Reduction of Oxygen Production by Algal Cells in the Presence of O-Chlorobenzylidene Malononitrile
Abstract
1. Introduction
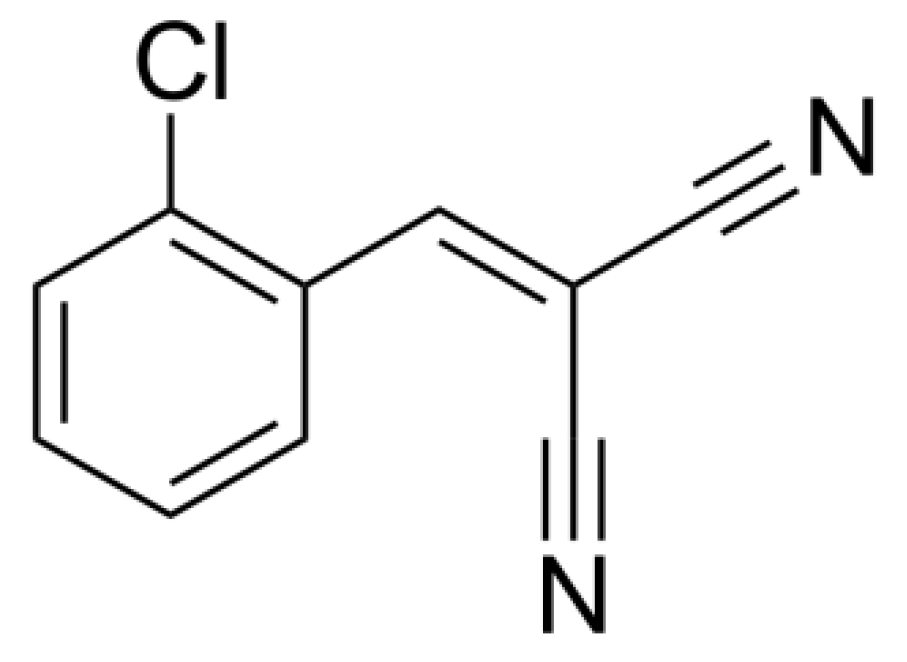
2. The Toxicity Analysis
3. Materials and Methods
3.1. Experimental Design
3.2. Biological Medium and Algae Cells
3.3. Preparation of CBM Concentrations
3.4. Preparation of the Bioreactors with Biological Samples Contaminated with CBM for Testing the Dissolved Oxygen
3.5. Determination of Oxygen Production of Algal Culture in Chemical Stress Generated by CBM
3.5.1. The Percentage of Cell Growth Inhibition
3.5.2. The Percent Inhibition in Yield (%I) Calculated with Equation (6)
3.6. Contents of Photosynthetic Pigments
3.6.1. Preparation of Bioreactors for Chlorophyll “a” and Chlorophyll “b” Analysis
3.6.2. Chlorophyll Quantification
3.6.3. FTIR and Fluorescence Analysis of the Chlorophyll Extract
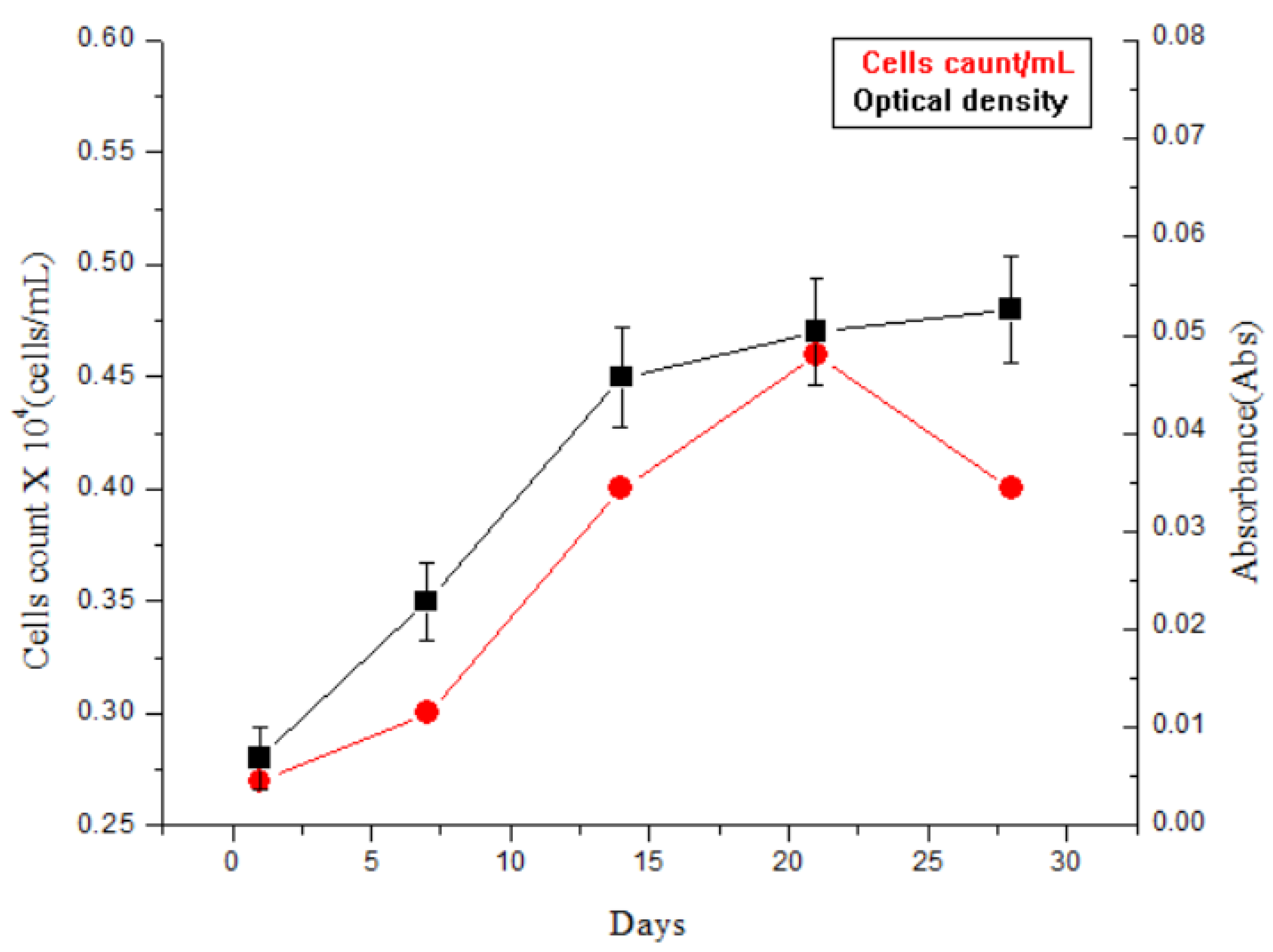
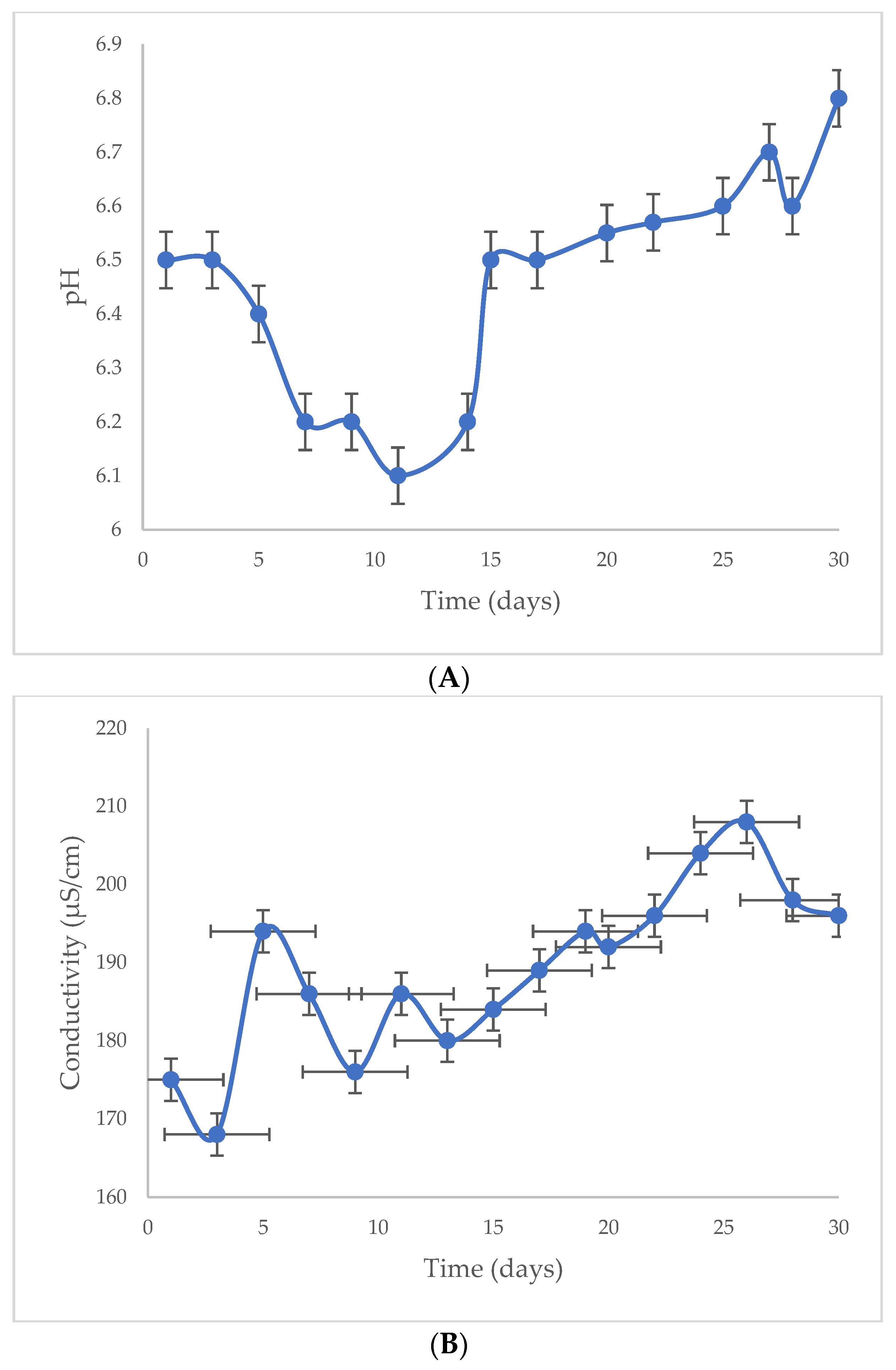
