ziran, B. Orthopaedic trauma patients and depression: A prospective cohort. J. Orthop. Trauma 2014, 28, e242–e246. [Google Scholar] [CrossRef] [PubMed]
- Vranceanu, A.M.; Bachoura, A.; Weening, A.; Vrahas, M.; Smith, R.M.; Ring, D. Psychological factors predict disability and pain intensity after skeletal trauma. J. Bone Jt. Surg. Am. Vol. 2014, 96, e20. [Google Scholar] [CrossRef] [PubMed]
- Munin, M.C.; Espejo-De Guzman, M.C.; Boninger, M.L.; Fitzgerald, S.G.; Penrod, L.E.; Singh, J. Predictive factors for successful early prosthetic ambulation among lower-limb amputees. J. Rehabil. Res. Dev. 2001, 38, 379–384. [Google Scholar] [PubMed]
- Helmerhorst, G.T.T.; Vranceanu, A.M.; Vrahas, M.; Smith, M.; Ring, D. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. J. Bone Jt. Surg. Am. Vol. 2014, 96, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Biddiss, E.A.; Chau, T.T. Upper limb prosthesis use and abandonment: A survery of the last 25 years. Prosthet. Orthot. Int. 2007, 31, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Stafford, N.; Huang, S.; Hu, X.; Ferris, D.P.; Huang, H.H. Myoelectric control of robotic lower limb prostheses: A review of electromyography interfaces, control paradigms, challenges and future directions. J. Neural Eng. 2021, 18, 041004. [Google Scholar] [CrossRef] [PubMed]
- Fougner, A.; Stavdahl, Ø.; Kyberd, P.J.; Losier, Y.G.; Parker, P.A. Control of Upper Limb Prostheses: Terminology and Proportional Myoelectric Control—A Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 663–677. [Google Scholar] [CrossRef]
- Roche, A.D.; Rehbaum, H.; Farina, D.; Aszmann, O.C. Prosthetic Myoelectric Control Strategies: A Clinical Perspective. Curr. Surg. Rep. 2014, 2, 44. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Menon, C. A review of force myography research and development. Sensors 2019, 19, 4557. [Google Scholar] [CrossRef]
- Castellini, C. State of the Art and Perspectives of Ultrasound Imaging as a Human-Machine Interface. In Neuro-Robotics: From Brain Machine Interfaces to Rehabilitation Robotics; Artemiadis, P., Ed.; Trends in Augmentation of Human Performance; Springer: Dordrecht, The Netherlands, 2014; pp. 37–58. [Google Scholar] [CrossRef]
- Castellini, C.; Artemiadis, P.; Wininger, M.; Ajoudani, A.; Alimusaj, M.; Bicchi, A.; Caputo, B.; Craelius, W.; Dosen, S.; Englehart, K.; et al. Proceedings of the first workshop on Peripheral Machine Interfaces: Going beyond traditional surface electromyography. Front. Neurorobotics 2014, 8, 22. [Google Scholar] [CrossRef]
- McClanahan, A.; Moench, M.; Fu, Q. Dimensionality analysis of forearm muscle activation for myoelectric control in transradial amputees. PLoS ONE 2020, 15, e0242921. [Google Scholar] [CrossRef] [PubMed]
- Gijsberts, A.; Castellini, C.; Caputo, B.; Hager, A.G.M.; Elsig, S.; Giatsidis, G.; Bassetto, F.; Müller, H. Effect of clinical parameters on the control of myoelectric robotic prosthetic hands. J. Rehabil. Res. Dev. 2016, 53, 345–358. [Google Scholar]
- Pasquina, P.F.; Evangelista, M.; Carvalho, A.J.; Lockhart, J.; Griffin, S.; Nanos, G.; McKay, P.; Hansen, M.; Ipsen, D.; Vandersea, J.; et al. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J. Neurosci. Methods 2015, 244, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Heidari, H.; Farina, D.; Nazarpour, K. Miniaturized magnetic sensors for implantable magnetomyography. Adv. Mater. Technol. 2020, 5, 2000185. [Google Scholar] [CrossRef]
- Tarantino, S.; Clemente, F.; Barone, D.; Controzzi, M.; Cipriani, C. The myokinetic control interface: Tracking implanted magnets as a means for prosthetic control. Sci. Rep. 2017, 7, 17149. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.A.; Barlow, A.K.; Hargrove, L.; Dumanian, G.A. Targeted Muscle Reinnervation for the Upper and Lower Extremity. Tech. Orthop. 2017, 32, 109–116. [Google Scholar] [CrossRef]
- Russell, C.; Roche, A.D.; Chakrabarty, S. Peripheral nerve bionic interface: A review of electrodes. Int. J. Intell. Robot. Appl. 2019, 3, 11–18. [Google Scholar] [CrossRef]
- Loeb, G.E.; Peck, R.A. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J. Neurosci. Methods 1996, 64, 95–103. [Google Scholar] [CrossRef]
- Tyler, D.; Durand, D. A slowly penetrating interfascicular nerve electrode for selective activation of peripheral nerves. IEEE Trans. Rehabil. Eng. 1997, 5, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Ceballos, D.; Rodrıguez, F.J.; Stieglitz, T.; Navarro, X. Long term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomaterials 2005, 26, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Vu, P.P.; Vaskov, A.K.; Irwin, Z.T.; Henning, P.T.; Lueders, D.R.; Laidlaw, A.T.; Davis, A.J.; Nu, C.S.; Gates, D.H.; Gillespie, R.B.; et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci. Transl. Med. 2020, 12, eaay2857. [Google Scholar] [CrossRef] [PubMed]
- Clites, T.R.; Carty, M.J.; Ullauri, J.B.; Carney, M.E.; Mooney, L.M.; Duval, J.F.; Srinivasan, S.S.; Herr, H.M. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 2018, 10, eaap8373. [Google Scholar] [CrossRef] [PubMed]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, eaax2352. [Google Scholar] [CrossRef]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef]
