Diverse Shape Design and Physical Property Evaluation of In-Body Tissue Architecture-Induced Tissues
Abstract
1. Introduction
2. Diverse Shaped iBTA-Induced Tissues
2.1. Ethical Approval
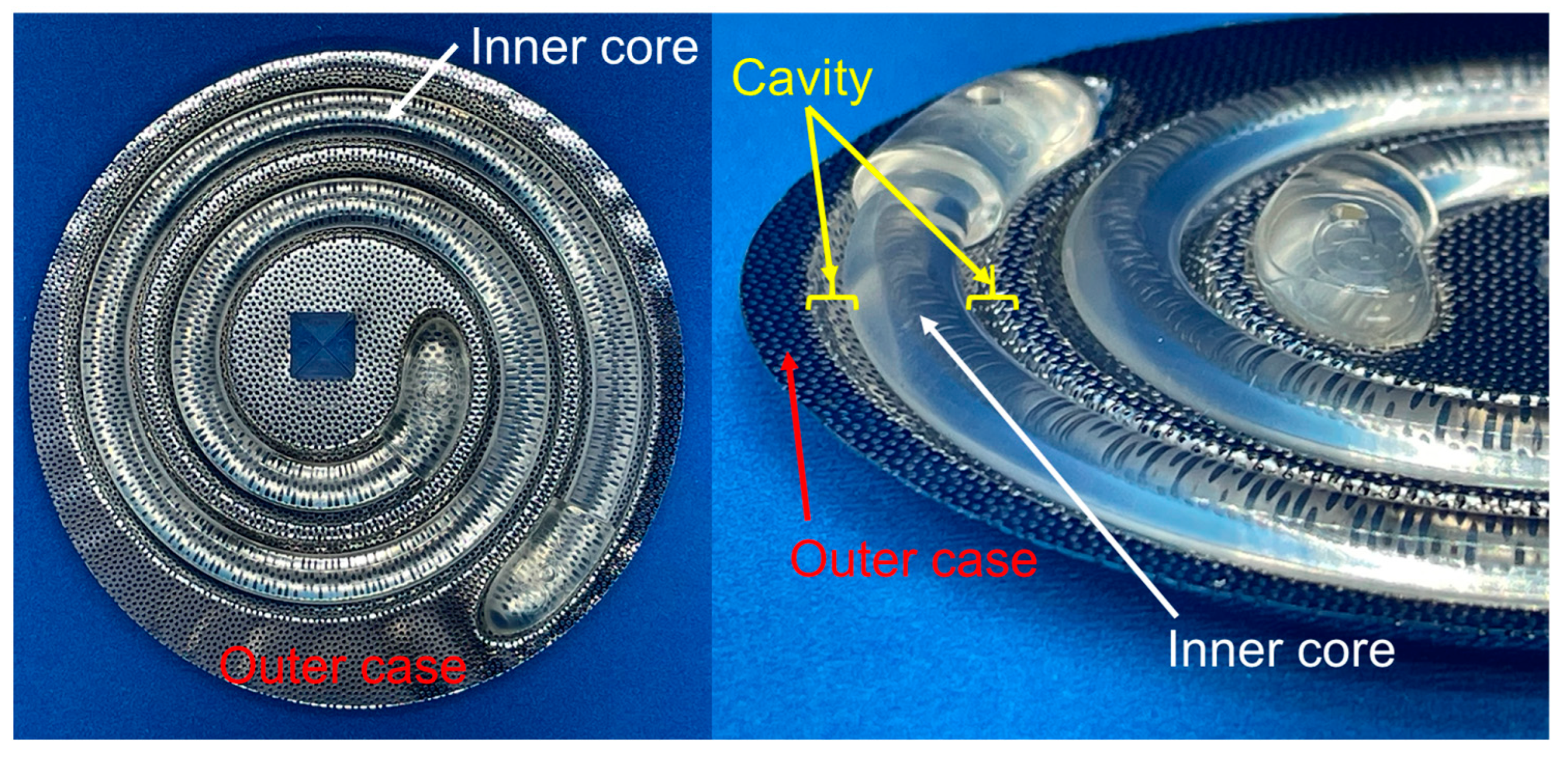
