Exploring the Potentials of Wearable Technologies in Managing Vestibular Hypofunction
Abstract
1. Introduction
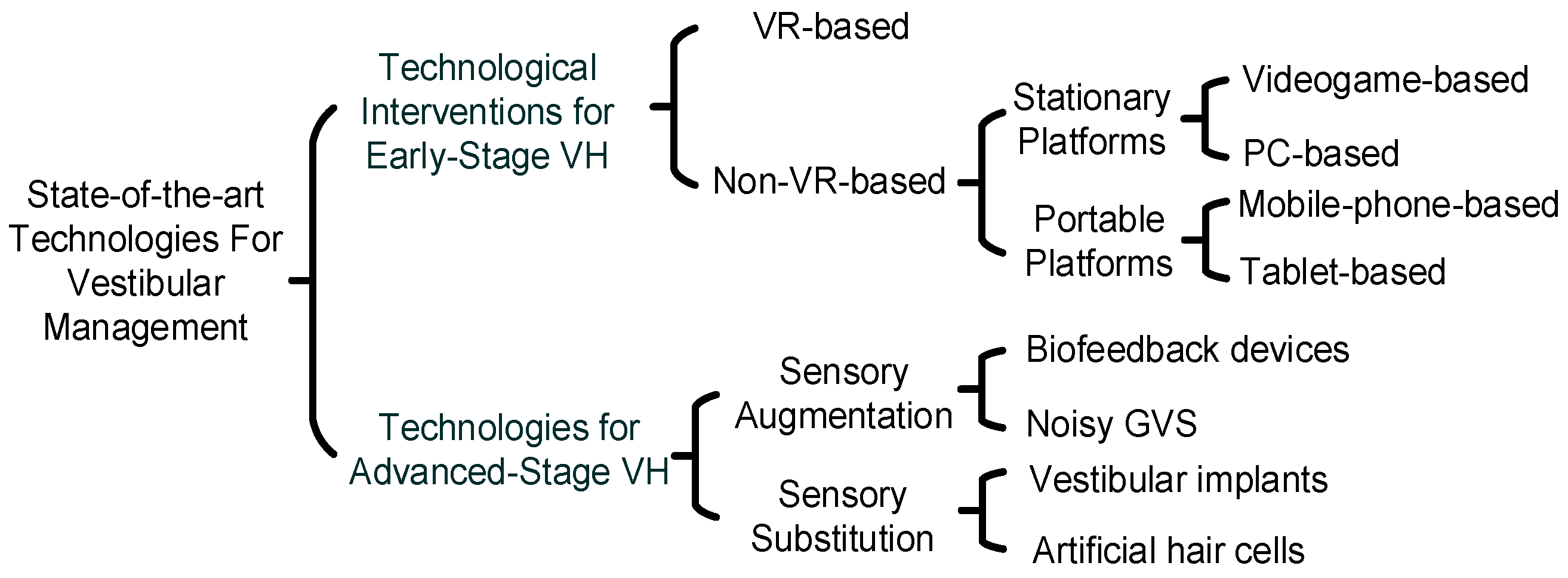
- It provides an overview of the management of VH using assistive technologies at different stages of VH. This work presents the first instance in which technologies used for VH management are categorized based on the stage in which they are used—early- or advanced-stage intervention. For early-stage intervention, it highlights the role of these technologies in facilitating VRT and classifies them based on their mobility and portability. Various platforms, including virtual reality (VR), PC-based, videogame-based, mobile phone-based, and tablet-based methods, are discussed.
- For advanced-stage technological interventions for VH, it explores the use of sensory augmentation and sensory substitution technologies. It discusses these using mathematical analogies and flow diagrams. To the authors’ best knowledge, this is the first time the content has been organized from an engineering perspective by using mathematical analogies and flow diagrams to show how these techniques can enhance or replace vestibular function by providing new avenues for vestibular perception.
- Based on the limitations of current wearable technologies for VH management presented in the article, it outlines the key technological advancements that would be required for VH management technologies of the future.
2. Understanding Vestibular Hypofunction
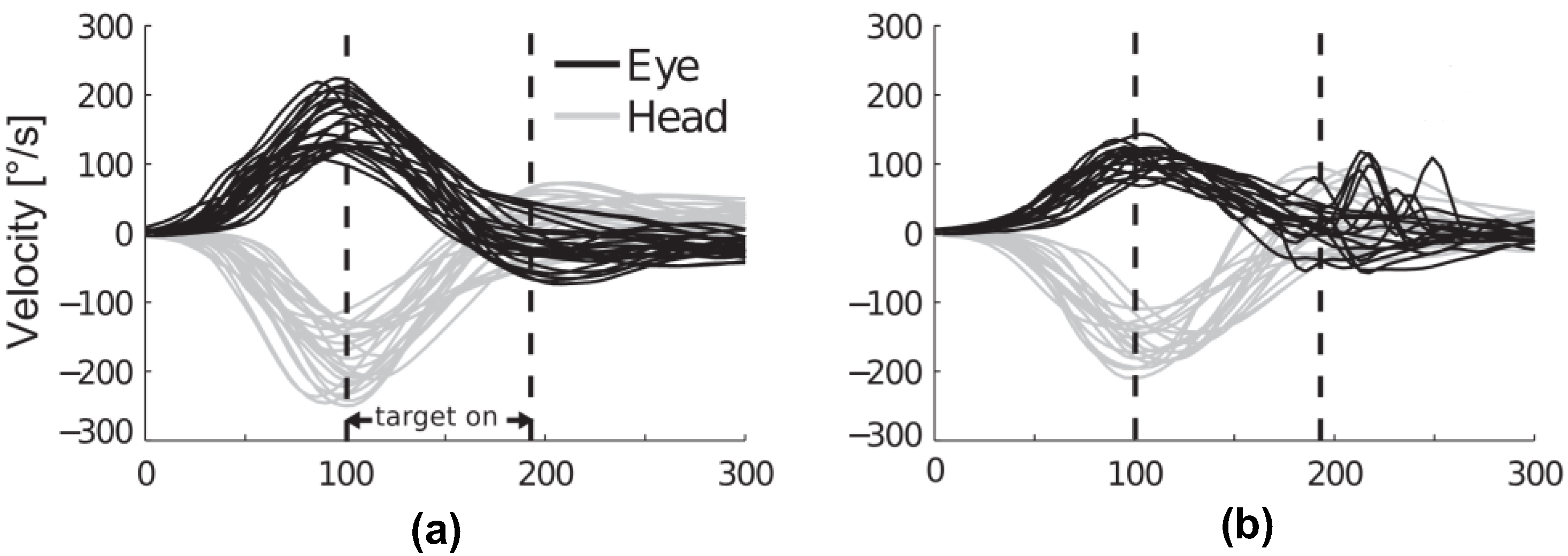
3. Technological Interventions for Early-Stage VH
3.1. VR-Based Methods
3.2. Non-VR Based Methods
3.2.1. Stationary Platforms
3.2.2. Portable Platforms
4. Advanced-Stage Intervention Technologies: Sensory Augmentation and Sensory Substitution
4.1. Sensory Augmentation
4.1.1. Biofeedback Devices
- A balance assessment sensor;
- An algorithm extracting meaningful postural information;
- An actuation device that provides relevant information to the subject in relation to the activity. This actuation signal may take the form of visual, auditory, or vibrotactile cues [10].