- Tan, D.W.; Schiefer, M.A.; Keith, M.W.; Anderson, J.R.; Tyler, J.; Tyler, D.J. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014, 6, 257ra138. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Wark, H.A.C.; Hutchinson, D.T.; Warren, D.J.; O’Neill, K.; Scheinblum, T.; Clark, G.A.; Normann, R.A.; Greger, B. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 2016, 13, 036001. [Google Scholar] [CrossRef]
- Petrini, F.M.; Valle, G.; Bumbasirevic, M.; Barberi, F.; Bortolotti, D.; Cvancara, P.; Hiairrassary, A.; Mijovic, P.; Sverrisson, A.ö.; Pedrocchi, A.; et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 2019, 11, eaav8939. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Rozbruch, S.R.; Handal, M.B.; Coughlan, A.; Al Muderis, M. Osseointegration for Amputees. JBJS Rev. 2020, 8, e0043. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.; Fatone, S. Residual Limb Volume Change: Systematic of Measurement and Management. J. Rehabil. Res. Dev. 2011, 48, 949–986. [Google Scholar] [CrossRef]
- Nehler, M.R.; Coll, J.R.; Hiatt, W.R.; Regensteiner, J.G.; Schnickel, G.T.; Klenke, W.A.; Strecker, P.K.; Anderson, M.W.; Jones, D.N.; Whitehill, T.A.; et al. Functional outcome in a contemporary series of major lower extremity amputations. J. Vasc. Surg. 2003, 38, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Muderis, M.A.; Lu, W.; Glatt, V.; Tetsworth, K. Two-stage osseointegrated reconstruction of post-traumatic unilateral transfemoral amputees. Mil. Med. 2018, 183, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Van de Meent, H.; Hopman, M.T.; Frölke, J.P. Walking Ability and Quality of Life in Subjects with Transfemoral Amputation: A Comparison of Osseointegration with Socket Prostheses. Arch. Phys. Med. Rehabil. 2013, 94, 2174–2178. [Google Scholar] [CrossRef] [PubMed]
- Frossard, L.; Hagberg, K.; Häggström, E.; Gow, D.L.; Brånemark, R.; Pearcy, M. Functional Outcome of Transfemoral Amputees Fitted with an Osseointegrated Fixation: Temporal Gait Characteristics. J. Prosthet. Orthot. 2010, 22, 11. [Google Scholar] [CrossRef]
- Van Eck, C.F.; McGough, R.L. Clinical outcome of osseointegrated prostheses for lower extremity amputations: A systematic review of the literature. Curr. Orthop. Pract. 2015, 26, 349–357. [Google Scholar] [CrossRef]
- Örgel, M.; Elareibi, M.; Graulich, T.; Krettek, C.; Neunaber, C.; Aschoff, H.H.; Ranker, A.; Winkelmann, M. Osseoperception in transcutaneous osseointegrated prosthetic systems (TOPS) after transfemoral amputation: A prospective study. Arch. Orthop. Trauma Surg. 2023, 143, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Hagberg, K.; Bullington, J. My prosthesis as a part of me: A qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet. Orthot. Int. 2011, 35, 207–214. [Google Scholar] [CrossRef]
- Gerzina, C.; Potter, E.; Haleem, A.M.; Dabash, S. The future of the amputees with osseointegration: A systematic review of literature. J. Clin. Orthop. Trauma 2020, 11, S142–S148. [Google Scholar] [CrossRef]
- Young, A.J.; Ferris, D.P. State of the Art and Future Directions for Lower Limb Robotic Exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, E.L.; Resnik, L.; Schiefer, M.A.; Schmitt, M.S.; Tyler, D.J. Home Use of a Neural-connected Sensory Prosthesis Provides the Functional and Psychosocial Experience of Having a Hand Again. Sci. Rep. 2018, 8, 9866. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.A.; Hirons, R. Preparing our Paralympians: Research and development at Össur, UK. Prosthet. Orthot. Int. 2012, 36, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Weyand, P.G.; Bundle, M.W. Point: Artificial limbs do make artificially fast running speeds possible. J. Appl. Physiol. 2010, 108, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P. The potential transformation of our species by neural enhancement. J. Mot. Behav. 2015, 47, 73–78. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.E.; Lebedev, M.A.; Ifft, P.J.; Zhuang, K.Z.; Shokur, S.; Bleuler, H.; Nicolelis, M.A. Active tactile exploration using a brain–machine–brain interface. Nature 2011, 479, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Gopura, R.A.R.C.; Bandara, D.S.V.; Kiguchi, K.; Mann, G.K.I. Developments in hardware systems of active upper-limb exoskeleton robots: A review. Robot. Auton. Syst. 2016, 75, 203–220. [Google Scholar] [CrossRef]
- Heo, P.; Gu, G.M.; Lee, S.J.; Rhee, K.; Kim, J. Current hand exoskeleton technologies for rehabilitation and assistive engineering. Int. J. Precis. Eng. Manuf. 2012, 13, 807–824. [Google Scholar] [CrossRef]
- Yap, H.K.; Lim, J.H.; Nasrallah, F.; Yeow, C.H. Design and Preliminary Feasibility Study of a Soft Robotic Glove for Hand Function Assistance in Stroke Survivors. Front. Neurosci. 2017, 11, 547. [Google Scholar] [CrossRef]
- Correia, C.; Nuckols, K.; Wagner, D.; Zhou, Y.M.; Clarke, M.; Orzel, D.; Solinsky, R.; Paganoni, S.; Walsh, C.J. Improving Grasp Function After Spinal Cord Injury with a Soft Robotic Glove. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1407–1415. [Google Scholar] [CrossRef]
