Management of Dry Eye Disease for Intraocular Lens Power Calculation in Cataract Surgery: A Systematic Review
Abstract
1. Introduction
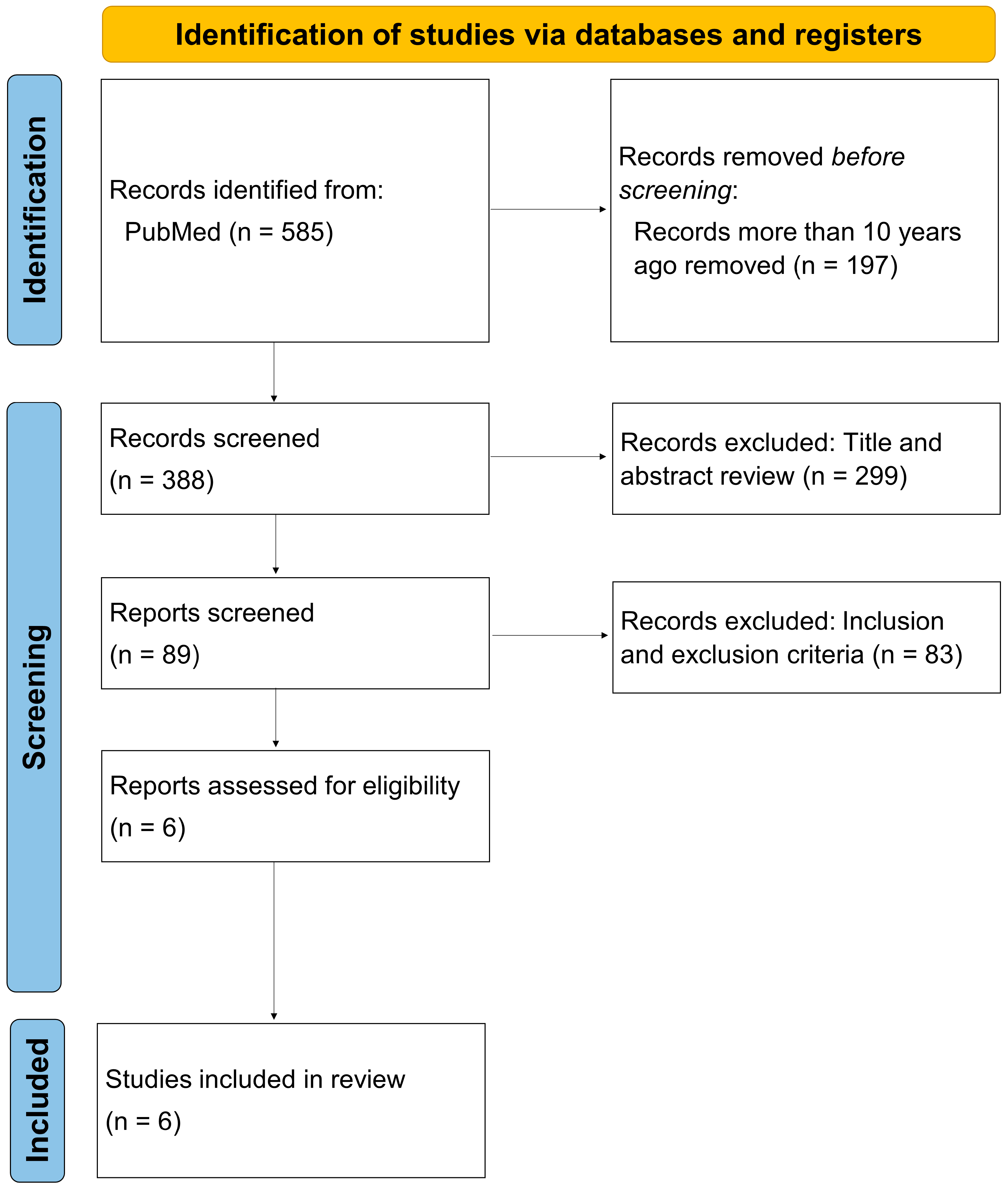
2. Materials and Methods
3. Results
3.1. Preoperative Diagnosis of DED
3.2. Preoperative Management of DED and Refractive Outcomes
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.W.; Day, A.C.; O’Brart, D.P. Femtosecond laser-assisted cataract surgery: A review. Eur. J. Ophthalmol. 2020, 30, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Yoo, H.S.; An, Y.; Yoon, S.Y.; Park, S.P.; Kim, Y.K. Inter-ocular and inter-visit differences in ocular biometry and refractive outcomes after cataract surgery. Sci. Rep. 2020, 10, 14673. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, X. Comparison of the Orbscan II topographer and the iTrace aberrometer for the measurements of keratometry and corneal diameter in myopic patients. BMC Ophthalmol. 2016, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Papas, E.B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol. Opt. 2021, 41, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, C.S. Effects of Aging in Dry Eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef]
- Kawahara, A. Treatment of Dry Eye Disease (DED) in Asia: Strategies for Short Tear Film Breakup Time-Type DED. Pharmaceutics 2023, 15, 2591. [Google Scholar] [CrossRef]
- Trattler, W.B.; Majmudar, P.A.; Donnenfeld, E.D.; McDonald, M.B.; Stonecipher, K.G.; Goldberg, D.F. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: The effect of dry eye. Clin. Ophthalmol. 2017, 11, 1423–1430. [Google Scholar] [CrossRef]
- Meyer, L.M.; Kronschläger, M.; Wegener, A.R. Schleimpflug photography detects alterations in corneal density and thickness in patients with dry eye disease. Ophthalmologe 2014, 111, 914–919. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Ning, X.; Liu, J.; Wang, J. Effect of dry eye on the reliability of keratometry for cataract surgery planning. J. Français d’Ophtalmologie 2024, 47, 103999. [Google Scholar] [CrossRef]
- Labetoulle, M.; Rousseau, A.; Baudouin, C. Management of dry eye disease to optimize cataract surgery outcomes: Two tables for a daily clinical practice. J. Français d’Ophtalmologie 2019, 42, 907–912. [Google Scholar] [CrossRef]
- Kim, S.; Shin, J.; Lee, J.E. A randomized, prospective study of the effects of 3% diquafosol on ocular sur-face following cataract surgery. Sci. Rep. 2021, 11, 9124. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Behrens, A.; Doyle, J.J.; Stern, L.; Chuck, R.S.; McDonnell, P.J.; Azar, D.T.; Dua, H.S.; Hom, M.; Karpecki, P.M.; Laibson, P.R.; et al. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations. Cornea 2006, 25, 900–907. [Google Scholar] [CrossRef]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 2007, 75–92.