2.2. Fundamental of Moulds for Preparation of iBTA-Induced Tissues
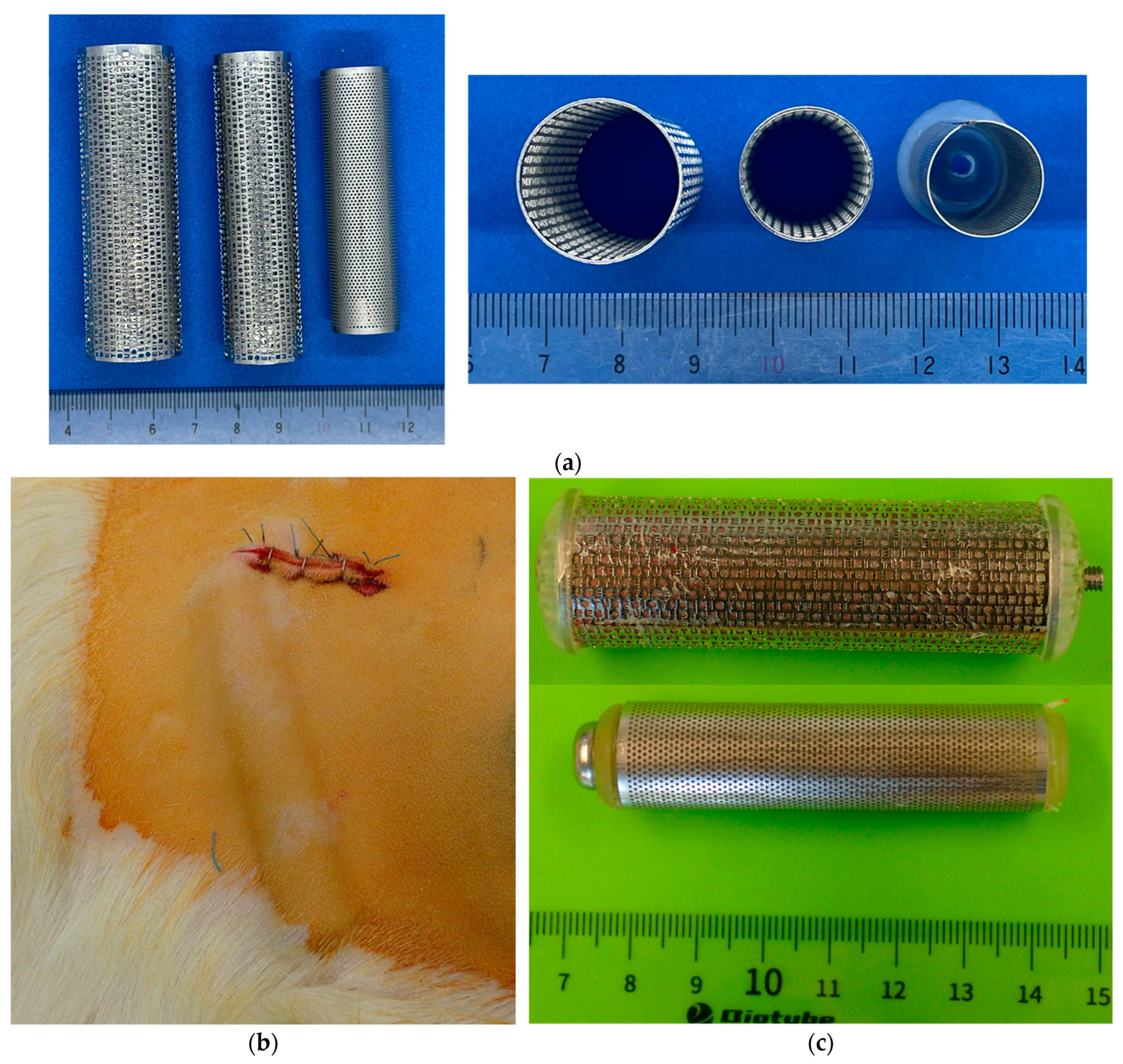
2.3. Fabrication Method for Ring-, Rod-, or Net-Shaped iBTA Tissues