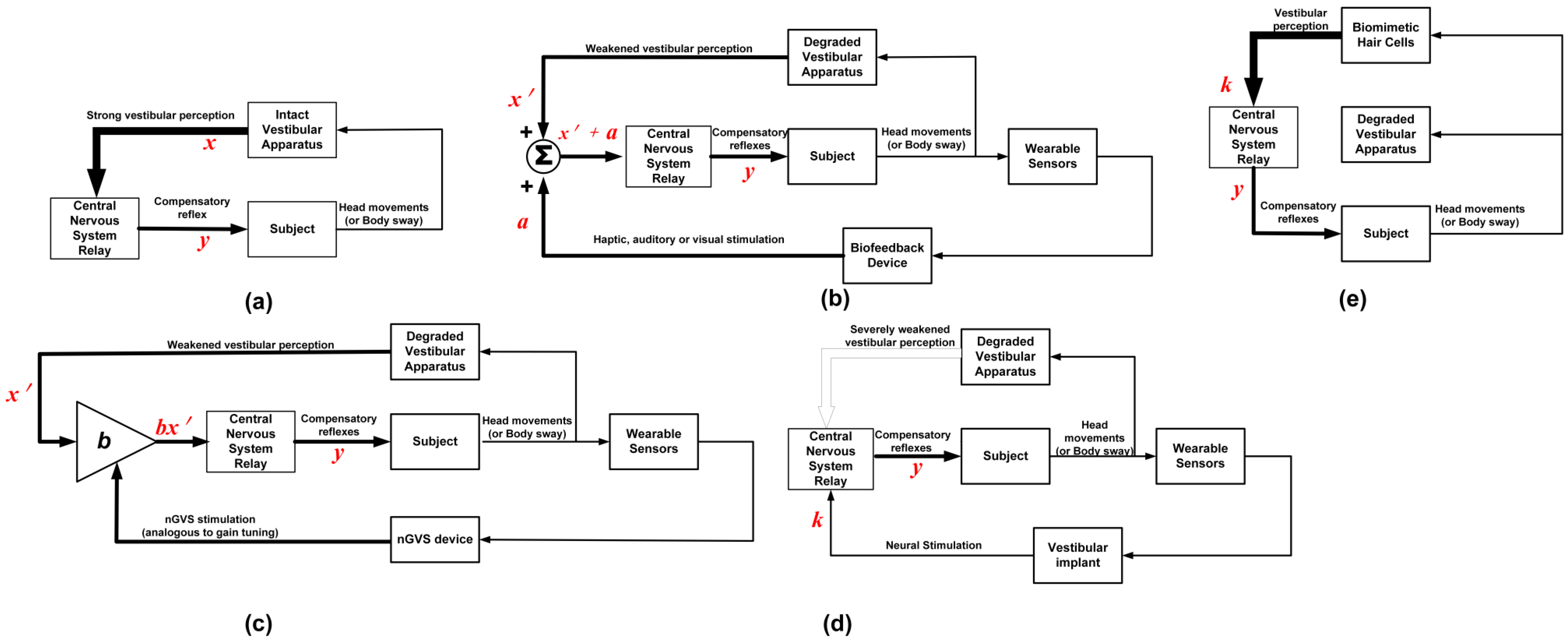
4.1.2. Noisy GVS

4.2. Sensory Substitution
4.2.1. Vestibular Implants
4.2.2. Biomimetic Hair Cells
| Year (Ref.) | Experiment | Device Description | Outcome |
|---|---|---|---|
| 2024 ([79]) | A total of 16 patients with progressive supranuclear palsy were administered nGVS. | nGVS was administered bilaterally, using an amplitude of 0 to 0.7 mA, a frequency range of 0 to 30 Hz, and some additive noise. | Nine out of sixteen patients showed improvements in balance and reduction in body sway, which demonstrates that nGVS has utility beyond VH. |
| 2024 ([80]) | A total of 19 patients with BVH were administered nGVS. | nGVS was administered bilaterally, using an amplitude of 0 to 0.7 mA, a frequency range of 0 to 30 Hz, and some additive noise. | More than half of the assessed patients showed robust improvements in postural balance when treated with nGVS. |
| 2023 ([81]) | A total of 20 healthy subjects were administered with nGVS, and their center of pressure sway path length was measured. | nGVS was administered bilaterally, using an amplitude of 0.2 mA and 0 mA for normal and sham stimulation, respectively, a frequency range of 0.1 to 640 Hz, and some additive noise. | nGVS demonstrated potential for improving the standing balance function. |
| 2023 ([9]) | In 11 patients with BVH, optimal balance stabilization was achieved using nGVS. | nGVS was administered bilaterally, using an amplitude of 0 to 0.7 mA, a frequency range of 0 to 30 Hz, and some additive noise. | Improvements in the body’s balance was due to enhancements in vestibular perception. |
| 2022 ([57]) | nGVS timing was synchronized with the visual motion for 47 participants using a VR head-mounted display. | Used a VR head-mounted display to present real-world 360° videos from a moving first-person viewpoint. nGVS were specifically tuned to match the motion displayed in the videos. | GVS significantly lessened discomfort for VR users prone to cybersickness, enhancing their immersive experience. |
| 2022 ([66]) | A total of 12 out of 23 patients with BVH received nGVS | nGVS was administered bilaterally, using an amplitude of 0 to 0.7 mA, a frequency range of 0 to 30 Hz, and some additive noise. | BVH patients’ balance improved immediately with nGVS. However, nGVS and VRT did not show synergistic effects, suggesting they might be complementary therapies. |
| 2021 ([82]) | The standing stability of subjects administered with nGVS was investigated, consisting of 10 BVH patients and 16 healthy patients. | nGVS was applied bilaterally. The stimulation had amplitudes of 0–1 mA and a frequency range of 0.02–10 Hz with additive noise. | nGVS significantly reduced swaying in both healthy individuals and BVH patients, particularly under visually deprived conditions. |
| 2020 ([64]) | The center of pressure and muscle activity for 17 healthy patients were tested with and without nGVS. | nGVS was administered bilaterally, using an amplitude of 1 mA and 0 mA for normal and sham stimulation, respectively, with a stimulation time of 40 s. | nGVS enhanced balance and muscle activity in the legs, regardless of whether the eyes were closed or open. |
| 2019 ([83]) | A total of 30 patients were administered posture-improving nGVS. | nGVS was applied bilaterally. The stimulation had amplitudes of 0–1 mA and a frequency range of 0.02–10 Hz with additive noise. | nGVS improved posture in balance-impaired subjects and enhanced natural balance mechanisms, even in those balancing with closed eyes. |
| 2018 ([84]) | Comparison between three nGVS experiments involving 18, 24, and 16 patients using sham (0 mA), 0.4 mA, and 1.0 mA stimulation, respectively. | nGVS (0.1–640 Hz) was delivered bilaterally at 0.4 and 1.0 mA. | nGVS used cathodal and anodal currents to enhance balance in daily activities like standing, with lasting benefits post-use. This was measured using the center of pressure sway. |