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Hiraoka, T.; Asano, H.; Ogami, T.; Nakano, S.; Okamoto, Y.; Yamada, Y.; Oshika, T. Influence of Dry Eye Disease on the Measurement Repeatability of Corneal Curvature Radius and Axial Length in Patients with Cataract. J. Clin. Med. 2022, 11, 710. [Google Scholar] [CrossRef]
- Rochet, E.; Levron, A.; Agard, E.; Chehab, H.E.; Plas, H.; Bouvarel, H.; Chirpaz, N.; Billant, J.; Dot, C. Should Artificial Tears Be Used during the Preoperative Assessment of Toric IOLs before Age-Related Cataract Surgery? The TORIDE Study. J. Refract. Surg. 2021, 37, 759–766. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.K.; Ha, Y.; Paik, H.J.; Kim, D.H. Improved accuracy of intraocular lens power calculation by preoperative management of dry eye disease. BMC Ophthalmol. 2021, 21, 364. [Google Scholar] [CrossRef]
- Hovanesian, J.; Epitropoulos, A.; Donnenfeld, E.D.; Holladay, J.T. The Effect of Lifitegrast on Refractive Accuracy and Symptoms in Dry Eye Patients Undergoing Cataract Surgery. Clin. Ophthalmol. 2020, 14, 2709–2716. [Google Scholar] [CrossRef]
- Epitropoulos, A.T.; Matossian, C.; Berdy, G.J.; Malhotra, R.P.; Potvin, R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J. Cataract. Refract. Surg. 2015, 41, 1672–1677. [Google Scholar] [CrossRef]
- Starr, C.E.; Gupta, P.K.; Farid, M.; Beckman, K.A.; Chan, C.C.; Yeu, E.; Gomes, J.A.P.; Ayers, B.D.; Berdahl, J.P.; Holland, E.J.; et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J. Cataract. Refract. Surg. 2019, 45, 669–684. [Google Scholar] [CrossRef]
- Sakane, Y.; Yamaguchi, M.; Yokoi, N.; Uchino, M.; Dogru, M.; Oishi, T.; Ohashi, Y.; Ohashi, Y. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol. 2013, 131, 1331–1338. [Google Scholar] [CrossRef]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Benítez Del Castillo, J.M.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e1. [Google Scholar] [CrossRef]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.N.; Søndergaard, A.P.; Pommerencke, C.; Møller, F. Variations in keratometric values (K-value) after administration of three different eye drops—Effects on the intraocular lens calculations in relation to cataract surgery. Acta Ophthalmol. 2020, 98, 613–617. [Google Scholar] [CrossRef]
- Röggla, V.; Leydolt, C.; Schartmüller, D.; Schwarzenbacher, L.; Meyer, E.; Abela-Formanek, C.; Menapace, R. Influence of Artificial Tears on Keratometric Measurements in Cataract Patients. Am. J. Ophthalmol. 2021, 221, 1–8. [Google Scholar] [CrossRef]