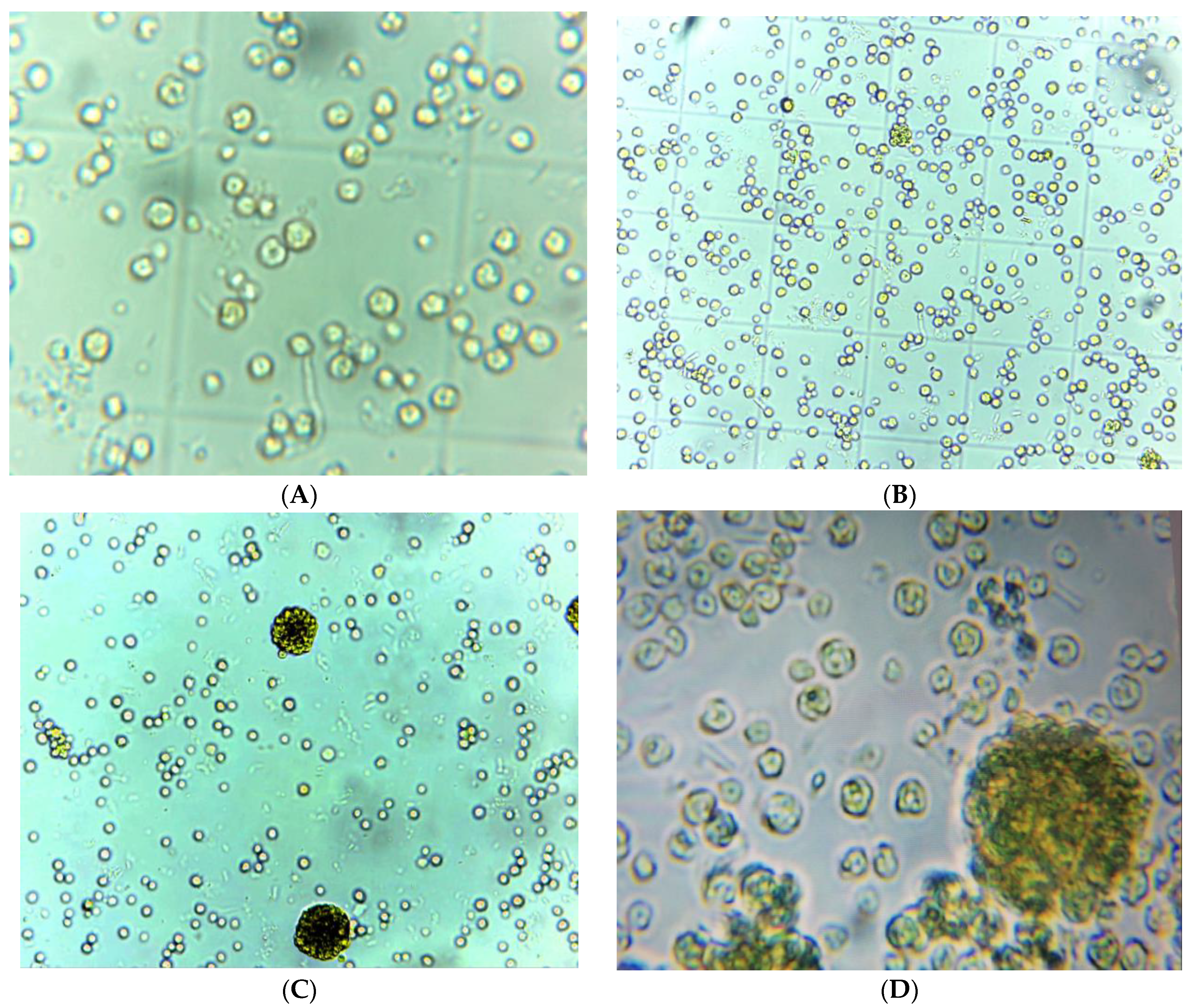
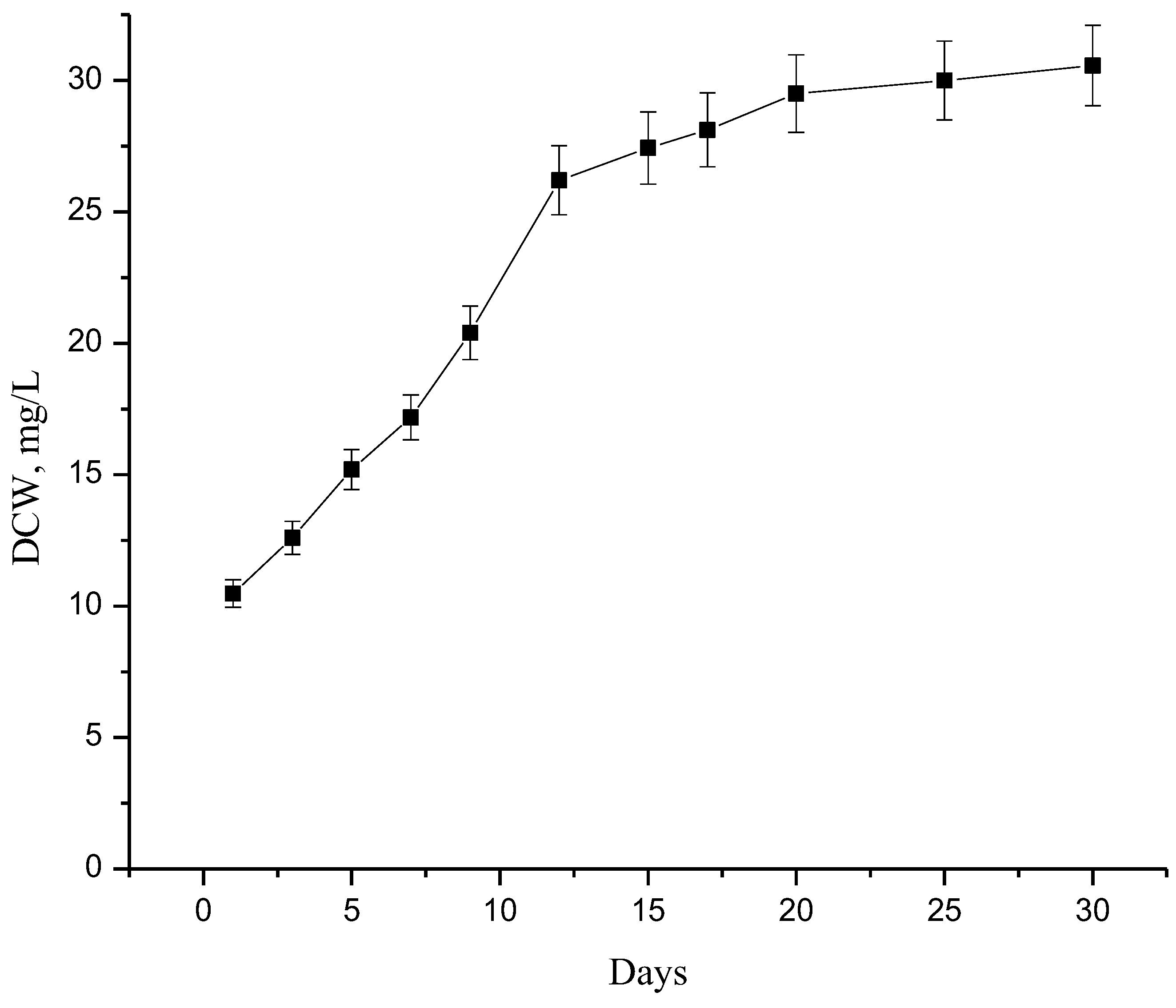
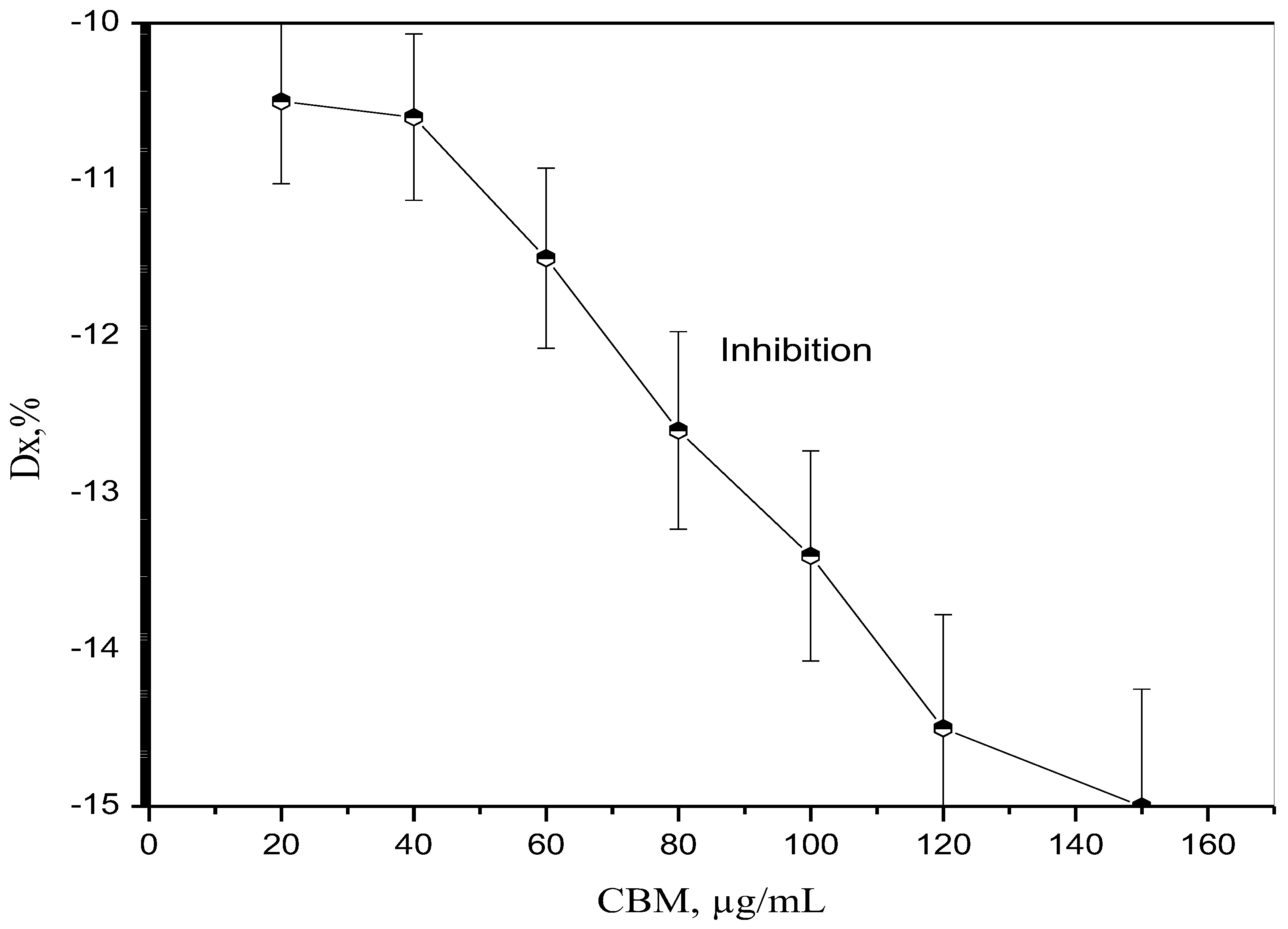
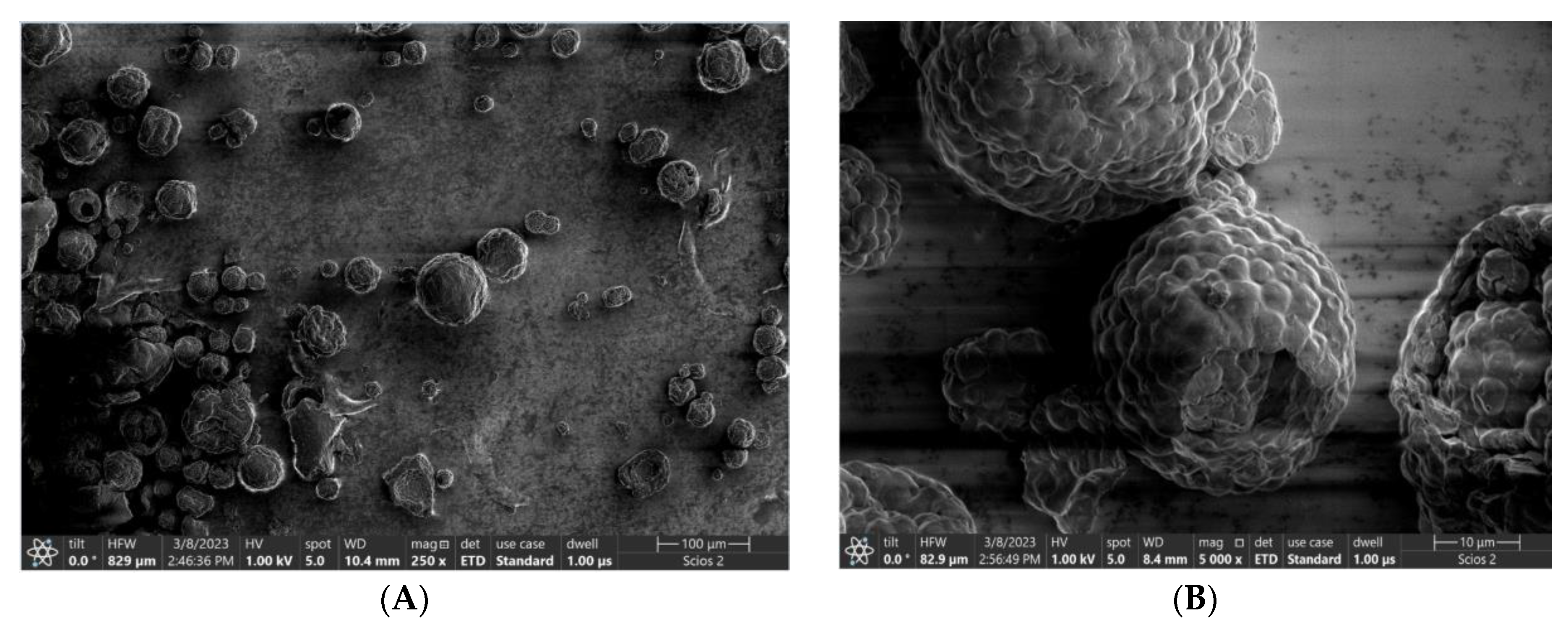
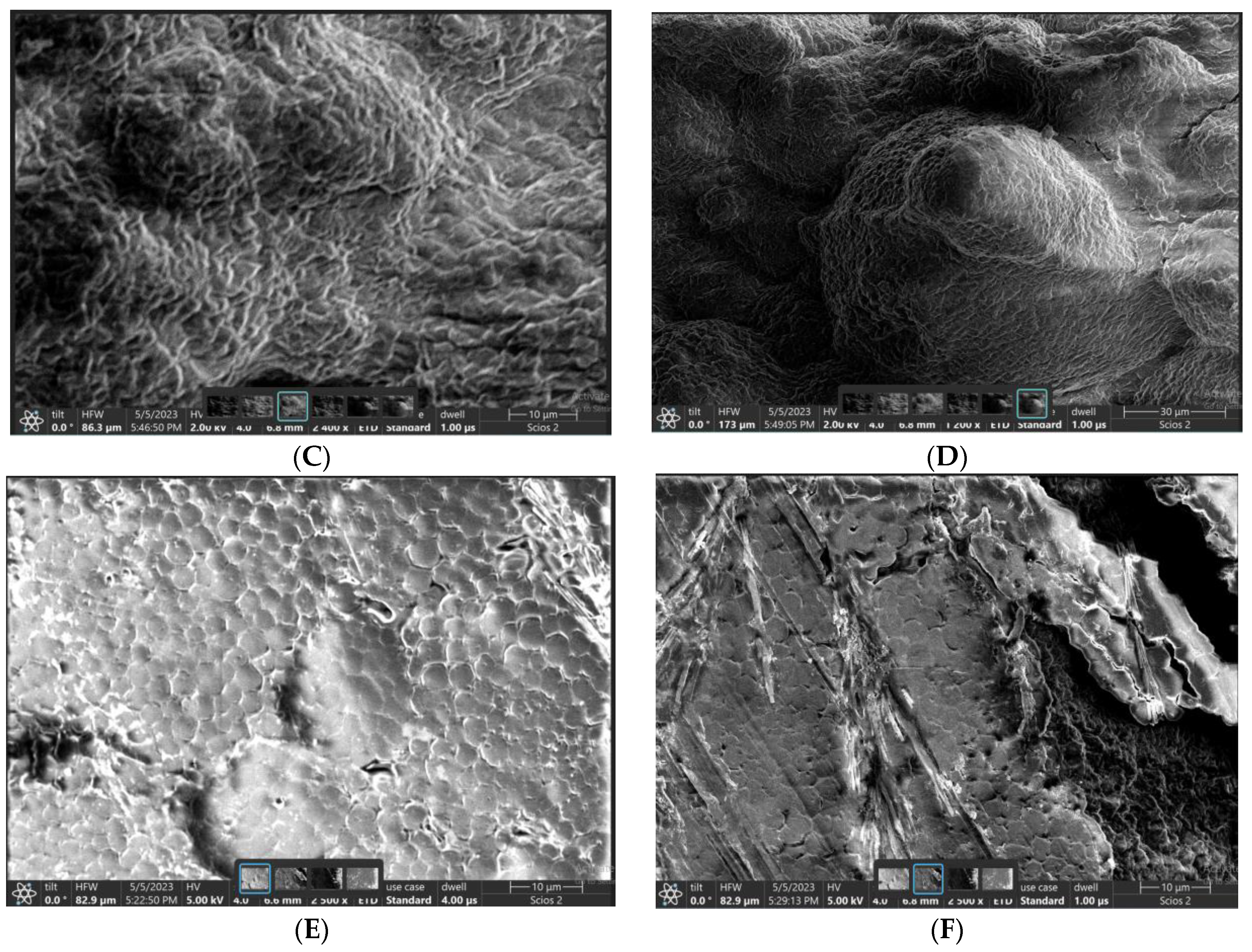
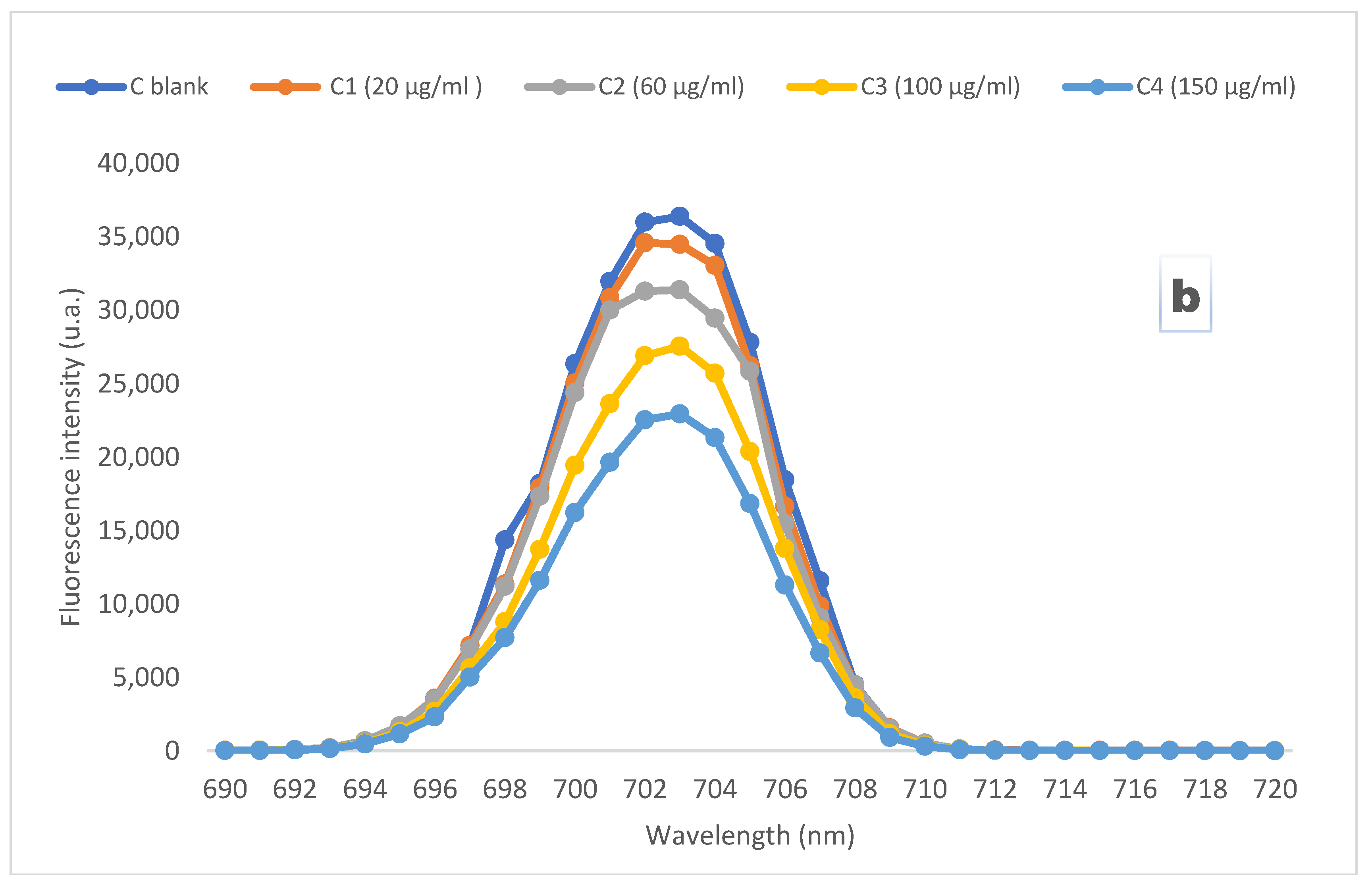
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaszeta, D. Restrict use of riot-control chemicals. Nature 2019, 573, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Zekri, A.M.B.; King, W.W.K.; Yeung, R.; Taylor, W.R.J. Acute mass burns caused by o-chlorobenzylidene malononitrile (CS) tear gas. Burns 1995, 21, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Lau, G.; Taylor, W.; Critchley, J. Acute effects of the potent lacrimator o-chlorobenzylidene malononitrile (CS) tear gas. Hum. Exp. Toxicol. 1996, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.C.; Li, L.; Tsang, R.K. Health risks of exposure to CS gas (tear gas): An update for healthcare practitioners in Hong Kong. Hong Kong Med. J. 