| 2018 ([65]) | Postural movements were monitored for 13 BVH patients that received nGVS. | nGVS was applied bilaterally. The stimulation had amplitudes of 0–1 mA and a frequency range of 0.02–10 Hz with additive noise. | nGVS offered immediate stability and balance in BVH patients, with effects lasting several hours post-stimulation. |
| 2017 ([85]) | A VR wearer controlled in a room using nGVS. | GVS induced “galvanic body sway” in standing users and impacts balance in walking users, causing them to stagger in anodal direction. In GalVR, GVS is used for smooth turning during walking. | The study demonstrated GVS’s ability to smoothly divert a user’s planned path in a VR environment (i.e., as a navigation interface). |
| 2016 ([86]) | nGVS was administered to VH patients. | nGVS was applied bilaterally. | nGVS led to lasting postural stability improvements, persisting even after the stimulus was discontinued. |
| 2015 ([87]) | Three nGVS experiments were conducted involving six, eight, and five participants, respectively. | The roll, pitch, and yaw motion of the head were dictated by current paths created by GVS administered between the two mastoids and the forehead. | The study demonstrated the potential for directionally induced head motion using GVS. |
| Vestibular Implant (VI) | Sensor | Stimulation Profile | Electrode Placement | Outcome |
|---|---|---|---|---|
| VI UW/Nucleus freedom | None | Fixed pulse rate. | Inserted at the perilymphatic space adjacent to the ampulla of the three semicircular canals. | Modulation of eye movements. |
| VI Geneva-Maastricht | 3D gyroscope | Stimulation amplitude and frequency linearly modulated using movement. | Inserted in the ampulla of the three semicircular canals. | Restoration of VOR, VCR, VSR, and cognitive response in patients. |
| LD-MVI | 3D gyroscope | Used a 32-point piecewise-linear sigmoid curve to map motion to the stimulation profile (amplitude and frequency). | Inserted in the three semicircular canals ampullae. | VOR restoration, gait, position, and stabilization. Significant improvement in the quality of life. |
| Bionic vest | None | Fixed pulse rate and amplitude. | Inserted adjacent to the saccular nerve. | Gait and gaze stabilization, with improvements in the quality of life. |
5. The Future of Wearable Technologies for VH Management
5.1. Essential Requirements for Wearable Systems for Vestibular Management
5.1.1. Objective and Comprehensive Data Collection
5.1.2. Advanced Data Analysis
5.1.3. Real-Time Monitoring and Prediction
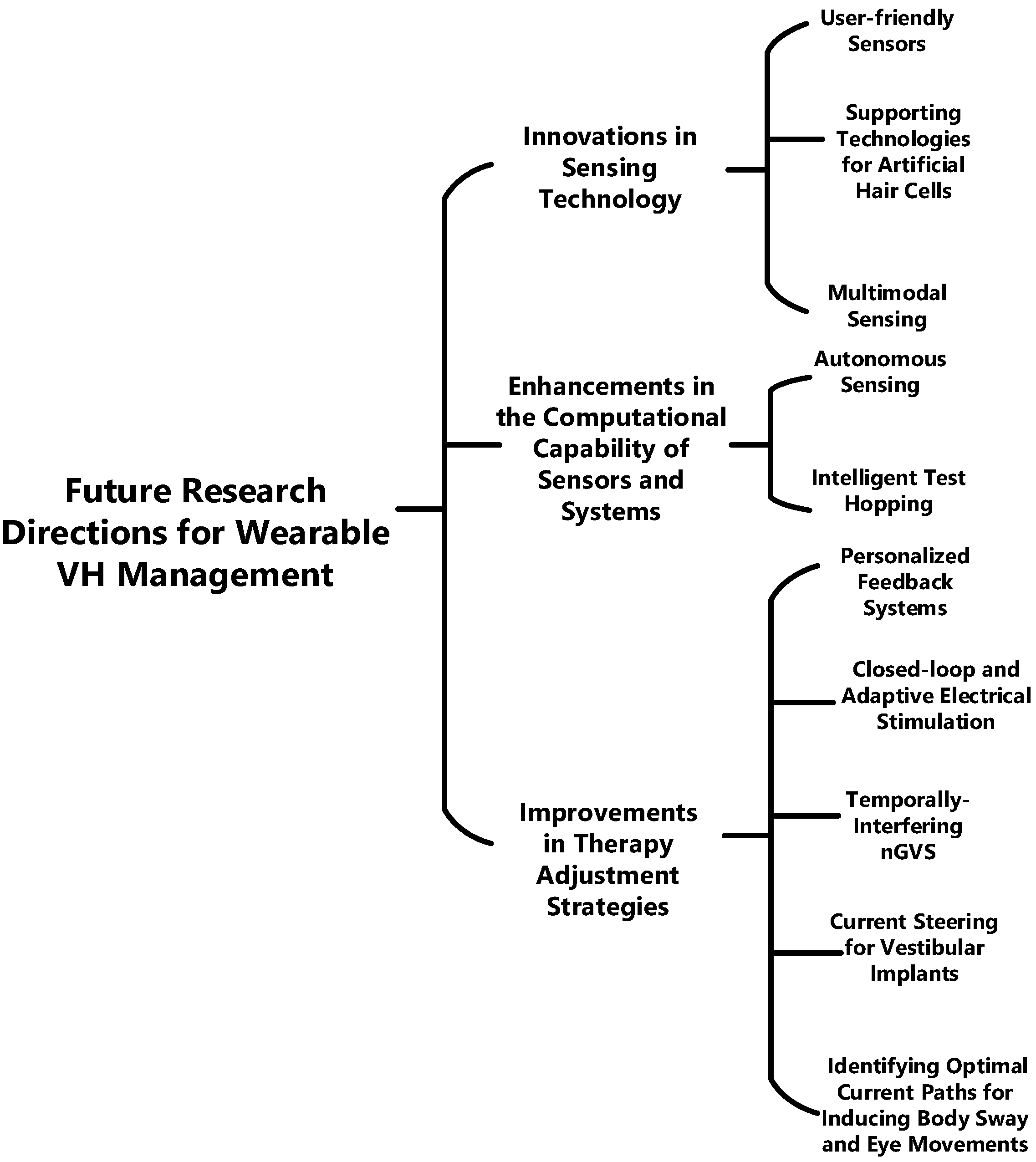
5.2. Future Research Directions for Vestibular Management Wearables
5.2.1. Innovations in Sensing Technology
- (A)
- User-friendly Sensors: For proper VH management using wearables, it is very likely that devices will be worn by users for an extended period of time. Thus, user-friendly sensors are crucial for VH management. This makes factors like comfort, size, weight, and power essential for these devices. For instance, eye tracking, which is a key component of all clinical tests for VH, is predominantly used in these wearables. However, traditional eye-tracking methods may block vision and interfere with normal daily routines, posing a significant challenge. Therefore, it is essential to explore innovative technologies that can measure eye movements indirectly or track alternative indicators representative of eye movements. These sensors, combined with miniaturized, contactless readout electronics, require less energy, extending the device’s battery life. Such advancements would not only enhance user comfort but also ensure that wearables seamlessly integrate into users’ lives without disrupting their vision and daily activities. This approach would significantly improve the usability and acceptance of these wearables, thereby facilitating more effective management of VH.