- Bach Baunsgaard, C.; Vig Nissen, U.; Katrin Brust, A.; Frotzler, A.; Ribeill, C.; Kalke, Y.B.; León, N.; Gómez, B.; Samuelsson, K.; Antepohl, W.; et al. Gait training after spinal cord injury: Safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord 2018, 56, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Wade, R.; Sumrell, R.; Villadelgado, L.; Khalil, R.E.; Lavis, T. Exoskeleton training may improve level of physical activity after spinal cord injury: A case series. Top. Spinal Cord Inj. Rehabil. 2017, 23, 245–255. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, M.; Kuindersma, S.; Walsh, C.J. Human-in-the-loop optimization of hip assistance with a soft exosuit during walking. Sci. Robot. 2018, 3, eaar5438. [Google Scholar] [CrossRef]
- Huysamen, K.; de Looze, M.; Bosch, T.; Ortiz, J.; Toxiri, S.; O’Sullivan, L.W. Assessment of an active industrial exoskeleton to aid dynamic lifting and lowering manual handling tasks. Appl. Ergon. 2018, 68, 125–131. [Google Scholar] [CrossRef]
- Malcolm, P.; Derave, W.; Galle, S.; De Clercq, D. A simple exoskeleton that assists plantarflexion can reduce the metabolic cost of human walking. PLoS ONE 2013, 8, e56137. [Google Scholar] [CrossRef] [PubMed]
- Mooney, L.M.; Rouse, E.J.; Herr, H.M. Autonomous exoskeleton reduces metabolic cost of human walking during load carriage. J. Neuroeng. Rehabil. 2014, 11, 80. [Google Scholar] [CrossRef]
- Khazoom, C.; Véronneau, C.; Bigué, J.P.L.; Grenier, J.; Girard, A.; Plante, J.S. Design and Control of a Multifunctional Ankle Exoskeleton Powered by Magnetorheological Actuators to Assist Walking, Jumping, and Landing. IEEE Robot. Autom. Lett. 2019, 4, 3083–3090. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Chen, X.; Xiong, C.; Hasegawa, Y. Supernumerary Robotic Limbs: A Review and Future Outlook. IEEE Trans. Med. Robot. Bionics 2021, 3, 623–639. [Google Scholar] [CrossRef]
- Aliman, N.; Ramli, R.; Haris, S.M. Design and development of lower limb exoskeletons: A survey. Robot. Auton. Syst. 2017, 95, 102–116. [Google Scholar] [CrossRef]
- Steger, R.; Kim, S.H.; Kazerooni, H. Control scheme and networked control architecture for the Berkeley lower extremity exoskeleton (BLEEX). In Proceedings of the Proceedings 2006 IEEE International Conference on Robotics and Automation, 2006. ICRA 2006, Orlando, FL, USA, 15–19 May 2006; pp. 3469–3476. [Google Scholar]
- Kim, H.; Seo, C.; Shin, Y.J.; Kim, J.; Kang, Y.S. Locomotion control strategy of hydraulic lower extremity exoskeleton robot. In Proceedings of the 2015 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Busan, Republic of Korea, 7–11 July 2015; pp. 577–582. [Google Scholar] [CrossRef]
- Karlin, S. Raiding Iron Man’s closet [Geek Life]. IEEE Spectr. 2011, 48, 25. [Google Scholar] [CrossRef]
- Swank, C.; Sikka, S.; Driver, S.; Bennett, M.; Callender, L. Feasibility of integrating robotic exoskeleton gait training in inpatient rehabilitation. Disabil. Rehabil. Assist. Technol. 2020, 15, 409–417. [Google Scholar] [CrossRef]
- Cecchini, M.G.; Wetterwald, A.; van der Pluijm, G.; Thalmann, G.N. Molecular and Biological Mechanisms of Bone Metastasis. EAU Updat. Ser. 2005, 3, 214–226. [Google Scholar] [CrossRef]
- Read, E.; Woolsey, C.; McGibbon, C.A.; O’Connell, C. Physiotherapists’ experiences using the Ekso bionic exoskeleton with patients in a neurological rehabilitation hospital: A qualitative study. Rehabil. Res. Pract. 2020, 2020, 2939573. [Google Scholar] [CrossRef] [PubMed]
- Høyer, E.; Opheim, A.; Jørgensen, V. Implementing the exoskeleton Ekso GTTM for gait rehabilitation in a stroke unit–feasibility, functional benefits and patient experiences. Disabil. Rehabil. Assist. Technol. 2022, 17, 473–479. [Google Scholar] [CrossRef]
- Edwards, D.J.; Forrest, G.; Cortes, M.; Weightman, M.M.; Sadowsky, C.; Chang, S.H.; Furman, K.; Bialek, A.; Prokup, S.; Carlow, J.; et al. Walking improvement in chronic incomplete spinal cord injury with exoskeleton robotic training (WISE): A randomized controlled trial. Spinal Cord 2022, 60, 522–532. [Google Scholar] [CrossRef]
- Rojek, A.; Mika, A.; Oleksy, Ł.; Stolarczyk, A.; Kielnar, R. Effects of exoskeleton gait training on balance, load distribution, and functional status in stroke: A randomized controlled trial. Front. Neurol. 2020, 10, 489493. [Google Scholar] [CrossRef] [PubMed]
- Asbeck, A.T.; De Rossi, S.M.; Holt, K.G.; Walsh, C.J. A biologically inspired soft exosuit for walking assistance. Int. J. Robot. Res. 2015, 34, 744–762. [Google Scholar] [CrossRef]
- Cappello, L.; Meyer, J.T.; Galloway, K.C.; Peisner, J.D.; Granberry, R.; Wagner, D.A.; Engelhardt, S.; Paganoni, S.; Walsh, C.J. Assisting hand function after spinal cord injury with a fabric-based soft robotic glove. J. Neuroeng. Rehabil. 2018, 15, 59. [Google Scholar] [CrossRef]
- Kang, B.B.; Lee, H.; In, H.; Jeong, U.; Chung, J.; Cho, K.J. Development of a polymer-based tendon-driven wearable robotic hand. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 3750–3755. [Google Scholar]
- Sasaki, D.; Noritsugu, T.; Takaiwa, M. Development of pneumatic lower limb power assist wear driven with wearable air supply 1277 system. In Proceedings of the 2013 IEEE/RSJ International Conference on Intelligent Robots and Systems, Tokyo, Japan, 3–7 November 2013; pp. 4440–4445. [Google Scholar]