- Mrukwa Kominek, E.; Sarnat-Kucharczyk, M.; Patel, S. The impact of exposure on the magnitude of astigmatism formed within the precorneal tear film over the central optical zone of the cornea in ocular surface disease. Contact Lens Anterior Eye 2020, 43, 261–267. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Georgiev, G.A. Tear-film-oriented diagnosis for dry eye. Jpn. J. Ophthalmol. 2019, 63, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Oka, K.; Inai, M. Efficacy and Safety of the Long-Acting Diquafosol Ophthalmic Solution DE-089C in Patients with Dry Eye: A Randomized, Double-Masked, Placebo-Controlled Phase 3 Study. Adv. Ther. 2022, 39, 3654–3667. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sasaki, T.; Maruyama, T.; Murayama, K.; Shinoda, K. Effectiveness and Adherence of Dry Eye Patients Who Switched from Short- to Long-Acting Diquafosol Ophthalmic Solution. J. Clin. Med. 2023, 12, 4495. [Google Scholar] [CrossRef]
- von Kügelgen, I. Molecular pharmacology of P2Y receptor subtypes. Biochem. Pharmacol. 2021, 187, 114361. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kageyama, T.; Sakamoto, A.; Shiba, T.; Nakamura, M.; Maeno, T. Comparison of Short-Term Effects of Diquafosol and Rebamipide on Mucin 5AC Level on the Rabbit Ocular Surface. J. Ocul. Pharmacol. Ther. 2017, 33, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Arita, R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: A randomized clinical study. Sci. Rep. 2019, 9, 9091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, H.; Qin, G.; Wu, Y.; Song, Y.; Yang, L.; Yu, S.; He, X.; Moore, J.E.; Moutari, S.; et al. Impact of Diquafosol Ophthalmic Solution on Tear Film and Dry Eye Symptom in Type 2 Diabetic Dry Eye: A Pilot Study. J. Ocul. Pharmacol. Ther. 2022, 38, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Sonomura, Y.; Kato, H.; Komuro, A.; Kinoshita, S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren’s syndrome. Eye 2015, 29, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.S.; Yoo, Y.S.; Kwon, J.Y.; Joo, J.S.; Lim, S.A.; Whang, W.J.; Mok, J.W.; Choi, J.S.; Joo, C.K. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp. Eye Res. 2016, 143, 89–97. [Google Scholar] [CrossRef]
- Wu, D.; Chen, W.Q.; Li, R.; Wang, Y. Efficacy and safety of topical diquafosol ophthalmic solution for treatment of dry eye: A systematic review of randomized clinical trials. Cornea 2015, 34, 644–650. [Google Scholar] [CrossRef]

| Author | Type of Study | N | Comparison | Subjective Symptoms of DED | Objective Clinical Signs of DED |
|---|---|---|---|---|---|
| Yang et al., 2024 [13] | Non-randomized controlled clinical study | 83 | DED versus non-DED group | OSD SPEED II questionnaire | TBUT less than 10 s and positive corneal and conjunctival fluorescein staining |
| Hiraoka et al., 2022 [20] | Observational prospective non-randomized study | 114 | DED versus non-DED group | DEQS questionnaire | TBUT less than 5 s and positive corneal fluorescein staining |
| Rochct et al., 2021 [21] | Comparative, monocentric, prospective study | 73 | Comparison of the same eye before and after eye drops | None stated | Subjects not limited to DED, but 54.8% with TBUT less than 5 s |
