Utilizing a Pathomics Biomarker to Predict the Effectiveness of Bevacizumab in Ovarian Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Image Dataset
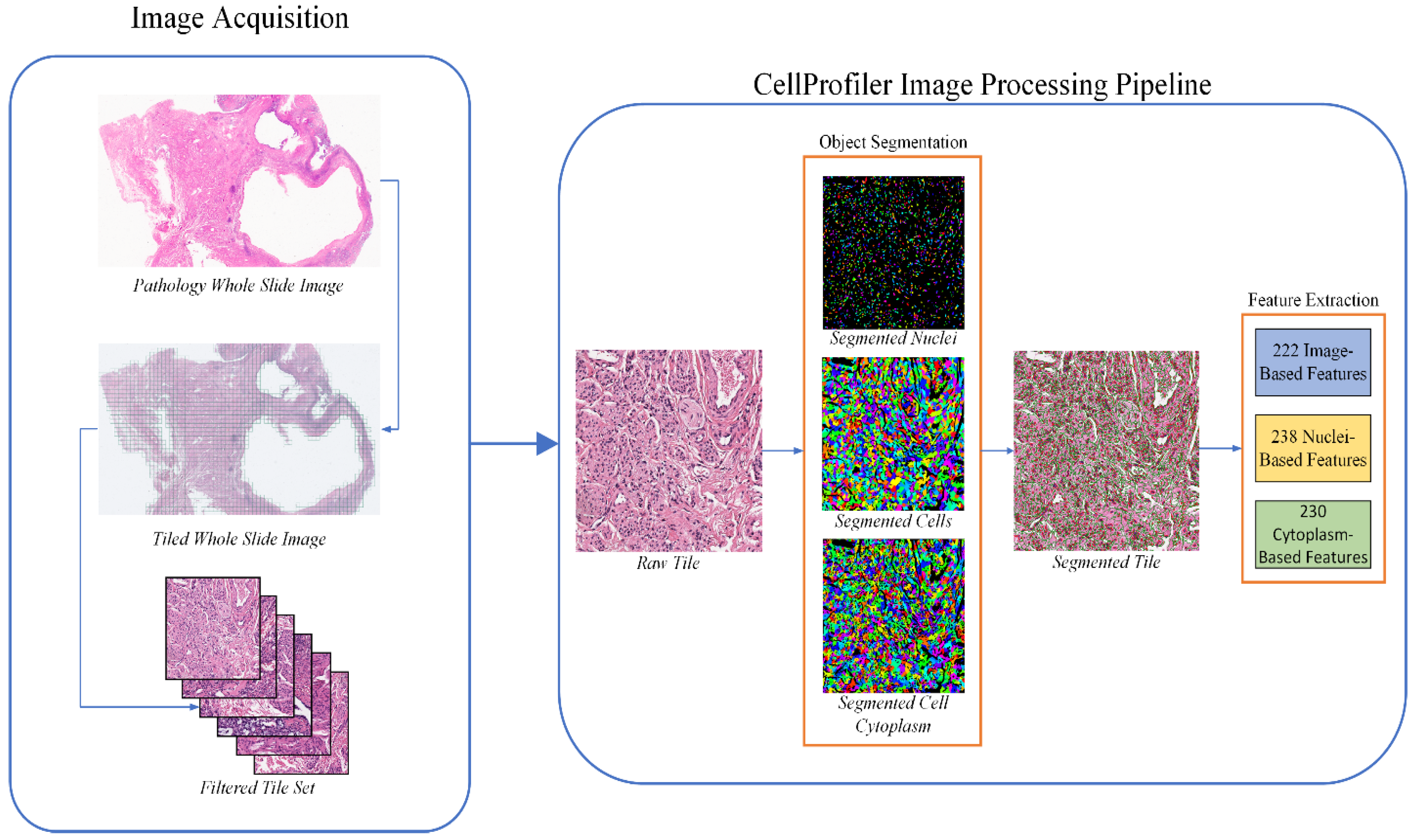