- Wang, S.; Wang, L.; Meijneke, C.; Van Asseldonk, E.; Hoellinger, T.; Cheron, G.; Ivanenko, Y.; La Scaleia, V.; Sylos-Labini, F.; Molinari, M.; et al. Design and control of the MINDWALKER exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 277–286. [Google Scholar] [CrossRef]
- Rea, R.; Beck, C.; Rovekamp, R.; Neuhaus, P.; Diftler, M. X1: A robotic exoskeleton for in-space countermeasures and dynamometry. In Proceedings of the AIAA Space 2013 Conference and Exposition, San Diego, CA, USA, 10–12 September 2013; p. 5510. [Google Scholar]
- Siviy, C.; Baker, L.M.; Quinlivan, B.T.; Porciuncula, F.; Swaminathan, K.; Awad, L.N.; Walsh, C.J. Opportunities and challenges in the development of exoskeletons for locomotor assistance. Nat. Biomed. Eng. 2023, 7, 456–472. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Wang, Z.; Ye, X.; Liu, Y.; Wu, X. A novel lightweight wearable soft exosuit for reducing the metabolic rate and muscle fatigue. Biosensors 2021, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Goršič, M.; Song, Y.; Dai, B.; Novak, D. Evaluation of the HeroWear Apex back-assist exosuit during multiple brief tasks. J. Biomech. 2021, 126, 110620. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.K.D.; Accoto, D. Review of Current Spinal Robotic Orthoses. Healthcare 2021, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Abrahamsen, B. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2022, 34, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, J.; Panizzolo, F.A.; Zhou, Y.M.; Baker, L.M.; Galiana, I.; Malcolm, P.; Walsh, C.J. Reducing the metabolic cost of running with a tethered soft exosuit. Sci. Robot. 2017, 2, eaan6708. [Google Scholar] [CrossRef] [PubMed]
- Proietti, T.; O’Neill, C.; Gerez, L.; Cole, T.; Mendelowitz, S.; Nuckols, K.; Hohimer, C.; Lin, D.; Paganoni, S.; Walsh, C. Restoring arm function with a soft robotic wearable for individuals with amyotrophic lateral sclerosis. Sci. Transl. Med. 2023, 15, eadd1504. [Google Scholar] [CrossRef]
- Schmidt, K.; Duarte, J.E.; Grimmer, M.; Sancho-Puchades, A.; Wei, H.; Easthope, C.S.; Riener, R. The Myosuit: Bi-articular Anti-gravity Exosuit That Reduces Hip Extensor Activity in Sitting Transfers. Front. Neurorobotics 2017, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Collo, A.; Bonnet, V.; Venture, G. A quasi-passive lower limb exoskeleton for partial body weight support. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 643–648. [Google Scholar]
- Cai, V.A.D.; Bidaud, P.; Hayward, V.; Gosselin, F.; Desailly, E. Self-adjusting, isostatic exoskeleton for the human knee joint. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 612–618. [Google Scholar]
- Kilicarslan, A.; Prasad, S.; Grossman, R.G.; Contreras-Vidal, J.L. High accuracy decoding of user intentions using EEG to control a lower-body exoskeleton. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5606–5609. [Google Scholar]
- Zoss, A.B.; Kazerooni, H.; Chu, A. Biomechanical design of the Berkeley lower extremity exoskeleton (BLEEX). IEEE/ASME Trans. Mechatron. 2006, 11, 128–138. [Google Scholar] [CrossRef]
- Kazerooni, H.; Steger, R. The Berkeley Lower Extremity Exoskeleton. J. Dyn. Syst. Meas. Control 2005, 128, 14–25. [Google Scholar] [CrossRef]
- Cenciarini, M.; Dollar, A.M. Biomechanical considerations in the design of lower limb exoskeletons. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6. [Google Scholar]
- Kim, W.; Lee, H.; Kim, D.; Han, J.; Han, C. Mechanical design of the Hanyang exoskeleton assistive robot (HEXAR). In Proceedings of the 2014 14th International Conference on Control, Automation and Systems (ICCAS 2014), Gyeonggi-do, Republic of Korea, 22–25 October 2014; pp. 479–484. [Google Scholar]
- Park, Y.L.; Santos, J.; Galloway, K.G.; Goldfield, E.C.; Wood, R.J. A soft wearable robotic device for active knee motions using flat pneumatic artificial muscles. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 4805–4810, ISSN 1050-4729. [Google Scholar] [CrossRef]
- Vadakkepat, P.; Goswami, D. Biped Locomotion: Stability, Analysis and Control. Int. J. Smart Sens. Intell. Syst. 2008, 1, 187–207. [Google Scholar] [CrossRef]
- Virk, G.S.; Haider, U.; Indrawibawa, I.N.; Thekkeparampumadom, R.K.; Masud, N. Exo-legs for elderly persons. In Mobile Service Robotics; World Scientific: Singapore, 2014; pp. 85–92. [Google Scholar]
- Kwon, J.; Park, J.H.; Ku, S.; Jeong, Y.; Paik, N.J.; Park, Y.L. A soft wearable robotic ankle-foot-orthosis for post-stroke patients. IEEE Robot. Autom. Lett. 2019, 4, 2547–2552. [Google Scholar] [CrossRef]
- Pratt, J.; Krupp, B.; Morse, C.; Collins, S. The RoboKnee: An exoskeleton for enhancing strength and endurance during walking. In Proceedings of the IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA ’04. 2004, New Orleans, LA, USA, 26 April–1 May 2004; Volume 3, pp. 2430–2435, ISSN 1050-4729. [Google Scholar] [CrossRef]
- Shima, K.; Eguchi, R.; Shiba, K.; Tsuji, T. CHRIS: Cybernetic Human-Robot Interface Systems. In Proceedings of International Symposium on Robotics; Hiroshima University: Higashi-Hiroshima, Japan, 2005; Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2176e27d2f0a6e52ceda1330d1a8ae43a173598c (accessed on 8 April 2024).