2020, 26, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Riches, J.R.; Read, R.W.; Black, R.M.; Harrison, J.M.; Shand, D.A.; Tomsett, E.V.; Newsome, C.R.; Bailey, N.C.; Roughley, N.; Gravett, M.R.; et al. The development of an analytical method for urinary metabolites of the riot control agent 2-chlorobenzylidene malononitrile (CS). J. Chromatogr. B 2013, 928, 125–130. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Rachiotis, G.; Hadjichristodoulou, C. Exposure to the Riot Control Agent CS and Potential Health Effects: A Systematic Review of the Evidence. Int. J. Environ. Res. Public Health 2015, 12, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Kluchinsky, T.A.; Savage, P.B.; Fitz, R.; Smith, P.A. Liberation of hydrogen cyanide and hydrogen chloride during high-temperature dispersion of cs riot control agent. Am. Ind. Hyg. Assoc. J. 2002, 63, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Blain, P.G. Tear Gases and Irritant Incapacitants. Toxicol. Rev. 2003, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Olajos, E.J.; Salem, H. Riot control agents: Pharmacology, toxicology, biochemistry and chemistry. J. Appl. Toxicol. 2001, 21, 355–391. [Google Scholar] [CrossRef]
- Blaho-Owens, K. Chemical crowd control agents. Encycl. Forensic Leg. Med. 2005, 319–325. [Google Scholar] [CrossRef]
- Possible Lethal Effects of CS Tear Gas on Possible Lethal Effects of CS Tear Gas on Branch Davidians during the Branch Davidians during the FBI raid on the Mount Carmel Compound FBI Raid on the Mount Carmel Compound near Waco, Texas near Waco, Twxas April 19, 1993. Available online: http://www.veritagiustizia.it/docs/gas_cs/CS_Effects_Waco.pdf (accessed on 14 May 2024).
- Directive 2008/32/EC of the European Parliament and of the Council of 11 March 2008 amending Directive 2000/60/EC estab-lishing a framework for Community action in the field of water policy, as regards