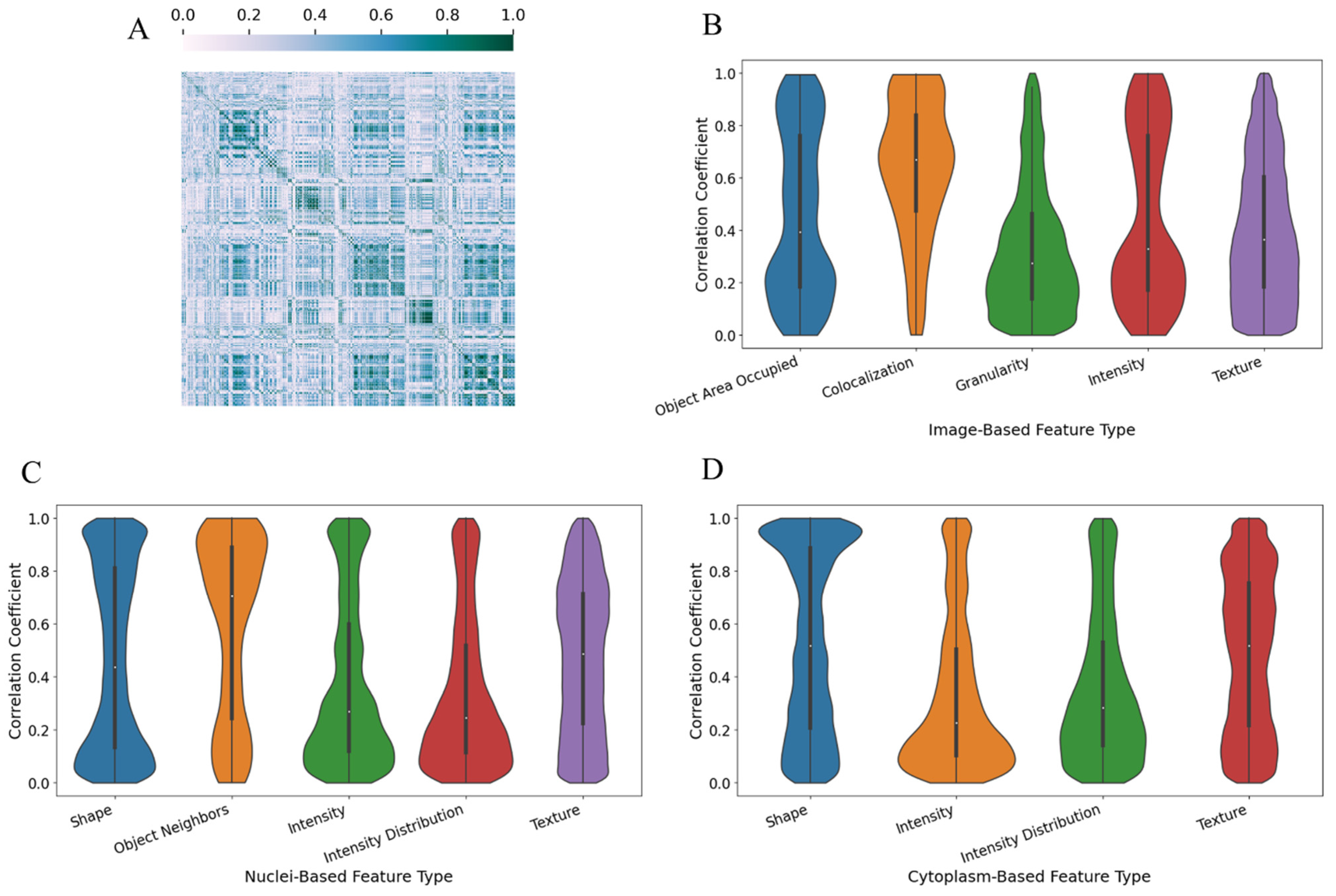
2.2. Histopathology Image Feature Extraction
2.3. Model Training
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Cancer Facts and Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023.