- Tucker, M.R.; Shirota, C.; Lambercy, O.; Sulzer, J.S.; Gassert, R. Design and characterization of an exoskeleton for perturbing the knee during gait. IEEE Trans. Biomed. Eng. 2017, 64, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, Y. In quest of mobility–Honda to develop walking assist devices. Ind. Robot Int. J. 2009, 36, 537–539. [Google Scholar] [CrossRef]
- Kawamoto, H.; Lee, S.; Kanbe, S.; Sankai, Y. Power assist method for HAL-3 using EMG-based feedback controller. In Proceedings of the SMC’03 Conference Proceedings. 2003 IEEE International Conference on Systems, Man and Cybernetics. Conference Theme-System Security and Assurance (Cat. No. 03CH37483), Washington, DC, USA, 8 October 2003; Volume 2, pp. 1648–1653. [Google Scholar]
- Crea, S.; Nann, M.; Trigili, E.; Cordella, F.; Baldoni, A.; Badesa, F.J.; Catalán, J.M.; Zollo, L.; Vitiello, N.; Aracil, N.G.; et al. Feasibility and safety of shared EEG/EOG and vision-guided autonomous whole-arm exoskeleton control to perform activities of daily living. Sci. Rep. 2018, 8, 10823. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Xu, J.; Chen, C.; Long, X.; Tao, D.; Wu, X. Vision-Assisted Autonomous Lower-Limb Exoskeleton Robot. IEEE Trans. Syst. Man Cybern. Syst. 2021, 51, 3759–3770. [Google Scholar] [CrossRef]
- Laschowski, B.; McNally, W.; Wong, A.; McPhee, J. Preliminary Design of an Environment Recognition System for Controlling Robotic Lower-Limb Prostheses and Exoskeletons. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 868–7901. [Google Scholar] [CrossRef]
- Zhang, J.; Fiers, P.; Witte, K.A.; Jackson, R.W.; Poggensee, K.L.; Atkeson, C.G.; Collins, S.H. Human-in-the-loop optimization of exoskeleton assistance during walking. Science 2017, 356, 1280–1284. [Google Scholar] [CrossRef]
- Grazi, L.; Crea, S.; Parri, A.; Yan, T.; Cortese, M.; Giovacchini, F.; Cempini, M.; Pasquini, G.; Micera, S.; Vitiello, N. Gastrocnemius myoelectric control of a robotic hip exoskeleton. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3881–3884. [Google Scholar] [CrossRef]
- Livolsi, C.; Conti, R.; Guanziroli, E.; Friðriksson, Þ.; Alexandersson, Á.; Kristjánsson, K.; Esquenazi, A.; Molino Lova, R.; Romo, D.; Giovacchini, F.; et al. An impairment-specific hip exoskeleton assistance for gait training in subjects with acquired brain injury: A feasibility study. Sci. Rep. 2022, 12, 19343. [Google Scholar] [CrossRef] [PubMed]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PMR 2018, 10, S220–S232. [Google Scholar] [CrossRef] [PubMed]
- d’Elia, N.; Vanetti, F.; Cempini, M.; Pasquini, G.; Parri, A.; Rabuffetti, M.; Ferrarin, M.; Molino Lova, R.; Vitiello, N. Physical human-robot interaction of an active pelvis orthosis: Toward ergonomic assessment of wearable robots. J. Neuroeng. Rehabil. 2017, 14, 29. [Google Scholar] [CrossRef]
- Monaco, V.; Tropea, P.; Aprigliano, F.; Martelli, D.; Parri, A.; Cortese, M.; Molino-Lova, R.; Vitiello, N.; Micera, S. An ecologically-controlled exoskeleton can improve balance recovery after slippage. Sci. Rep. 2017, 7, 46721. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z. 25th anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Tegin, J.; Wikander, J. Tactile sensing in intelligent robotic manipulation—A review. Ind. Robot Int. J. 2005, 32, 64–70. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.S.; Kim, R.H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hwang, D.; Yu, Z.; Takei, K.; Park, J.; Chen, T.; Ma, B.; Javey, A. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 2016, 11, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.H.; Sun, Q.; Kim, S.Y.; Han, J.T.; Kim, D.H.; Cho, J.H. Stretchable and multimodal all graphene electronic skin. Adv. Mater. 2016, 28, 2601–2608. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, eaaq0508. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.K.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, R. Electronic Skin with Multifunction Sensors Based on Thermosensation. Adv. Mater. 2017, 29, 1606151. [Google Scholar] [CrossRef]