the implementing powers conferred on the Commission. DIRECTIVE 2000/60/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 23 October 2000 establishing a framework for Community action in the field of water policy (OJ L 327, 22.12.2000, p. 1). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20141120 (accessed on 14 May 2024).
- Rice, p.; Jones, D.; Stanton, D. A Literature Review of the Solvents Suitable for the Police CS Spray Device; Chemical & Biological Defence Establishment: Salisbury, UK, 1997. [Google Scholar]
- Agrawal, Y.; Thornton, D.; Phipps, A. CS gas—Completely safe? A burn case report and literature review. Burns 2009, 35, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Evaluation Report Enzymatic Decontamination of Chemical Warfare Agents united states environmental protection agency research triangle park, north carolina 2771 EPA 600/R-12/033 | 2013. EPA, UNITED NATIONS ENVIRONMENTAL PROTECTION AGENCY. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100JD73.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2011+Thru+2015&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C11thru15%5CTxt%5C00000010%5CP100JD73.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 14 May 2024).
- Salem, H.; Gutting, B.W.; Kluchinsky, T.; Boardman, C.; Tuorinsky, S.; Hout, J. Riot Control Agents. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 137–154. [Google Scholar] [CrossRef]
- Borusiewicz, R. Chromatographic analysis of the traces of 2-chlorobenzalmalononitrile with passive adsorption from the headspace on Tenax TA and Carbotrap 300. Forensic Sci. Int. 2019, 303, 109933. [Google Scholar] [CrossRef] [PubMed]
- Analysis of the Toxicity Hazards of Methylene Chloride Associated with the Use of Tear Gas at the Branch Davidian Compound at Waco, Texas, on April 19, 1993. Available online: https://www.apologeticsindex.org/pdf/lucier.pdf (accessed on 14 May 2024).