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Chien, J.; Poole, E.M. Ovarian Cancer Prevention, Screening, and Early Detection: Report From the 11th Biennial Ovarian Cancer Research Symposium. Int. J. Gynecol. Cancer 2017, 27 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Doubeni, A.R.B.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Wang, C.-W.; Chang, C.-C.; Khalil, M.A.; Lin, Y.-J.; Liou, Y.-A.; Hsu, P.-C.; Lee, Y.-C.; Wang, C.-H.; Chao, T.-K. Histopathological whole slide image dataset for classification of treatment effectiveness to ovarian cancer. Sci. Data 2022, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M.; Msc, M.B.; (Lon), A.M.O.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.-L.; Seow, K.-M.; Chen, K.-H. The Utilization of Bevacizumab in Patients with Advanced Ovarian Cancer: A Systematic Review of the Mechanisms and Effects. Int. J. Mol. Sci. 2022, 23, 6911. [Google Scholar] [CrossRef] [PubMed]
- Micha, J.P.; Goldstein, B.H.; Rettenmaier, M.A.; Genesen, M.; Graham, C.; Bader, K.; Lopez, K.L.; Nickle, M.; Brown, J.V. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int. J. Gynecol. Cancer 2007, 17, 771–776. [Google Scholar] [CrossRef]
- Boussios, S.; Sadauskaite, A.; Kanellos, F.S.; Tsiouris, A.K.; Karathanasi, A.; Sheriff, M. A narrative review of neoadjuvant, HIPEC and maintenance treatment in ovarian and peritoneal serous cancer: Current status. Gynecol. Pelvic Med. 2020, 3. [Google Scholar] [CrossRef]
- Secord, A.A.; Burdett, K.B.; Owzar, K.; Tritchler, D.; Sibley, A.B.; Liu, Y.; Starr, M.D.; Brady, J.C.; Lankes, H.A.; Hurwitz, H.I.; et al. Predictive Blood-Based Biomarkers in Patients with Epithelial Ovarian Cancer Treated with Carboplatin and Paclitaxel with or without Bevacizumab: Results from GOG-0218. Clin. Cancer Res. 2020, 26, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, A.M.; Lurje, G.; Rhodes, K.E.; Zhang, W.; Yang, D.; Garcia, A.A.; Morgan, R.; Gandara, D.; Scudder, S.; Oza, A.; et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin. Cancer Res. 2008, 14, 7554–7563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thai, T.; Moore, K.; Ding, K.; McMeekin, S.; Liu, H.; Zheng, B. Quantitative measurement of adiposity using CT images to predict the benefit of bevacizumab-based chemotherapy in epithelial ovarian cancer patients. Oncol. Lett. 2016, 12, 680–686. [Google Scholar] [CrossRef][Green Version]