- Chou, H.H.; Nguyen, A.; Chortos, A.; To, J.W.F.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.G.; Tok, J.B.H.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6, 8011. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Liao, Q.; Xun, X.; Gao, F.; Xu, L.; Kang, Z.; Zhang, Y. Self-powered user-interactive electronic skin for programmable touch operation platform. Sci. Adv. 2020, 6, eaba4294. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Li, J.; Zhou, Q.; Xiao, Y.; Zhang, K.; Luo, B.; Zhou, J.; Hu, B. Dual-Mode Electronic Skin with Integrated Tactile Sensing and Visualized Injury Warning. ACS Appl. Mater. Interfaces 2017, 9, 37493–37500. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Walia, S.; Naznee, S.; Taha, M.; Nirantar, S.; Rahman, F.; Bhaskaran, M.; Sriram, S. Artificial somatosensors: Feedback receptors for electronic skins. Adv. Intell. Syst. 2020, 2, 2000094. [Google Scholar] [CrossRef]
- Song, Y.; Tay, R.Y.; Li, J.; Xu, C.; Min, J.; Sani, E.S.; Kim, G.; Heng, W.; Kim, I.; Gao, W. 3D-printed epifluidic electronic skin for machine leaning-powered multimodal health surveillance. Sci. Adv. 2023, 9, eadi6492. [Google Scholar] [CrossRef]
- Kim, Y.; Suh, J.M.; Shin, J.; Liu, Y.; Yeon, H.; Qiao, K.; Kum, H.S.; Kim, C.; Lee, H.E.; Choi, C.; et al. Chipless wireless electronic skins by remote epitaxial freestanding compound semiconductors. Science 2022, 377, 859–864. [Google Scholar] [CrossRef]
- Fernandez, E. Development of visual Neuroprostheses: Trends and challenges. Bioelectron. Med. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.O.; Jalil, A.; Stanga, P.E. Electronic retinal implants and artificial vision: Journey and present. Eye 2017, 31, 1383–1398. [Google Scholar] [CrossRef] [PubMed]
- Merabet, L.B.; Rizzo III, J.F.; Pascual-Leone, A.; Fernandez, E. ‘Who is the ideal candidate?’: Decisions and issues relating to visual neuroprothesis development, patient testing and neuroplasty. J. Neural Eng. 2007, 4, S130. [Google Scholar] [CrossRef]
- Rizzo, J.F.; Wyatt, J.; Loewenstein, J.; Kelly, S.; Shire, D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5362–5369. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, C.; Huang, W.; Li, L.; Ren, Q. Current research of C-Sight visual prosthesis for the blind. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5875–5878. [Google Scholar]
- Killian, N.J.; Vurro, M.; Keith, S.B.; Kyada, M.J.; Pezaris, J.S. Perceptual learning in a non-human primate model of artificial vision. Sci. Rep. 2016, 6, 36329. [Google Scholar] [CrossRef] [PubMed]
- Normann, R.A.; Fernandez, E. Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J. Neural Eng. 2016, 13, 061003. [Google Scholar] [CrossRef]
- Huff, T.; Mahabadi, N.; Tadi, P. Neuroanatomy, visual cortex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Beauchamp, M.S.; Oswalt, D.; Sun, P.; Foster, B.L.; Magnotti, J.F.; Niketeghad, S.; Pouratian, N.; Bosking, W.H.; Yoshor, D. Dynamic stimulation of visual cortex produces form vision in sighted and blind humans. Cell 2020, 181, 774–783.e5. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, G.; Colombo, E.; Barsotti, J.; Benfenati, F.; Lanzani, G. Photochemistry of organic retinal prostheses. Annu. Rev. Phys. Chem. 2019, 70, 99–121. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S.; et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef]
- Schuster, G.M.; Arianpour, A.; Cookson, S.; Zhang, A.; Hendrik, L.; O’Brien, T.; Alvarez, A.; Ford, J.E. Wink-controlled polarization-switched telescopic contact lenses. Appl. Opt. 2015, 54, 9597–9605. [Google Scholar] [CrossRef]
- Arianpour, A.; Schuster, G.M.; Tremblay, E.J.; Stamenov, I.; Groisman, A.; Legerton, J.; Meyers, W.; Amigo, G.A.; Ford, J.E. Wearable telescopic contact lens. Appl. Opt. 2015, 54, 7195–7204. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.J.; Stamenov, I.; Beer, R.D.; Arianpour, A.; Ford, J.E. Switchable telescopic contact lens. Opt. Express 2013, 21, 15980–15986. [Google Scholar] [CrossRef]
- Vincent, S.J. The use of contact lens telescopic systems in low vision rehabilitation. Contact Lens Anterior Eye 2017, 40, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K. Biohacking. Trends Biotechnol. 2018, 36, 744–747. [Google Scholar] [CrossRef]
- Catherwood, P.A.; Finlay, D.D.; McLaughlin, J.A. Intelligent subcutaneous body area networks: Anticipating implantable devices. IEEE Technol. Soc. Mag. 2016, 35, 73–80. [Google Scholar] [CrossRef]
- Gasson, M.; Hutt, B.; Goodhew, I.; Kyberd, P.; Warwick, K. Invasive neural prosthesis for neural signal detection and nerve stimulation. Int. J. Adapt. Control Signal Process. 2005, 19, 365–375. [Google Scholar] [CrossRef]
- Three Square Market Offers to Microchip Employees at ‘Chip Party’. PR Newswire. Available online: https://www.prnewswire.com/news-releases/three-square-market-offers-to-microchip-employees-at-chip-party-300496735.html (accessed on 6 April 2024).