- Gheorghe, V.; Gheorghe, C.G.; Bondarev, A.; Somoghi, R. Ecotoxicity of o-Chlorobenzylidene Malononitrile (CBM) and Toxicological Risk Assessment for SCLP Biological Cultures (Saccharomyces sp., Chlorella sp., Lactobacillus sp., Paramecium sp.). Toxics 2023, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Peng, Y.-P.; Chen, K.-F.; Chen, T.-Y.; Tang, C.-T. The effect of different in situ chemical oxidation (ISCO) technologies on the survival of indigenous microbes and the remediation of petroleum hydrocarbon-contaminated soil. Process. Saf. Environ. Prot. 2022, 163, 105–115. [Google Scholar] [CrossRef]
- Gheorghe, V.; Gheorghe, C.G.; Bondarev, A.; Toader, C.N.; Bombos, M.; Vasile, M. The Contamination Effects and Toxicological Characterization of o-Chlorobenzylidene Manolonitrile. Rev. Chim. 2021, 71, 67–75. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Venkateswarlu, K.; Venkidusamy, K.; Palanisami, T.; Naidu, R.; Megharaj, M. Bioremediation of soil long-term contaminated with PAHs by algal–bacterial synergy of Chlorella sp. MM3 and Rhodococcus wratislaviensis strain 9 in slurry phase. Sci. Total Environ. 2019, 659, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J.; He, Z.; Zheng, C.; Pan, X. Study on soil physical structure after the bioremediation of Pb pollution using microbial-induced carbonate precipitation methodology. J. Hazard. Mater. 2021, 411, 125103. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.M.; Jusoh, A.; Manan, H.; Kasan, N.A.; Kamaruzzan, A.S.; Ghani, W.A.W.A.K.; Kurniawan, S.B.; Lananan, F. Utilization of microalgae, Chlorella sp. UMT LF2 for bioremediation of Litopenaeus vannamei culture system and harvesting using bio-flocculant, Aspergillus niger. Biocatal. Agric. Biotechnol. 2023, 47, 1025960. [Google Scholar] [CrossRef]
- Gheorghe, V.; Gheorghe, C.G.; Popovici, D.R.; Mihai, S.; Calin, C.; Sarbu, E.E.; Doukeh, R.; Grigoriu, N.; Toader, C.N.; Epure, C.; et al. Synthesis, Purity Check, Hydrolysis and Removal of o-Chlorobenzyliden Malononitrile (CBM) by Biological Selective Media. Toxics 2023, 11, 672. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Li, X.; Wu, X.; Chen, L.; Wang, G. Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate. Int. J. Environ. Res. Public Health 2022, 20, 386. [Google Scholar] [CrossRef]
- Posadas, E.; del Mar Morales, M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem. Eng. J. 2015, 265, 232–239. [Google Scholar] [CrossRef]
- Takáčová, A.; Bajuszová, M.; Šimonovičová, A.; Šutý, S.; Nosalj, S. Biocoagulation of Dried Algae Chlorella sp. and Pellets of Aspergillus Niger in Decontamination Process of Wastewater, as a Presumed Source of Biofuel. J. Fungi 2022, 8, 1282. [Google Scholar] [CrossRef] [PubMed]
- SR 13328; Wather quality. Aquatic organisms tests. Pollutants toxicity determinations compared to green algae ICS 1306040, Test de inhibitie a cresterii algelor. Test de inhibitie a cresterii algelor. Jurnalul Ofícial al Uniunii Europene: Bucuresti, Romania, 1996.
- Dusescu, C.; Bolocan, I. New catalysts for the glycerol hydrogenolysis. Rev. De Chim. 2012, 63, 732–738. [Google Scholar]
- Gheorghe, C.G.; Gheorghe, V. Schreening behavioral responses of certain microorganisms to CBM toxic used in military and law enforcement operations. In Proceedings of the 5th International Colloquium Energy and Environmental Protection, Ploiesti, Romania, 4–6 November 2020. [Google Scholar]
- Gheorghe, C.G.; Pantea, O.; Matei, V.; Bombos, D.; Borcea, A.F. Testing of Bacterial and Fungal Resistance in the Water Pollution with Cationic Detergents. Chem. J. 2011, 62, 707–711. [Google Scholar]
- Gheorghe, C.G.; Dusescu, C.; Carbureanu, M. Asphaltenes biodegradation in biosystems adapted on selective media. Rev. Chim. 2016, 67, 2106–2110. [Google Scholar]
- Gheorghe, C.G.; Pantea, O.; Matei, V.; Bombos, D.; Borcea, A.F. Testing the behavior of pure bacterial suspension (Bacillus subtilis, Pseudomonas aeruginosa and Micrococcus luteus) în case of hydrocarbons contaminators. Rev. Chim. 2011, 62, 926–929. [Google Scholar]
- Gheorghe, C.G.; Pantea, O.; Bombos, V.M.D.; Borcea, A.F. The Efficiency of Flocculants in Biological Treatment with Activated Sludge. Rev. De Chim. 2011, 62, 1023–1026. [Google Scholar]
- Yu, H.; Du, X.; Zhao, Q.; Yin, C.; Song, W. Weighted gene Co-expression network analysis (WGCNA) reveals a set of hub genes related to chlorophyll metabolism process in chlorella (Chlorella vulgaris) response androstenedione. Environ. Pollut. 2022, 306, 119360. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wei, S.; Li, F.; Zhu, N.; Wei, X.; Wu, P.; Dang, Z. Biomass production of C. pyrenoidosa by filled sphere carrier reactor: Performance and mechanism. Bioresour. Technol. 2023, 383, 129195. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and conventional microplastics posed similar toxicity to marine algae Chlorella vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef]
- Murdock, J.N.; Wetzel, D.L. FT-IR Microspectroscopy Enhances Biological and Ecological Analysis of Algae. Appl. Spec-Troscopy Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Surendhiran, D.; Vijay, M. Influence of bioflocculation parameters on harvesting Chlorella salina and its optimization using response surface methodology. J. Environ. Chem. Eng. 2013, 1, 1051–1056. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, L.; Sainsbury, G.L.; Utley, D. Ortho-Chlorobenzylmalononitrile: A metabolite formed from or-tho-chloro-benzylidenemalononitrile (CS) Toxicol. Appl. Pharmacol. 1973, 25, 111. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M. Green Microalgae Water Extract as Foliar Feeding to Wheat Plants. Pak. J. Biol. Sci. 2001, 4, 628–632. [Google Scholar] [CrossRef]