- Wimberger, P.; Gerber, M.J.; Pfisterer, J.; Erdmann, K.; Füessel, S.; Link, T.; du Bois, A.; Kommoss, S.; Heitz, F.; Sehouli, J.; et al. Bevacizumab May Differentially Improve Prognosis of Advanced Ovarian Cancer Patients with Low Expression of VEGF-A165b, an Antiangiogenic VEGF-A Splice Variant. Clin. Cancer Res. 2022, 28, 4660–4668. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Tsibulak, I.; Reimer, D.U.; Zeimet, A.G.; Fiegl, H.; Hackl, H.; Marth, C. An angiogenic tumor phenotype predicts poor prognosis in ovarian cancer. Gynecol. Oncol. 2023, 170, 290–299. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Gupta, R.; Kurc, T.; Sharma, A.; Almeida, J.S.; Saltz, J. The Emergence of Pathomics. Curr. Pathobiol. Rep. 2019, 7, 73–84. [Google Scholar] [CrossRef]
- Lu, C.; Shiradkar, R.; Liu, Z. Integrating pathomics with radiomics and genomics for cancer prognosis: A brief review. Chin. J. Cancer Res. 2021, 33, 563–573. [Google Scholar] [CrossRef]
- Kothari, S.; Phan, J.H.; Stokes, T.H.; Wang, M.D. Pathology imaging informatics for quantitative analysis of whole-slide images. J. Am. Med. Informatics Assoc. 2013, 20, 1099–1108. [Google Scholar] [CrossRef]
- Wang, C.-W.; Lee, Y.-C.; Lin, Y.-J.; Chang, C.-C.; Sai, A.-K.-O.; Wang, C.-H.; Chao, T.-K. Interpretable attention-based deep learning ensemble for personalized ovarian cancer treatment without manual annotations. Comput. Med. Imaging Graph. 2023, 107, 102233. [Google Scholar] [CrossRef]
- Wang, C.-W.; Lee, Y.-C.; Chang, C.-C.; Lin, Y.-J.; Liou, Y.-A.; Hsu, P.-C.; Chang, C.-C.; Sai, A.-K.-O.; Wang, C.-H.; Chao, T.-K. A Weakly Supervised Deep Learning Method for Guiding Ovarian Cancer Treatment and Identifying an Effective Biomarker. Cancers 2022, 14, 1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-W.; Lee, Y.-C.; Lin, Y.-J.; Chang, C.-C.; Sai, A.-K.-O.; Wang, C.-H.; Chao, T.-K. Ensemble biomarkers for guiding anti-angiogenesis therapy for ovarian cancer using deep learning. Clin. Transl. Med. 2023, 13, e1162. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-H.; Zhang, C.; Berry, G.J.; Altman, R.B.; Ré, C.; Rubin, D.L.; Snyder, M. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun. 2016, 7, 12474. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, C.D.A.; Devaraj, D.; Venkatesulu, M. Gene Expression Data Classification Using Support Vector Machine and Mutual Information-based Gene Selection. Procedia Comput. Sci. 2015, 47, 13–21. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Park, H.-G.; Kim, C.-H.; Prakash, D.; Madusanka, N.; So, J.-H.; Cho, N.-H.; Choi, H.-K. Quantitative Analysis of Benign and Malignant Tumors in Histopathology: Predicting Prostate Cancer Grading Using SVM. Appl. Sci. 2019, 9, 2969. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2013, 2, 27. [Google Scholar] [CrossRef]