- Dela Rosa, Y.J. The Introduction of ‘Voluntary’ Microchip Technology in the Workplace: An Innovative Solution or Invasion of Privacy? Institute of Business Ethics, University of Bath: Bath, UK, 2019. [Google Scholar]
- Yahoo News. Microchips under Skin. 2021. Available online: https://www.yahoo.com/news/microchips-under-skin-technophile-swedes-033147071.html?guccounter=2 (accessed on 6 April 2024).
- Maeda, K.; Henbest, K.B.; Cintolesi, F.; Kuprov, I.; Rodgers, C.T.; Liddell, P.A.; Gust, D.; Timmel, C.R.; Hore, P.J. Chemical compass model of avian magnetoreception. Nature 2008, 453, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Nye, C. Biohacker: Meet the People ‘Hacking’ Their Bodies. BBC News, 2018. Available online: https://www.bbc.com/news/technology-46442519 (accessed on 8 April 2024).
- Ma, Y.; Bao, J.; Zhang, Y.; Li, Z.; Zhou, X.; Wan, C.; Huang, L.; Zhao, Y.; Han, G.; Xue, T. Mammalian near-infrared image vision through injectable and self-powered retinal nanoantennae. Cell 2019, 177, 243–255. [Google Scholar] [CrossRef]
- Doetschman, T.; Georgieva, T. Gene editing with CRISPR/Cas9 RNA-directed nuclease. Circ. Res. 2017, 120, 876–894. [Google Scholar] [CrossRef]
- Garneau, J.E.; Dupuis, M.È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. 2010, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 2012, 482, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Zhang, F. Genome engineering using CRISPR-Cas9 system. Chromosom. Mutagen. 2015, 1239, 197–217. [Google Scholar]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID) a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, N. High-resolution interrogation of functional elements in the noncoding genome. Eur. J. Hum. Genet. 2019, 27, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Li, B.; Meng, Z.; Jung, I.; Lee, A.Y.; Dixon, J.; Maliskova, L.; Guan, K.l.; Shen, Y.; Ren, B. A new class of temporarily phenotypic enhancers identified by CRISPR/Cas9-mediated genetic screening. Genome Res. 2016, 26, 397–405. [Google Scholar] [CrossRef]
- Zhang, C.; Quan, R.; Wang, J. Development and application of CRISPR/Cas9 technologies in genomic editing. Hum. Mol. Genet. 2018, 27, R79–R88. [Google Scholar] [CrossRef]
- 7.23B: Applications of Genetic Engineering. LibreTexts Biology. Available online: https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_(Boundless)/07%3A_Microbial_Genetics/7.23%3A_Genetic_Engineer-ing_Products/7.23B%3A__Applications_of_Genetic_Engineering#:~:text=In%20medicine%2C%20genetic%20engineering%20has,the%20functions%20of%20certain%20genes (accessed on 6 April 2024).
- Yi, L.; Li, J. CRISPR-Cas9 therapeutics in cancer: Promising strategies and present challenges. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2016, 1866, 197–207. [Google Scholar] [CrossRef]
- Genetic Therapies Benefits and Risks. National Heart, Lung, and Blood Institute. Available online: https://www.nhlbi.nih.gov/health/genetic-therapies/benefits-risks (accessed on 8 April 2024).
- Aartsma-Rus, A.; Fokkema, I.; Verschuuren, J.; Ginjaar, I.; Van Deutekom, J.; van Ommen, G.J.; Den Dunnen, J.T. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 2009, 30, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Tabebordbar, M.; Zhu, K.; Cheng, J.K.; Chew, W.L.; Widrick, J.J.; Yan, W.X.; Maesner, C.; Wu, E.Y.; Xiao, R.; Ran, F.A.; et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016, 351, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Amoasii, L.; Mireault, A.A.; McAnally, J.R.; Li, H.; Sanchez-Ortiz, E.; Bhattacharyya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 2016, 351, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.E.; Hakim, C.H.; Ousterout, D.G.; Thakore, P.I.; Moreb, E.A.; Rivera, R.M.C.; Madhavan, S.; Pan, X.; Ran, F.A.; Yan, W.X.; et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zeng, Y.; Du, H.; Gong, M.; Peng, J.; Zhang, B.; Lei, M.; Zhao, F.; Wang, W.; Li, X.; et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol. Genet. Genom. 2017, 292, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; He, W.; Huang, Y.; Yu, Q.; Chen, Y.; Gao, X.; Sun, X.; Fan, Y. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J. Assist. Reprod. Genet. 2016, 33, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.K.; Chortos, A.; Dunn, R.R.; Schwartz, G.; Eason, E.; Bao, Z. Tunable Flexible Pressure Sensors using Microstructured Elastomer Geometries for Intuitive Electronics. Adv. Funct. Mater. 2014, 24, 5427–5434. [Google Scholar] [CrossRef]
- Svensson, S.; Richter, J.L.; Maitre-Ekern, E.; Pihlajarinne, T.; Maigret, A.; Dalhammar, C. The emerging ‘Right to repair’ legislation in the EU and the US. In Proceedings of the Going Green–Care Innovation, Vienna, Austria, 27–29 November 2018; pp. 27–29. [Google Scholar]
- Lindgren, L.; Kesselheim, A.S.; Kramer, D.B. The Right to Repair Software-Dependent Medical Devices. J. Law Med. Ethics 2023, 50, 857–859. [Google Scholar] [CrossRef]
- Strickland, E.; Harris, M. Their Bionic Eyes Are Now Obsolete and Unsupported. IEEE Spectr. 2022, 15. [Google Scholar]
- SmileDirectClub Is Shutting Down. Where Does That Leave Its Customers? 2023. AP News. Available online: https://apnews.com/article/smile-direct-club-shuts-down-bankruptcy-ecff8127b0bf54812a98217dde5a2170 (accessed on 10 April 2024).