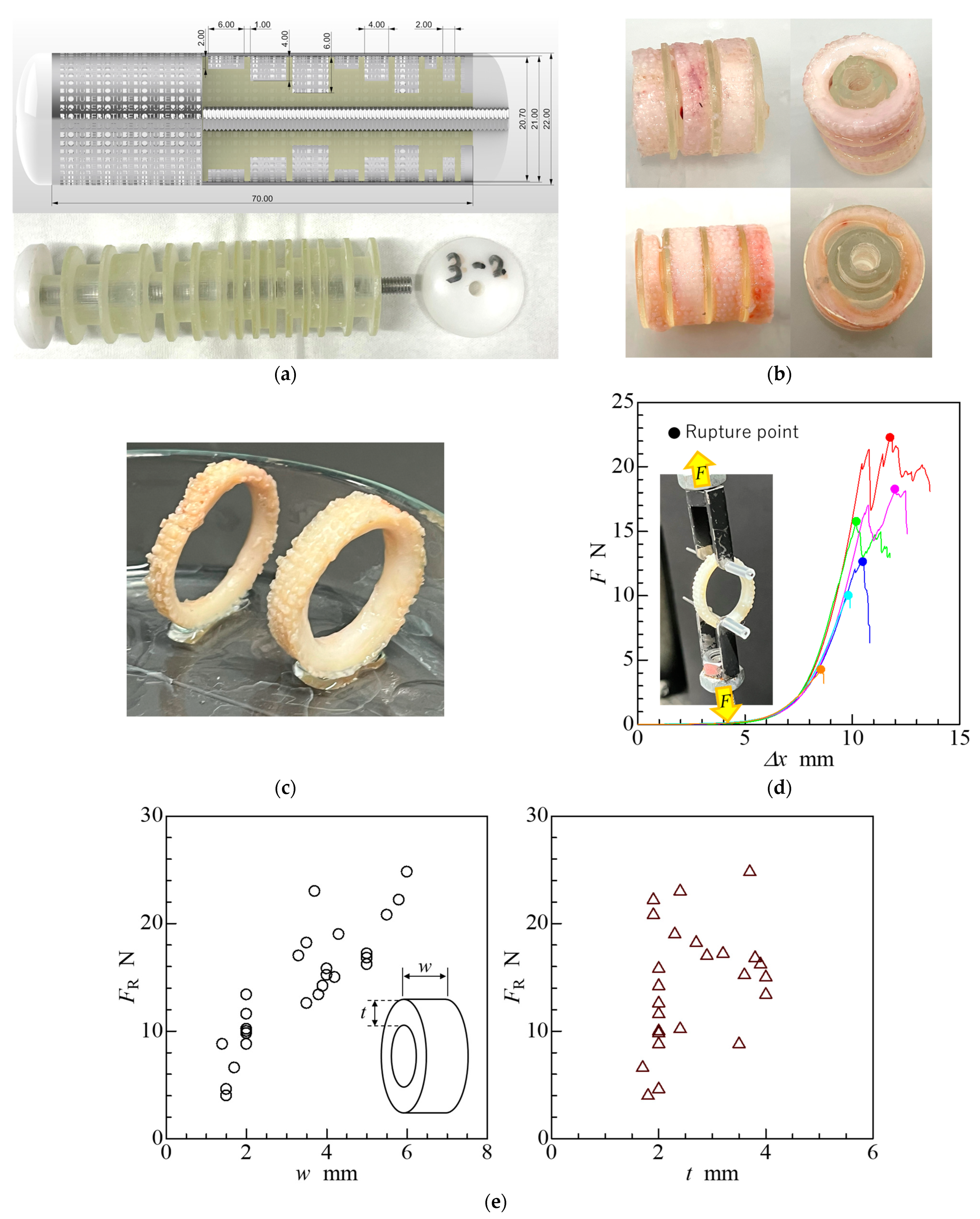
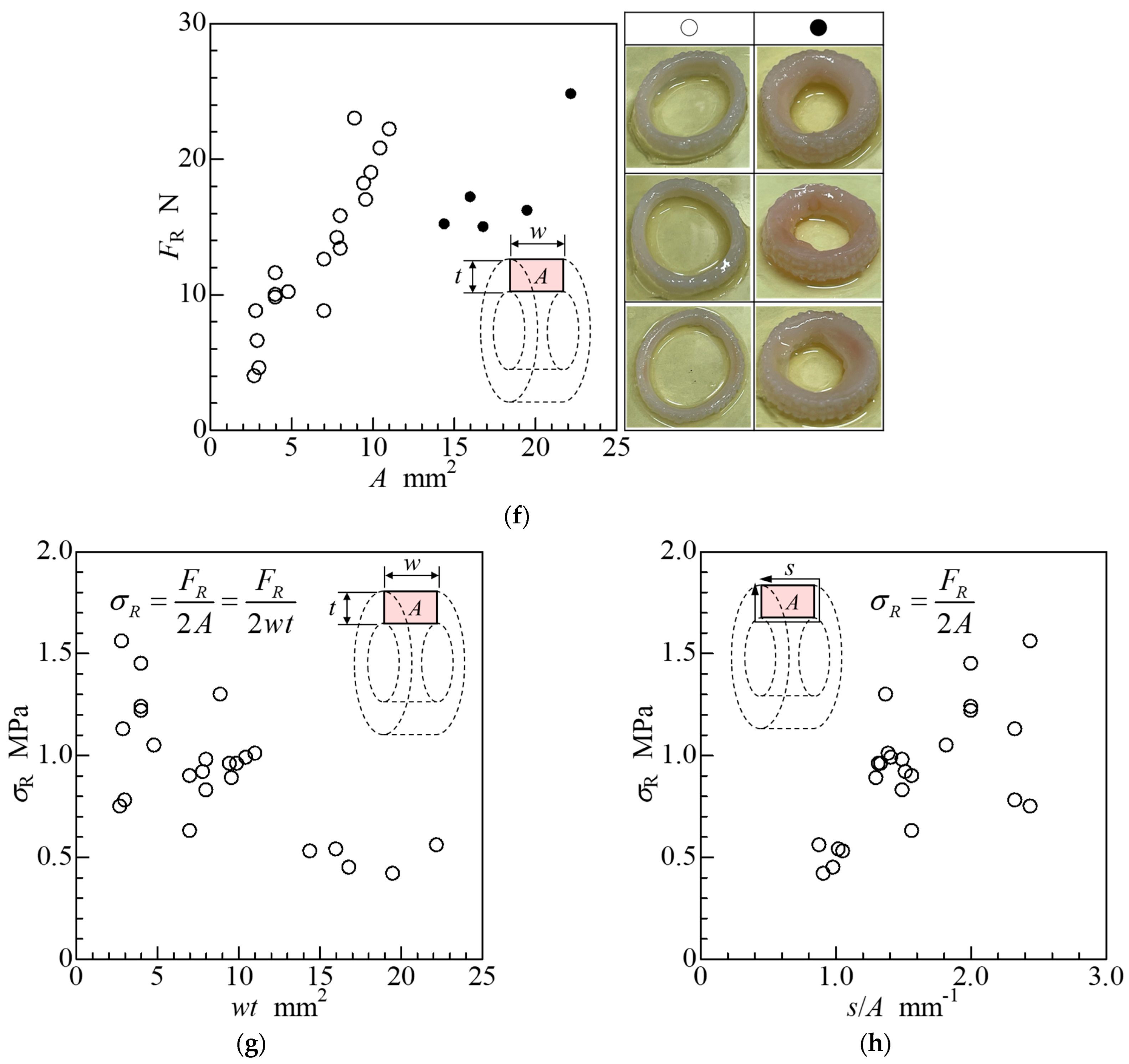
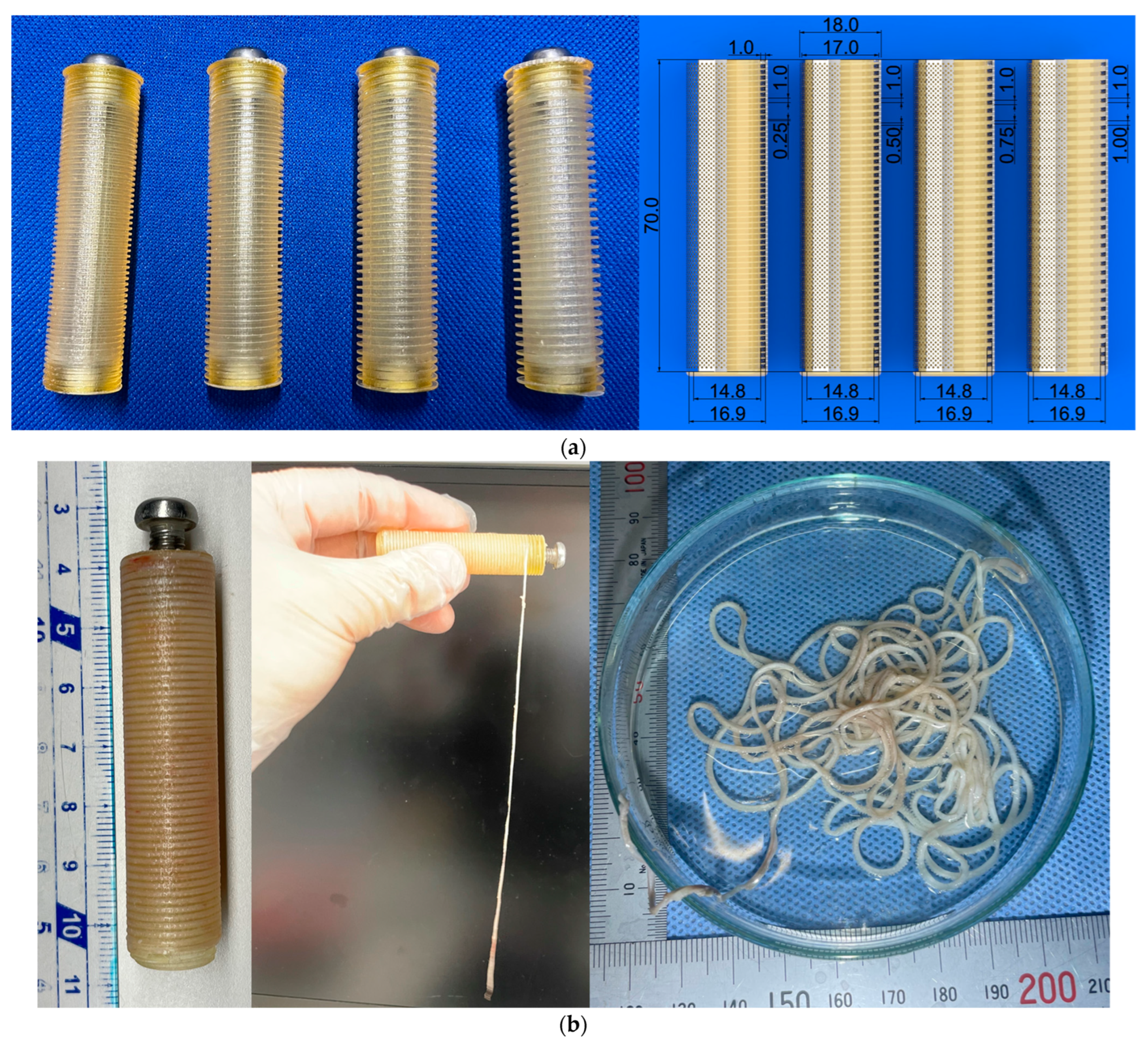