- Li, Q.; Doi, K. Reduction of bias and variance for evaluation of computer-aided diagnostic schemes. Med. Phys. 2006, 33, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; He, J.; Chalise, P. Nested and Repeated Cross Validation for Classification Model with High-Dimensional Data. Rev. Colomb. Estad. 2020, 43, 103–125. [Google Scholar] [CrossRef]
- Jubb, A.M.; Harris, A.L. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010, 11, 1172–1183. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Bamias, A.; Psaltopoulou, T.; Sotiropoulou, M.; Haidopoulos, D.; Lianos, E.; Bournakis, E.; Papadimitriou, C.; Rodolakis, A.; Vlahos, G.; Dimopoulos, M.A. Mucinous but not clear cell histology is associated with inferior survival in patients with advanced stage ovarian carcinoma treated with platinum-paclitaxel chemotherapy. Cancer 2010, 116, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Sotiropoulou, M.; Zagouri, F.; Trachana, P.; Sakellariou, K.; Kostouros, E.; Kakoyianni, K.; Rodolakis, A.; Vlahos, G.; Haidopoulos, D.; et al. Prognostic evaluation of tumour type and other histopathological characteristics in advanced epithelial ovarian cancer, treated with surgery and paclitaxel/carboplatin chemotherapy: Cell type is the most useful prognostic factor. Eur. J. Cancer 2012, 48, 1476–1483. [Google Scholar] [CrossRef] [PubMed]

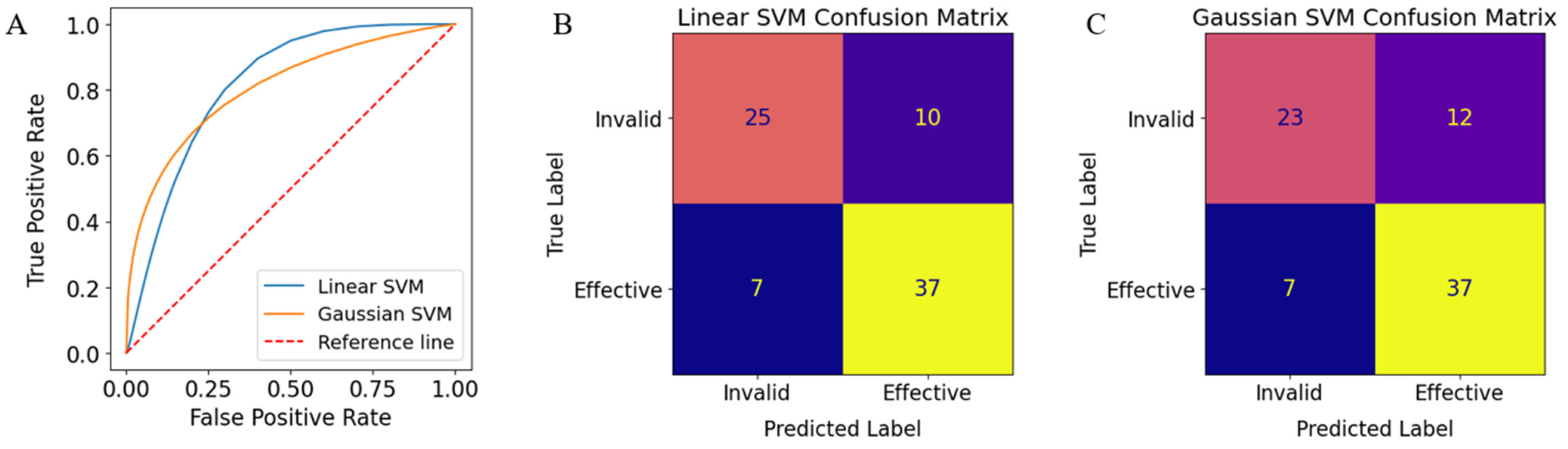
| Model Type | AUC | Accuracy | Precision | Recall | F-Score |
|---|---|---|---|---|---|
| Linear | 0.8312 | 0.7848 | 0.7872 | 0.8409 | 0.8132 |
| Gaussian | 0.8253 | 0.7595 | 0.7551 | 0.8409 | 0.7957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilley, P.; Zhang, K.; Abdoli, N.; Sadri, Y.; Adhikari, L.; Fung, K.-M.; Qiu, Y. Utilizing a Pathomics Biomarker to Predict the Effectiveness of Bevacizumab in Ovarian Cancer Treatment. Bioengineering 2024, 11, 678. https://doi.org/10.3390/bioengineering11070678
Gilley P, Zhang K, Abdoli N, Sadri Y, Adhikari L, Fung K-M, Qiu Y. Utilizing a Pathomics Biomarker to Predict the Effectiveness of Bevacizumab in Ovarian Cancer Treatment. Bioengineering. 2024; 11(7):678. https://doi.org/10.3390/bioengineering11070678
Chicago/Turabian StyleGilley, Patrik, Ke Zhang, Neman Abdoli, Youkabed Sadri, Laura Adhikari, Kar-Ming Fung, and Yuchen Qiu. 2024. "Utilizing a Pathomics Biomarker to Predict the Effectiveness of Bevacizumab in Ovarian Cancer Treatment" Bioengineering 11, no. 7: 678. https://doi.org/10.3390/bioengineering11070678
APA StyleGilley, P., Zhang, K., Abdoli, N., Sadri, Y., Adhikari, L., Fung, K.-M., & Qiu, Y. (2024). Utilizing a Pathomics Biomarker to Predict the Effectiveness of Bevacizumab in Ovarian Cancer Treatment. Bioengineering, 11(7), 678. https://doi.org/10.3390/bioengineering11070678