- (B)
- Multimodal Sensing: Since VH is a result of the mismatch between the head and some components of the nervous system, sensors in multiple locations are required for proper VH monitoring. This leads to a more precise prediction of dizziness onset, risk of fall, and balance instability. In order to facilitate multimodal sensing, data from the multiple sensors needs to be integrated. Aside from multimodal sensing, even if a single feature like posture is being measured in a patient, say, using an IMU. The dynamic state of a subject affects the IMU sensitivity. This means that the IMU’s sensitivity and accuracy can vary depending on the subject’s activity [93]. Sensor fusion, consisting of multiple sensors, can be used to overcome the limitations of using a single sensor.
- (C)
- Supportive Technologies for Seamless Integration of Artificial Hair Cells with the Inner Ear: Even though studies like [8] have developed biomimetic hair cells capable of detecting low-frequency movements with high sensitivity, the challenge still remains integrating these sensors into the extracellular environment of the inner ear. This opens up several research opportunities on supportive technologies that could assist with the seamless integration of artificial hair cells with the inner ear; below are some potential areas:
- Material Technology: This could involve the development of biocompatible materials that can better interface with biological tissues of the inner ear without resulting in implant rejection or causing damage/inflammation.
- Nanotechnology: This could involve creating artificial hair cells that are nearly the same size and shape as the natural hair cells of the inner ear. This would ensure the seamless integration of the hair cells into the existing cellular configuration.
- MEMS Transducers for Sensorineural Coupling: Designing highly efficient MEMS interfaces that can effectively transmit signals from the artificial hair cells to the vestibulocochlear nerve. This could involve the development of novel microelectrode arrays or MEMS transducers that enable coupling between the artificial sensors and the nerve.
5.2.2. Enhancements in the Computational Capability of Sensors and Systems
- (A)
- Autonomous Sensing: VH is predominant among the elderly, who may be less inclined to frequently calibrate or recalibrate wearable devices. Making the need for more intelligent sensors that can process and interpret data at the point of collection essential. Autonomous sensors can fulfill this role in VH management by detecting abnormal patterns in a subject’s gait or eye movements, facilitating the tracking of disease progression. These sensors can be adaptive, adjusting the threshold for detecting abnormalities based on the patient’s condition. This adaptability is particularly useful when real-time therapy is administered. For instance, during the early stages of VH, when symptoms are more manageable, high precision may be required to provide therapy only when necessary, thereby minimizing side effects. Conversely, in advanced stages of VH, a higher sensitivity may be needed to identify most manifestations as the condition becomes less manageable. Also, autonomous sensors have limited bandwidth and power requirements since they make decisions onsite without the need to transmit them to another device.
- (B)
- Intelligent Test Hopping: Currently, clinics are using VH screening tests selectively, which may not offer a holistic assessment. The feasibility of conducting comprehensive tests each time is often limited by time and cost constraints. To circumvent this, the creation of heuristic-based algorithms or systems embodying the concept of intelligent test hopping could streamline the evaluation process. These systems, designed as wearable devices for ease of use and continuous monitoring, could displace the current series of narrow bandwidth tests. Intelligent test hopping allows for the selection of the most relevant tests based on real-time data, thereby optimizing the assessment process. This paves the way for home-based VH assessments or tests.
5.2.3. Improvements in Therapy Adjustment Strategies
- (A)
- Personalized Feedback Systems: Future systems for VH management could offer patient-specific therapy, providing feedback (visual, auditory, or haptic) based on patient status. This necessitates research on innovative feedback systems. Examples of these include the following:
- Dizziness Alerting System: Personalized for patients with dizziness, a major symptom of VH. An example is the Continuous Ambulatory Vestibular Assessment, which detects dizziness by recording head and eye movements [94]. However, it lacks a patient alerting mechanism, which could use auditory or haptic feedback.
- Balance Improvement using Sensory Feedback: Unsteadiness, a common VH symptom, can be managed using feedback like vibrotactile feedback. An example is a vibrotactile belt used to improve the perception of verticality in patients during daily activities [44]. These devices should be comfortable and not provide unwanted sensations.
- (B)
- Closed-loop and Adaptive Electrical Stimulation: Understanding the main causes of vestibulopathy is crucial for effective management [71]. This paves the way for personalized treatment plans. One such approach is the use of closed-loop and adaptive electrical stimulation. In these systems, the output is continuously monitored, and the input is adjusted based on deviations from a predetermined baseline. This strategy, employed in VH management through an amplitude-modulated signal from a vestibular prosthesis, holds promise for effective therapy adjustment. However, the optimal approach for closing the loop, be it sensory augmentation or sensory substitution, remains to be determined. The effectiveness of these systems hinges on advancements in sensing technology, feedback systems, and data processing algorithms.
- (C)
- Identifying Optimal Current Paths for Inducing Body Sway and Eye Movements: Studies have demonstrated the existence of current paths between the left and right mastoids, as well as the left and right forehead, for inducing body sway or eye movements [57,87]. Noisy GVS has the capacity to induce eye movements without any head or body motion [63] and generates body sway during standing or walking. This dual influence makes it a valuable tool for diagnosing and managing VH. For triggering complex trajectories in eye movement and body sway, it is essential to establish the optimal current path for inducing these effects. This could involve identifying the optimal number of electrodes based on the intended eye movement, the optimal position for the placement and arrangement of these electrodes, and the way electrical currents are distributed among the electrodes.