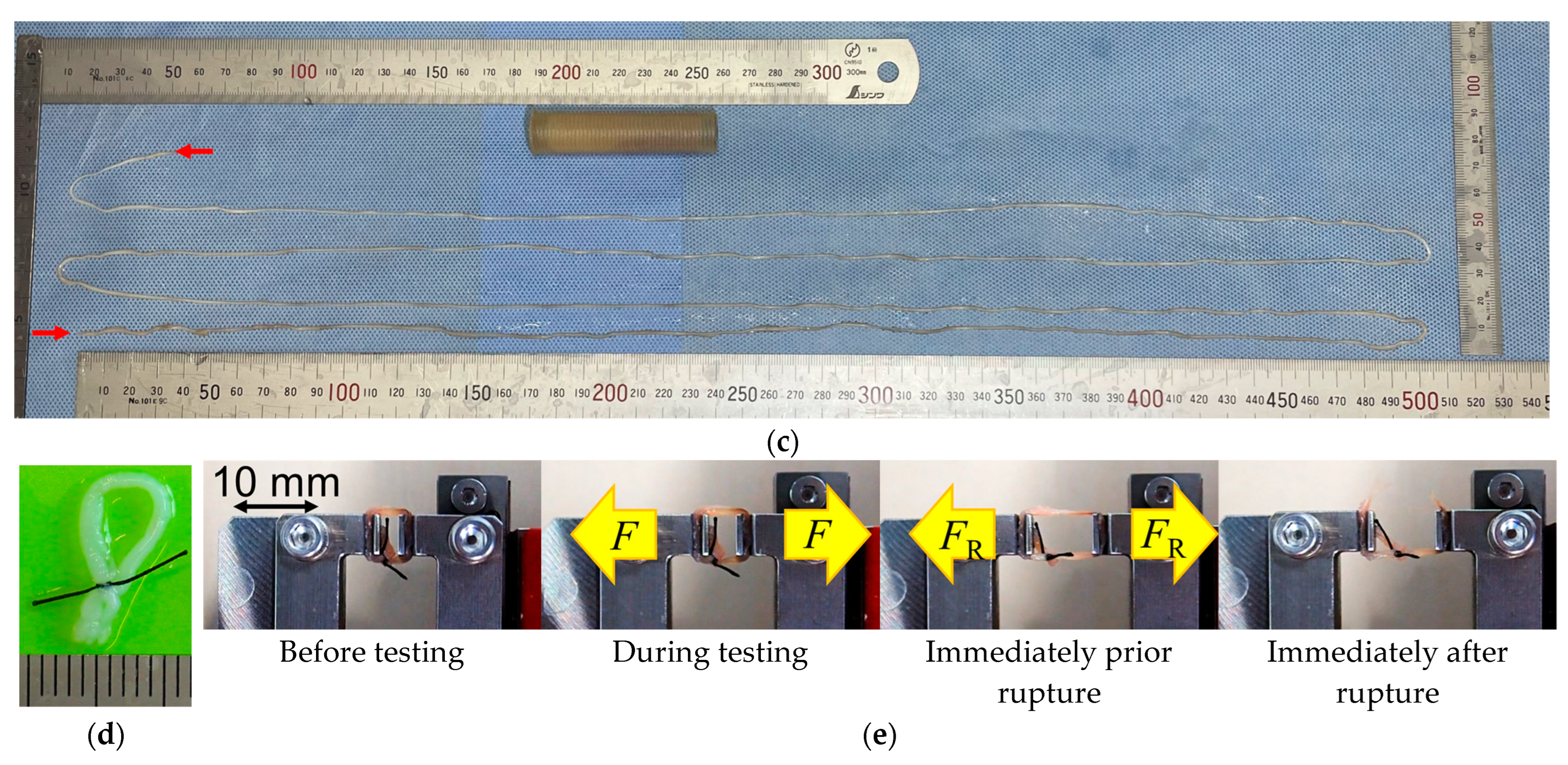
2.4. Ring-Shaped iBTA Tissues and Their Mechanical Properties