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R. Review of challenges for algae based wastewater treatment: Strain selection, wastewater characteristics, abiotic, and biotic factors. J. Hazard. Toxic Radioact. Waste 2021, 25. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Liang, Y.; Beardall, J.; Heraud, P. Changes in growth, chlorophyll fluorescence and fatty acid composition with culture age in batch cultures of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Bot. Mar. 2006, 49, 165–173. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Fan, Z.; Tang, P.; Wu, M.; Xiao, H.; Zeng, Z. Toxicity of Tetracycline and Metronidazole in Chlorella pyrenoidosa. Int. J. Environ. Res. Public Health 2023, 20, 3623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Fourier transform infrared spectroscopy (FTIR) analysis, chlorophyll content and antioxidant properties of native and defatted foliage of green leafy vegetables. J. Food Sci. Technol. 2015, 52, 8131–8139. [Google Scholar] [CrossRef] [PubMed]
- Hazeem, L.J.; Yesilay, G.; Bououdina, M.; Perna, S.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020, 156, 111278. [Google Scholar] [CrossRef]
- Bastert, J.; Korting, H.C.; Traenkle, P.; Schmalreck, A.F. Identification of Dermatophytes by Fourier Transform Infrared Spectros-copy. Mycoses 1999, 42, 525–528. [Google Scholar] [CrossRef]
- Hirschmugl, C.J.; Bayarri, Z.E.; Bunta, M.; Holt, J.B.; Giordano, M. Analysis of the nutritional status of algae by Fourier transform infrared chemical imaging. Infrared Phys. Technol. 2006, 49, 57–63. [Google Scholar] [CrossRef]
- Slovacek, R.E.; Hannan, P.J. In vivo fluorescence determinations of phytoplankton chlorophyll a. Limnol. Oceanogr. 1977, 22, 919–925. [Google Scholar] [CrossRef]
- Ribeiro Rodrigues, L.; Arenzon, A.; Raya-Rodriguez, M.; Fontoura, N. Algal density assessed by spectrophotometry: A calibration curve for the unicellular algae Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar]
- Volgusheva, A.A.; Todorenko, D.A.; Konyukhov, I.V.; Voronova, E.N.; Pogosyan, S.I.; Plyusnina, T.Y.; Khruschev, S.S.; Antal, T.K. Acclimation Response of Green Microalgae Chlorella Sorokiniana to 2,3′,4,4′,6-Pentachlorobiphenyl. Photochem. Photobiol. 2023, 99, 1106–1114. [Google Scholar] [PubMed]
- Dhivare, R.S.; Rajput, S. Malononitrile: A Versatile Active Methylene Group. Int. Lett. Chem. Phys. Astron. 2015, 57, 126–144. [Google Scholar] [CrossRef][Green Version]
- Park, S.-H.; Chung, E.-K.; Yi, G.-Y.; Chung, K.-J.; Shin, J.-A.; Lee, I.-S. A Study for Health Hazard Evaluation of Methylene Chloride Evaporated from the Tear Gas Mixture. Saf. Health Work. 2010, 1, 98–101. [Google Scholar] [CrossRef]
- Li, X.; Zhou, R.; Xu, K.; Xu, J.; Jin, J.; Fang, H.; He, Y. Rapid Determination of Chlorophyll and Pheophytin in Green Tea Using Fourier Transform Infrared Spectroscopy. Molecules 2018, 23, 1010. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Tian, X.; Xu, K.; Gong, S.; Zhang, Y.; Yang, Y.; Wang, Z.; Wang, S. Colorimetric and turn-on fluorescent chemosensor with large stokes shift for sensitively probing cyanide anion in real samples and living systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120882. [Google Scholar] [CrossRef]
- Chorvatova, A.M.; Uherek, M.; Mateasik, A.; Chorvat, D. Time-resolved endogenous chlorophyll fluorescence sensitivity to pH: Study on Chlorella sp. algae. Methods Appl. Fluoresc. 2020, 8, 024007. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, N.K.; Kang, S.; Huh, Y.; Jung, J.; Hur, J.K.; Kim, D. Latent turn-on fluorescent probe for the detection of toxic malononitrile in water and its practical applications. Anal. Chim. Acta 2019, 1095, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Valicaa, M.; Pipíškaa, M.; Hostina, S. Effectiveness of Chlorella vulgaris inactivation during electrochemical water treatment. Desalin. Water Treat 2019, 138, 190–199. [Google Scholar] [CrossRef]
- Vingiani, G.M.; De Luca, P.; Ianora, A.; Dobson, A.D.; Lauritano, C. Microalgal Enzymes with Biotechnological Applications. Mar. Drugs 2019, 17, 459. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Ecotoxicological effects of enrofloxacin and its removal by monoculture of microalgal species and their consortium. Environ. Pollut. 2017, 226, 486–493. [Google Scholar] [CrossRef]
- Dusescu, C.; Juganaru, T.; Bombos, D.; Mihai, O.; Popovici, D. Multilayered catalysts for fatty acid ester hydrotreatment into fuel range hydrocarbons. Comptes Rendus. Chim. 2018, 21, 288–302. [Google Scholar] [CrossRef]
- Gheorghe, V.; Gheorghe, C.G.; Popovici, D.R.; Mihai, S.; Elena, D.R.; Şomoghi, R. Modification of Oxygen Production of Algal Cells in the Presence of O-chlorobenzylidene Malononitrile, Biodegradation in the Eco-Friendly Way. Preprint 2023. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, V.; Gheorghe, C.G.; Popovici, D.R.; Mihai, S.; Dragomir, R.E.; Somoghi, R. Reduction of Oxygen Production by Algal Cells in the Presence of O-Chlorobenzylidene Malononitrile. Bioengineering 2024, 11, 623. https://doi.org/10.3390/bioengineering11060623
Gheorghe V, Gheorghe CG, Popovici DR, Mihai S, Dragomir RE, Somoghi R. Reduction of Oxygen Production by Algal Cells in the Presence of O-Chlorobenzylidene Malononitrile. Bioengineering. 2024; 11(6):623. https://doi.org/10.3390/bioengineering11060623
Chicago/Turabian StyleGheorghe, Viorel, Catalina Gabriela Gheorghe, Daniela Roxana Popovici, Sonia Mihai, Raluca Elena Dragomir, and Raluca Somoghi. 2024. "Reduction of Oxygen Production by Algal Cells in the Presence of O-Chlorobenzylidene Malononitrile" Bioengineering 11, no. 6: 623. https://doi.org/10.3390/bioengineering11060623
APA StyleGheorghe, V., Gheorghe, C. G., Popovici, D. R., Mihai, S., Dragomir, R. E., & Somoghi, R. (2024). Reduction of Oxygen Production by Algal Cells in the Presence of O-Chlorobenzylidene Malononitrile. Bioengineering, 11(6), 623. https://doi.org/10.3390/bioengineering11060623