- (D)
- Focused and Targeted Electrical Stimulation: There is a need for motion-modulated electrical stimulation in VH treatment, achievable through vestibular implants or non-invasive nGVS systems. This involves controlling the electrical stimulation that is delivered to the vestibular system. Given the inherent risks of invasive inner ear surgery with implants, nGVS may be a preferable alternative [30]. To target the semi-circular canal more precisely using nGVS, sophisticated algorithms, such as AI-based ones, or adaptive stimulation profiles could be used for beamforming or temporally interfering (TI) stimulation, minimizing side effects from electric current spread to nearby organs. TI uses a low-frequency envelope waveform generated by the superposition of two high-frequency sinusoidal currents of slightly different frequencies to stimulate specific targets inside the brain [95]. This method holds the advantages of both spatial targeting and non-invasive stimulation and could also be adopted for the vestibular system. For vestibular implants, current steering for improved spatial precision can be adopted.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Bilateral Vestibular Hypofunction | BVH |
| Galvanic Vestibular Stimulation | GVS |
| Inertial Measurement Unit | IMU |
| Noisy Galvanic Vestibular Stimulation | nGVS |
| Stochastic Resonance | SR |
| Unilateral Vestibular Hypofunction | UVH |
| Vestibular Hypofunction | VH |
| Vestibular Implant | VI |
| Vestibular Rehabilitation Therapy | VRT |
| Vestibulocollic Reflex | VCR |
| Vestibulo-ocular Reflex | VOR |
| Vestibulo-spinal Reflex | VSR |
| Virtual Reality | VR |
References
- Perez Fornos, A.; Guinand, N.; Van De Berg, R.; Stokroos, R.; Micera, S.; Kingma, H.; Pelizzone, M.; Guyot, J.-P. Artificial balance: Restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front. Neurol. 2014, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Starkov, D.; Strupp, M.; Pleshkov, M.; Kingma, H.; van de Berg, R. Diagnosing vestibular hypofunction: An update. J. Neurol. 2021, 268, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Burzynski, J.; Sulway, S.; Rutka, J.A. Vestibular Rehabilitation: Review of Indications, Treatments, Advances, and Limitations. Curr. Otorhinolaryngol. Rep. 2017, 5, 160–166. [Google Scholar]
- Orlov, I.V.; Stolbkov, Y.K.; Gerasimenko, Y.P. Vestibular Prosthetics: Concepts, Approaches, Results. Neurosci. Behav. Physiol. 2018, 48, 711–720. [Google Scholar] [CrossRef]
- Kovacs, E.; Wang, X.; Grill, E. Economic burden of vertigo: A systematic review. Health Econ. Rev. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Sharma, K.G.; Gupta, A.K. Efficacy and Comparison of Vestibular Rehabilitation Exercises on Quality of Life in Patients with Vestibular Disorders. Indian J. Otolaryngol. Head Neck Surg. 2020, 72, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Stultiens, J.J.A.; Lewis, R.F.; Phillips, J.O.; Boutabla, A.; Della Santina, C.C.; Glueckert, R.; van de Berg, R. The Next Challenges of Vestibular Implantation in Humans. JARO J. Assoc. Res. Otolaryngol. 2023, 24, 401–412. [Google Scholar] [PubMed]
- Moshizi, S.A.; Pastras, C.J.; Peng, S.; Wu, S.; Asadnia, M. Artificial Hair Cell Sensor Based on Nanofiber-Reinforced Thin Metal Films. Biomimetics 2024, 9, 18. [Google Scholar] [CrossRef]
- Wuehr, M.; Eder, J.; Keywan, A.; Jahn, K. Noisy galvanic vestibular stimulation improves vestibular perception in bilateral vestibulopathy. J. Neurol. 2023, 270, 938–943. [Google Scholar] [CrossRef]
- Sienko, K.; Whitney, S.; Carender, W.; Wall, C. The role of sensory augmentation for people with vestibular deficits: Real-time balance aid and/or rehabilitation device? J. Vestib. Res. 2017, 27, 63–76. [Google Scholar] [CrossRef]
- de Azevedo, Y.J.; Ledesma, A.L.L.; Pereira, L.V.; Oliveira, C.A.; Junior, F.B. Vestibular implant: Does it really work? A systematic review. Braz. J. Otorhinolaryngol. 2019, 85, 788–798. [Google Scholar] [CrossRef] [PubMed]
- da Costa Monsanto, R.; Pauna, H.F.; Cureoglu, S. The Anatomy of the Vestibular System. In Disorders of the Vestibular System; Springer: Cham, Switzerland, 2023; pp. 1–11. [Google Scholar] [CrossRef]
- Bonsu, A.N.; Nousi, S.; Lobo, R.; Strutton, P.H.; Arshad, Q.; Bronstein, A.M. Vestibulo-perceptual influences upon the vestibulo-spinal reflex. Exp. Brain Res. 2021, 239, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Forbes, P.A.; Kwan, A.; Rasman, B.G.; Mitchell, D.E.; Cullen, K.E.; Blouin, J.-S. Neural Mechanisms Underlying High-Frequency Vestibulocollic Reflexes in Humans and Monkeys. J. Neurosci. 2020, 40, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.J.; Hubner, P.P.; Hubner, P.; Schubert, M.C.; Migliaccio, A.A. StableEyes—A Portable Vestibular Rehabilitation Device. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, A.; Shiozaki, T.; Tanaka, H. Vestibulo-Ocular Reflex Is Modulated by Noisy Galvanic Vestibular Stimulation. Front. Neurol. 2022, 13, 826739. [Google Scholar] [CrossRef] [PubMed]
- Ramaioli, C.; Colagiorgio, P.; Sağlam, M.; Heuser, F.; Schneider, E.; Ramat, S.; Lehnen, N. The Effect of Vestibulo-Ocular Reflex Deficits and Covert Saccades on Dynamic Vision in Opioid-Induced Vestibular Dysfunction. PLoS ONE 2014, 9, e110322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grill, E.; Heuberger, M.; Strobl, R.; Saglam, M.; Holle, R.; Linkohr, B.; Ladwig, K.-H.; Peters, A.; Schneider, E.; Jahn, K.; et al. Prevalence, Determinants, and Consequences of Vestibular Hypofunction. Results From the KORA-FF4 Survey. Front. Neurol. 2018, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hain, T.C.; Cherchi, M.; Yacovino, D.A. Bilateral vestibular weakness. Front. Neurol. 2018, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Ribeiro, L.; Arshad, Q.; Seemungal, B.M. Age-related vestibular loss: Current understanding and future research directions. Front. Neurol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Fawzan, S.; Kozou, H.; Baki, F.; Asal, S. Fall risk assessment and effect of vestibular rehabilitation in the elderly population. Egypt. J. Otolaryngol. 2022, 38, 1–13. [Google Scholar] [CrossRef]