- Executive Order on Promoting the Competition in the American Economy. The White House, United States. Available online: https://www.whitehouse.gov/briefing-room/presidential-actions/2021/07/09/executive-order-on-promoting-competition-in-the-american-economy/ (accessed on 6 April 2024).
- Right to Repair: Making Repair Easier for Consumers. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_1794 (accessed on 10 April 2024).
- Rep. Morelle, J.D.D.N. Text—H.R.4006—117th Congress (2021–2022): Fair Repair Act, 2021. Archive Location: 17 June 2021. Available online: https://www.congress.gov/bill/117th-congress/house-bill/4006 (accessed on 10 April 2024).
- Right to Repair 2023 Legislation. Available online: https://www.ncsl.org/technology-and-communication/right-to-repair-2023-legislation (accessed on 10 April 2024).
- Susan Eggman. Right to Repair Act. 2023. Available online: https://sd05.senate.ca.gov/news/new-bill-would-make-electronics-easier-fix-reducing-waste (accessed on 10 April 2024).
- Marchman, J.; Hinrichsen, N.; Weinberg, R.; Titone, B. Consumer Right to Repair Agricultural Equipment. Available online: https://leg.colorado.gov/bills/hb23-1011 (accessed on 10 April 2024).
- Hinrichsen, N.; Weinberg, R.; Titone, B. Consumer Wheelchair Repair Bill of Rights. Available online: https://leg.colorado.gov/bills/hb22-1031 (accessed on 10 April 2024).
- Kupac, R.; Klein, M.; Latz, R.; Marty, J. Digital Fair Repair Act. 2023. Available online: https://www.revisor.mn.gov/bills/text.php?number=SF1598&version=latest&session=ls93&session_year=2023&session_number=0&format=pdf (accessed on 10 April 2024).
- Breslin, N.D.; Thomas, K.; Biaggi, A.; Brisport, J.; Brouk, S.G. Digital Fair Repair Act; 2022. Available online: https://www.nysenate.gov/legislation/bills/2021/S4104 (accessed on 10 April 2024).
- He, S.; Lai, D.; Lee, J. The medical right to repair: The right to save lives. Lancet 2021, 397, 1260–1261. [Google Scholar] [CrossRef]
- Tan, M.J.; Owh, C.; Chee, P.L.; Kyaw, A.K.K.; Kai, D.; Loh, X.J. Biodegradable electronics: Cornerstone for sustainable electronics and transient applications. J. Mater. Chem. C 2016, 4, 5531–5558. [Google Scholar] [CrossRef]
- Porter, A. Bioethics and Transhumanism. J. Med. Philos. Forum Bioeth. Philos. Med. 2017, 42, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.D.; Yeo, J.; Lee, Y.Y.; Ong, Q.; Han, M.; Tee, B.C.K. Advancing the frontiers of silk fibroin protein-based materials for futuristic electronics and clinical wound-healing (Invited review). Mater. Sci. Eng. C 2018, 86, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Cochlear implantation, enhancements, transhumanism and posthumanism: Some human questions. Sci. Eng. Ethics 2016, 22, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Cosetti, M.K.; Waltzman, S.B. Cochlear implants: Current status and future potential. Expert Rev. Med. Devices 2011, 8, 389–401. [Google Scholar] [CrossRef]
- Sarant, J. Cochlear Implants in Children: A Review. In Hearing Loss; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Cherney, J.L. Deaf culture and the cochlear implant debate: Cyborg politics and the identity of people with disabilities. Argum. Advocacy 1999, 36, 22–34. [Google Scholar] [CrossRef]
- Maynard, A.D.; Scragg, M. The Ethical and Responsible Development and Application of Advanced Brain Machine Interfaces. J. Med. Internet Res. 2019, 21, e16321. [Google Scholar] [CrossRef]
- Wiseman, L.; Kariyawasam, K. Restoring Human Dignity: Some Reflections on the Right to Repair & Medical Devices and Assistive Technologies. Griffith J. Law Hum. Dign. 2022, 10, 1. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manero, A.; Rivera, V.; Fu, Q.; Schwartzman, J.D.; Prock-Gibbs, H.; Shah, N.; Gandhi, D.; White, E.; Crawford, K.E.; Coathup, M.J. Emerging Medical Technologies and Their Use in Bionic Repair and Human Augmentation. Bioengineering 2024, 11, 695. https://doi.org/10.3390/bioengineering11070695
Manero A, Rivera V, Fu Q, Schwartzman JD, Prock-Gibbs H, Shah N, Gandhi D, White E, Crawford KE, Coathup MJ. Emerging Medical Technologies and Their Use in Bionic Repair and Human Augmentation. Bioengineering. 2024; 11(7):695. https://doi.org/10.3390/bioengineering11070695
Chicago/Turabian StyleManero, Albert, Viviana Rivera, Qiushi Fu, Jonathan D. Schwartzman, Hannah Prock-Gibbs, Neel Shah, Deep Gandhi, Evan White, Kaitlyn E. Crawford, and Melanie J. Coathup. 2024. "Emerging Medical Technologies and Their Use in Bionic Repair and Human Augmentation" Bioengineering 11, no. 7: 695. https://doi.org/10.3390/bioengineering11070695
APA StyleManero, A., Rivera, V., Fu, Q., Schwartzman, J. D., Prock-Gibbs, H., Shah, N., Gandhi, D., White, E., Crawford, K. E., & Coathup, M. J. (2024). Emerging Medical Technologies and Their Use in Bionic Repair and Human Augmentation. Bioengineering, 11(7), 695. https://doi.org/10.3390/bioengineering11070695