| Kim et al., 2021 [22] | Retrospective observational study | 105 | Preoperative DED treatment versus no treatment group | None stated | TBUT, ocular surface staining score, Schirmer test (no diagnostic criteria listed) |
| Hovanesian et al., 2020 [23] | Multicenter, prospective, open-label study | 58 | Comparison of the same eye before and after DED treatment | SPEED questionnaire | TBUT less than 10 s and positive corneal fluorescein staining |
| Epitropoulos et al., 2015 [24] | Observational prospective non-randomized study | 144 | Tear hyperosmolar versus normal osmolarity group | Verbal questions | Hyperosmolarity is tear osmolarity 316 mOsm/L or more, normal is less than 308 mOsm/L |
| Author | Therapeutic Eye Drops | Length of Treatment | Keratometry Measurement Biometry | Results |
|---|---|---|---|---|
| Yang et al., 2024 [13] | None | None | OA-2000 | Reproducibility of corneal curvature and astigmatic vectors was significantly lower in the DED group. |
| Hiraoka et al., 2022 [20] | None | None | IOLMaster 500 | The measurement repeatability of corneal curvature radius declined in eyes with DED. |
| Rochct et al., 2021 [21] | Artificial tears | 1 min | IOLMaster 700 | Predicted astigmatic error improved significantly after eye drops in the group with TBUT less than 5 s. |
| Kim et al., 2021 [22] | Steroid and cyclosporin * | 2 weeks | IOLMaster 500 | Preoperative treatment significantly reduced postoperative refractive error and number of refractive surprises. |
| Hovanesian et al., 2020 [23] | Lifitegrast | 4 weeks | IOLMaster 500 or 700 | Preoperative treatment significantly increased the accuracy of predicting post-operative spherical equivalent. |
| Epitropoulos et al., 2015 [24] | None | None | IOLMaster (No model mentioned) | High osmolarity group showed significant variation in mean keratometry values and anterior corneal astigmatism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, A. Management of Dry Eye Disease for Intraocular Lens Power Calculation in Cataract Surgery: A Systematic Review. Bioengineering 2024, 11, 597. https://doi.org/10.3390/bioengineering11060597
Kawahara A. Management of Dry Eye Disease for Intraocular Lens Power Calculation in Cataract Surgery: A Systematic Review. Bioengineering. 2024; 11(6):597. https://doi.org/10.3390/bioengineering11060597
Chicago/Turabian StyleKawahara, Atsushi. 2024. "Management of Dry Eye Disease for Intraocular Lens Power Calculation in Cataract Surgery: A Systematic Review" Bioengineering 11, no. 6: 597. https://doi.org/10.3390/bioengineering11060597
APA StyleKawahara, A. (2024). Management of Dry Eye Disease for Intraocular Lens Power Calculation in Cataract Surgery: A Systematic Review. Bioengineering, 11(6), 597. https://doi.org/10.3390/bioengineering11060597