- Meldrum, D.; Burrows, L.; Cakrt, O.; Kerkeni, H.; Lopez, C.; Tjernstrom, F.; Vereeck, L.; Zur, O.; Jahn, K. Vestibular rehabilitation in Europe: A survey of clinical and research practice. J. Neurol. 2020, 267, 24–35. [Google Scholar] [CrossRef] [PubMed]
- van de Berg, R.; van Tilburg, M.; Kingma, H. Bilateral Vestibular Hypofunction: Challenges in Establishing the Diagnosis in Adults. ORL 2015, 77, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Kim, H.J.; Kim, J.S. Bilateral Vestibular Dysfunction. Semin. Neurol. 2020, 40, 040–048. [Google Scholar]
- Strupp, M.; Dlugaiczyk, J.; Ertl-Wagner, B.B.; Rujescu, D.; Westhofen, M.; Dieterich, M. Vestibular Disorders: Diagnosis, New Classification and Treatment. Dtsch. Aerzteblatt Online 2020, 117, 300. [Google Scholar] [CrossRef]
- Meng, L.; Liang, Q.; Yuan, J.; Li, S.; Ge, Y.; Yang, J.; Tsang, R.C.C.; Wei, Q. Vestibular rehabilitation therapy on balance and gait in patients after stroke: A systematic review and meta-analysis. BMC Med. 2023, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Deveze, A.; Bernard-Demanze, L.; Xavier, F.; Lavieille, J.-P.; Elziere, M. Vestibular compensation and vestibular rehabilitation. Current concepts and new trends. Neurophysiol. Clin. 2014, 44, 49–57. [Google Scholar] [CrossRef]
- Ballardini, G.; Florio, V.; Canessa, A.; Carlini, G.; Morasso, P.; Casadio, M. Vibrotactile Feedback for Improving Standing Balance. Front. Bioeng. Biotechnol. 2020, 8, 94. [Google Scholar] [CrossRef]
- Kilic, G.; Temirbekov, D.; Ata, G.; Algun, Z. Effects of Vestibular Rehabilitation in Patients with Unilateral Vestibular Hypofunction. Indian J. Otol. 2023, 29, 33–38. [Google Scholar] [CrossRef]
- Raymond, V.D.B. Biophysics of the Vestibular Implant; Tomsk State University: Tomsk, Russia, 2021. [Google Scholar]
- Han, B.I. Vestibular Rehabilitation Therapy: Review of Indications, Mechanisms, and Key Exercises. In Simplified Vestibular Rehabilitation Therapy; Springer: Singapore, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, K.; Patro, N.; Chan, T.; Levin, M.; Gupta, M.K.; Archibald, J. Virtual Reality for Vestibular Rehabilitation: A Systematic Review. Otol. Neurotol. 2021, 42, 967–977. [Google Scholar] [CrossRef]
- Anson, E.R.; Gimmon, Y. Vestibular Rehabilitation: A Patient-Centered Approach. In Disorders of the Vestibular System; Springer: Cham, Switzerland, 2023; pp. 263–300. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Hsieh, W.-L.; Wei, S.-H.; Kao, C.-L. Interactive wiimote gaze stabilization exercise training system for patients with vestibular hypofunction. J. Neuroeng. Rehabil. 2012, 9, 77. [Google Scholar] [CrossRef]
- Solís, J.O.; Reynard, P.; Spruyt, K.; Bécaud, C.; Ionescu, E.; Thai-Van, H. Developing a serious game for gaze stability rehabilitation in children with vestibular hypofunction. J. Neuroeng. Rehabil. 2023, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Booth, L.; Fletcher, R.; Nunez, D.A. Vestibular rehabilitation potential of commercially available virtual reality video games. J. Otolaryngol. Head Neck Surg. 2023, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.G.; Yacovino, D.A.; Cherchi, M.; Nader, S.N.; Teixeira, L.J.; da Silva, D.A.; Verdecchia, D.H. The Role of the Smartphone in the Diagnosis of Vestibular Hypofunction: A Clinical Strategy for Teleconsultation during the COVID-19 Pandemic and Beyond. Int. Arch. Otorhinolaryngol. 2021, 25, e602–e609. [Google Scholar] [CrossRef] [PubMed]
- D’silva, L.J.; Phongsavath, T.; Partington, K.; Pickle, N.T.; Marschner, K.; Zehnbauer, T.P.; Rossi, M.; Skop, K.; Roos, P.E. A gaming app developed for vestibular rehabilitation improves the accuracy of performance and engagement with exercises. Front. Med. 2023, 10, 1269874. [Google Scholar] [CrossRef] [PubMed]
- Hovareshti, P.; Roeder, S.; Holt, L.S.; Gao, P.; Xiao, L.; Zalkin, C.; Ou, V.; Tolani, D.; Klatt, B.N.; Whitney, S.L. Vestaid: A tablet-based technology for objective exercise monitoring in vestibular rehabilitation. Sensors 2021, 21, 8388. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Decker, J.; Schniepp, R. Noisy galvanic vestibular stimulation: An emerging treatment option for bilateral vestibulopathy. J. Neurol. 2017, 264, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, F. Sensory Substitution and Augmentation: An Introduction. In Sensory Substitution and Augmentation; Macpherson, F., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 1–42. [Google Scholar] [CrossRef]
- Kemlin, C.; Verite, F.; Marchand-Pauvert, V.; Pradat, P.-F.; Pradat-Diehl, P.; Giron, A.; Bachta, W. Closed-Loop Control of the Centre of Pressure in Post-Stroke Patients with Balance Impairments. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 265–274. [Google Scholar] [CrossRef]
- Sienko, K.H.; Balkwill, M.D.; Oddsson, L.I.E.; Wall, C. The effect of vibrotactile feedback on postural sway during locomotor activities. J. Neuroeng. Rehabil. 2013, 10, 93. [Google Scholar] [CrossRef]
- Kingma, H.; Felipe, L.; Gerards, M.-C.; Gerits, P.; Guinand, N.; Perez-Fornos, A.; Demkin, V.; van de Berg, R. Vibrotactile feedback improves balance and mobility in patients with severe bilateral vestibular loss. J. Neurol. 2018, 266, 19–26. [Google Scholar] [CrossRef]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef]
- Wall, C.; Wrisley, D.M.; Statler, K.D. Vibrotactile tilt feedback improves dynamic gait index: A fall risk indicator in older adults. Gait Posture 2009, 30, 16–21. [Google Scholar] [CrossRef]
- Guyot, J.-P.; Fornos, A.P.; Guinand, N.; van de Berg, R.; Stokroos, R.; Kingma, H. Vestibular assistance systems: Promises and challenges. J. Neurol. 2016, 263, 30–35. [Google Scholar] [CrossRef][Green Version]
- Goebel, J.A.; Sinks, B.C.; Parker, B.E.J.; Richardson, N.T.; Olowin, A.B.; Cholewiak, R.W. Effectiveness of head-mounted vibrotactile stimulation in subjects with bilateral vestibular loss: A phase 1 clinical trial. Otol. Neurotol. 2009, 30, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Bechly, K.E.; Carender, W.J.; Myles, J.D.; Sienko, K.H. Determining the preferred modality for real-time biofeedback during balance training. Gait Posture 2013, 37, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Lauber, B.; Keller, M. Improving motor performance: Selected aspects of augmented feedback in exercise and health. Eur. J. Sport Sci. 2014, 14, 36–43. [Google Scholar] [CrossRef]
- Robinson, B.S.; Cook, J.L.; Richburg, C.M.; Price, S.E. Use of an electrotactile vestibular substitution system to facilitate balance and gait of an individual with gentamicin-induced bilateral vestibular hypofunction and bilateral transtibial amputation. J. Neurol. Phys. Ther. 2009, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.R.; Peterka, R.J.; Fino, P.C.; Parrington, L.; Wilhelm, J.L.; Pettigrew, N.C.; King, L.A. The effects of augmenting traditional rehabilitation with audio biofeedback in people with persistent imbalance following mild traumatic brain injury. Front. Neurol. 2022, 13, 926691. [Google Scholar] [CrossRef]
- Ardıç, F.N.; Alkan, H.; Tümkaya, F.; Ardıç, F. Effectiveness of whole-body vibration or biofeedback postural training as an add-on to vestibular exercises rehabilitation therapy in chronic unilateral vestibular weakness: A randomized controlled study. J. Vestib. Res. 2021, 31, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Klatt, B.N.; Carender, W.J.; Kinnaird, C.; Alsubaie, S.; Whitney, S.L.; Sienko, K.H. Effects of long-term vestibular rehabilitation therapy with vibrotactile sensory augmentation for people with unilateral vestibular disorders—A randomized preliminary study. J. Vestib. Res. 2019, 29, 323–334. [Google Scholar] [CrossRef]
- Martini, D.N.; Pettigrew, N.C.; Wilhelm, J.L.; Parrington, L.; King, L.A. Wearable Sensors for Vestibular Rehabilitation: A Pilot Study. J. Physiother. Res. 2021, 5, 1–8. [Google Scholar]
- Putman, E.J.; Galvan-Garza, R.C.; Clark, T.K. The Effect of Noisy Galvanic Vestibular Stimulation on Learning of Functional Mobility and Manual Control Nulling Sensorimotor Tasks. Front. Hum. Neurosci. 2021, 15, 756674. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Tauscher, J.-P.; Heesen, N.; Hattenbach, M.; Castillo, S.; Magnor, M. Omnidirectional Galvanic Vestibular Stimulation in Virtual Reality. IEEE Trans. Vis. Comput. Graph. 2022, 28, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, A.; Spliethoff, P.; Rother, M.; Machner, B.; Helmchen, C. Effects of perceptible and imperceptible galvanic vestibular stimulation on the postural control of patients with bilateral vestibulopathy. J. Neurol. 2020, 267, 2383–2397. [Google Scholar] [CrossRef]
- McLaren, R.; Smith, P.F.; Taylor, R.L.; Niazi, I.K.; Taylor, D. Scoping out noisy galvanic vestibular stimulation: A review of the parameters used to improve postural control. Front. Neurosci. 2023, 17, 1156796. [Google Scholar] [CrossRef]
- Schniepp, R.; Boerner, J.C.; Decker, J.; Jahn, K.; Brandt, T.; Wuehr, M. Noisy vestibular stimulation improves vestibulospinal function in patients with bilateral vestibulopathy. J. Neurol. 2018, 265, 57–62. [Google Scholar] [CrossRef]
- Wuehr, M.; Boerner, J.; Pradhan, C.; Decker, J.; Jahn, K.; Brandt, T.; Schniepp, R. Stochastic resonance in the human vestibular system—Noise-induced facilitation of vestibulospinal reflexes. Brain Stimul. 2018, 11, 261–263. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Gensberger, K.D.; Straka, H. Galvanic vestibular stimulation: From basic concepts to clinical applications. J. Neurophysiol. 2019, 121, 2237–2255. [Google Scholar] [CrossRef]
- Gensberger, K.D.; Kaufmann, A.-K.; Dietrich, H.; Branoner, F.; Banchi, R.; Chagnaud, B.P.; Straka, H. Galvanic Vestibular Stimulation: Cellular Substrates and Response Patterns of Neurons in the Vestibulo-Ocular Network. J. Neurosci. 2016, 36, 9097–9110. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, A.; Oku, K.; Mori, N. The Effects of Stochastic Galvanic Vestibular Stimulation on Body Sway and Muscle Activity. Front. Hum. Neurosci. 2020, 14, 591671. [Google Scholar] [CrossRef]
- Fujimoto, C.; Egami, N.; Kawahara, T.; Uemura, Y.; Yamamoto, Y.; Yamasoba, T.; Iwasaki, S. Noisy Galvanic Vestibular Stimulation Sustainably Improves Posture in Bilateral Vestibulopathy. Front. Neurol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Eder, J.; Kellerer, S.; Amberger, T.; Keywan, A.; Dlugaiczyk, J.; Wuehr, M.; Jahn, K. Combining vestibular rehabilitation with noisy galvanic vestibular stimulation for treatment of bilateral vestibulopathy. J. Neurol. 2022, 269, 5731–5737. [Google Scholar] [CrossRef]
- Khavarghazalani, B.; Ghahraman, M.A.; Hoseinabadi, R.; Jalaie, S.; Kouhi, A.; Yazdani, N. Combining Vestibular Rehabilitation and Noisy Galvanic Vestibular Stimulation for Treatment of Unilateral Vestibulopathy: A Randomized Controlled Trial. Audit. Vestib. Res. 2023, 32, 272–283. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular Functions and Parkinson’s Disease. Front. Neurol. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, K.; Marigold, D.S.; Valdés, B.A.; Menon, C. The potential of noisy galvanic vestibular stimulation for optimizing and assisting human performance. Neuropsychologia 2021, 152, 107751. [Google Scholar] [CrossRef] [PubMed]
- Moshizi, S.A.; Pastras, C.J.; Sharma, R.; Mahmud, M.P.; Ryan, R.; Razmjou, A.; Asadnia, M. Recent advancements in bioelectronic devices to interface with the peripheral vestibular system. Biosens. Bioelectron. 2022, 214, 114521. [Google Scholar] [CrossRef]
- Pires, A.P.B.d.; Silva, T.R.; Torres, M.S.; Diniz, M.L.; Tavares, M.C.; Gonçalves, D.U. Galvanic vestibular stimulation and its applications: A systematic review. Braz. J. Otorhinolaryngol. 2022, 88, S202–S211. [Google Scholar] [CrossRef]
- Zingler, V.C.; Cnyrim, C.; Jahn, K.; Weintz, E.; Fernbacher, J.; Frenzel, C.; Brandt, T.; Strupp, M. Causative factors and epidemiology of bilateral vestibulopathy in 255 patients. Ann. Neurol. 2007, 61, 524–532. [Google Scholar] [CrossRef]
- Guyot, J.-P.; Fornos, A.P. Milestones in the development of a vestibular implant. Curr. Opin. Neurol. 2019, 32, 145–153. [Google Scholar] [CrossRef]
- Sluydts, M.; Curthoys, I.; Vanspauwen, R.; Papsin, B.C.; Cushing, S.L.; Ramos, A.; de Miguel, A.R.; Barreiro, S.B.; Barbara, M.; Manrique, M.; et al. Electrical Vestibular Stimulation in Humans: A Narrative Review. Audiol. Neurotol. 2020, 25, 6–24. [Google Scholar] [CrossRef]
- Guinand, N.; van de Berg, R.; Cavuscens, S.; Stokroos, R.J.; Ranieri, M.; Pelizzone, M.; Kingma, H.; Guyot, J.-P.; Perez-Fornos, A. Vestibular Implants: 8 Years of Experience with Electrical Stimulation of the Vestibular Nerve in 11 Patients with Bilateral Vestibular Loss. ORL 2015, 77, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Pliego, A.; Vega, R. Vestibular prosthesis: From basic research to clinics. Front. Integr. Neurosci. 2023, 17, 1161860. [Google Scholar] [CrossRef] [PubMed]
- Marianelli, P.; Capogrosso, M.; Luciani, L.B.; Panarese, A.; Micera, S. A Computational Framework for Electrical Stimulation of Vestibular Nerve. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Peto, D.; Fietzek, U.M.; Katzdobler, S.; Nübling, G.; Zaganjori, M.; Brendel, M.; Levin, J.; Höglinger, G.U.; Zwergal, A. Low-intensity vestibular noise stimulation improves postural symptoms in progressive supranuclear palsy. J. Neurol. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Eder, J.; Kellerer, S.; Amberger, T.; Jahn, K. Mechanisms underlying treatment effects of vestibular noise stimulation on postural instability in patients with bilateral vestibulopathy. J. Neurol. 2024, 271, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Inukai, Y.; Miyaguchi, S.; Otsuru, N.; Kawakami, S.; Onishi, H. Effects of noisy galvanic vestibular stimulation on functional reach test. Neurosci. Lett. 2023, 810, 137336. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Jheng, Y.-C.; Wang, C.-C.; Huang, S.-E.; Yang, T.-H.; Hsu, P.-C.; Kuo, C.-H.; Lin, Y.-Y.; Lai, W.-Y.; Kao, C.-L. Effect of noisy galvanic vestibular stimulation on dynamic posture sway under visual deprivation in patients with bilateral vestibular hypofunction. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Kinoshita, M.; Kamogashira, T.; Egami, N.; Kawahara, T.; Uemura, Y.; Yamamoto, Y.; Yamasoba, T.; Iwasaki, S. Noisy galvanic vestibular stimulation has a greater ameliorating effect on posture in unstable subjects: A feasibility study. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Otsuru, N.; Masaki, M.; Saito, K.; Miyaguchi, S.; Kojima, S.; Onishi, H. Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimul. 2018, 11, 85–93. [Google Scholar] [CrossRef]
- Sra, M.; Xu, X.; Maes, P. GalVR: A novel collaboration interface using GVS. In Proceedings of the 23rd ACM Symposium on Virtual Reality Software and Technology, VRST, Gothenburg, Sweden, 8–10 November 2017; pp. 1–2. [Google Scholar] [CrossRef]
- Fujimoto, C.; Yamamoto, Y.; Kamogashira, T.; Kinoshita, M.; Egami, N.; Uemura, Y.; Togo, F.; Yamasoba, T.; Iwasaki, S. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci. Rep. 2016, 6, 37575. [Google Scholar] [CrossRef]
- Aoyama, K.; Iizuka, H.; Ando, H.; Maeda, T. Four-pole galvanic vestibular stimulation causes body sway about three axes. Sci. Rep. 2015, 5, 10168. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Connolly, J.P.; Condell, J.; McKelvey, N.; Gardiner, P. Review of Wearable Devices and Data Collection Considerations for Connected Health. Sensors 2021, 21, 5589. [Google Scholar] [CrossRef] [PubMed]
- Sunny, J.S.; Patro, C.P.K.; Karnani, K.; Pingle, S.C.; Lin, F.; Anekoji, M.; Jones, L.D.; Kesari, S.; Ashili, S. Anomaly Detection Framework for Wearables Data: A Perspective Review on Data Concepts, Data Analysis Algorithms and Prospects. Sensors 2022, 22, 756. [Google Scholar] [PubMed]
- Vivar, G.; Strobl, R.; Grill, E.; Navab, N.; Zwergal, A.; Ahmadi, S.-A. Using Base-ml to Learn Classification of Common Vestibular Disorders on DizzyReg Registry Data. Front. Neurol. 2021, 12, 681140. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bao, T.; Lee, U.H.; Kinnaird, C.; Carender, W.; Huang, Y.; Sienko, K.H.; Shull, P.B. Configurable, wearable sensing and vibrotactile feedback system for real-time postural balance and gait training: Proof-of-concept. J. Neuroeng. Rehabil. 2017, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Raphan, T. Vestibular, locomotor, and vestibulo-autonomic research: 50 years of collaboration with Bernard Cohen. J. Neurophysiol. 2020, 123, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yang, Y.; Mao, H.; Yang, D.; Wang, W. Effects of Dynamic IMU-to-Segment Misalignment Error on 3-DOF Knee Angle Estimation in Walking and Running. Sensors 2022, 22, 9009. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.L.; Phillips, J.S.; Cox, S.J. 1D Convolutional Neural Networks for Detecting Nystagmus. IEEE J. Biomed. Health Inform. 2021, 25, 1814–1823. [Google Scholar] [CrossRef]
- Guo, W.; He, Y.; Zhang, W.; Sun, Y.; Wang, J.; Liu, S.; Ming, D. A novel non-invasive brain stimulation technique: “Temporally interfering electrical stimulation”. Front. Neurosci. 2023, 17, 1092539. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.; Li, S.; Liu, X. Exploring the Potentials of Wearable Technologies in Managing Vestibular Hypofunction. Bioengineering 2024, 11, 641. https://doi.org/10.3390/bioengineering11070641
Mohammed A, Li S, Liu X. Exploring the Potentials of Wearable Technologies in Managing Vestibular Hypofunction. Bioengineering. 2024; 11(7):641. https://doi.org/10.3390/bioengineering11070641
Chicago/Turabian StyleMohammed, Ameer, Shutong Li, and Xiao Liu. 2024. "Exploring the Potentials of Wearable Technologies in Managing Vestibular Hypofunction" Bioengineering 11, no. 7: 641. https://doi.org/10.3390/bioengineering11070641
APA StyleMohammed, A., Li, S., & Liu, X. (2024). Exploring the Potentials of Wearable Technologies in Managing Vestibular Hypofunction. Bioengineering, 11(7), 641. https://doi.org/10.3390/bioengineering11070641