2.5. Cord-Shaped iBTA Tissues and Their Mechanical Properties
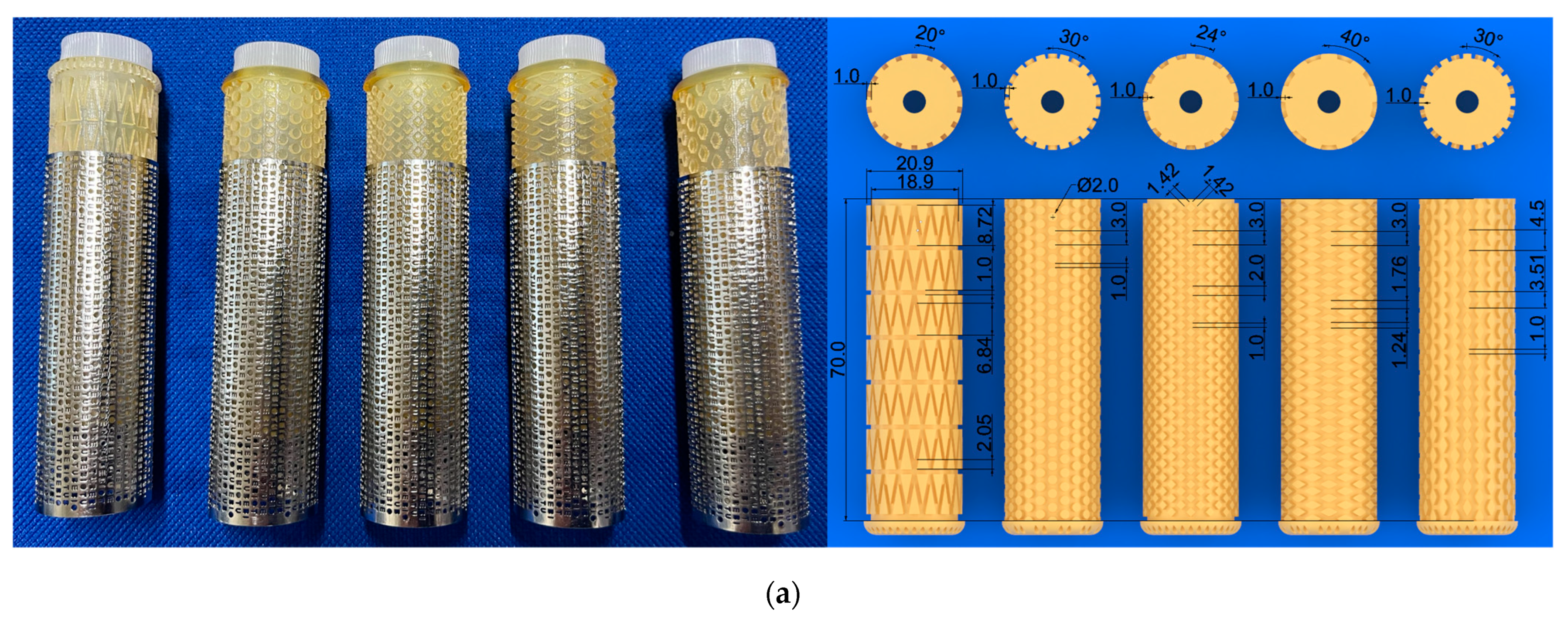
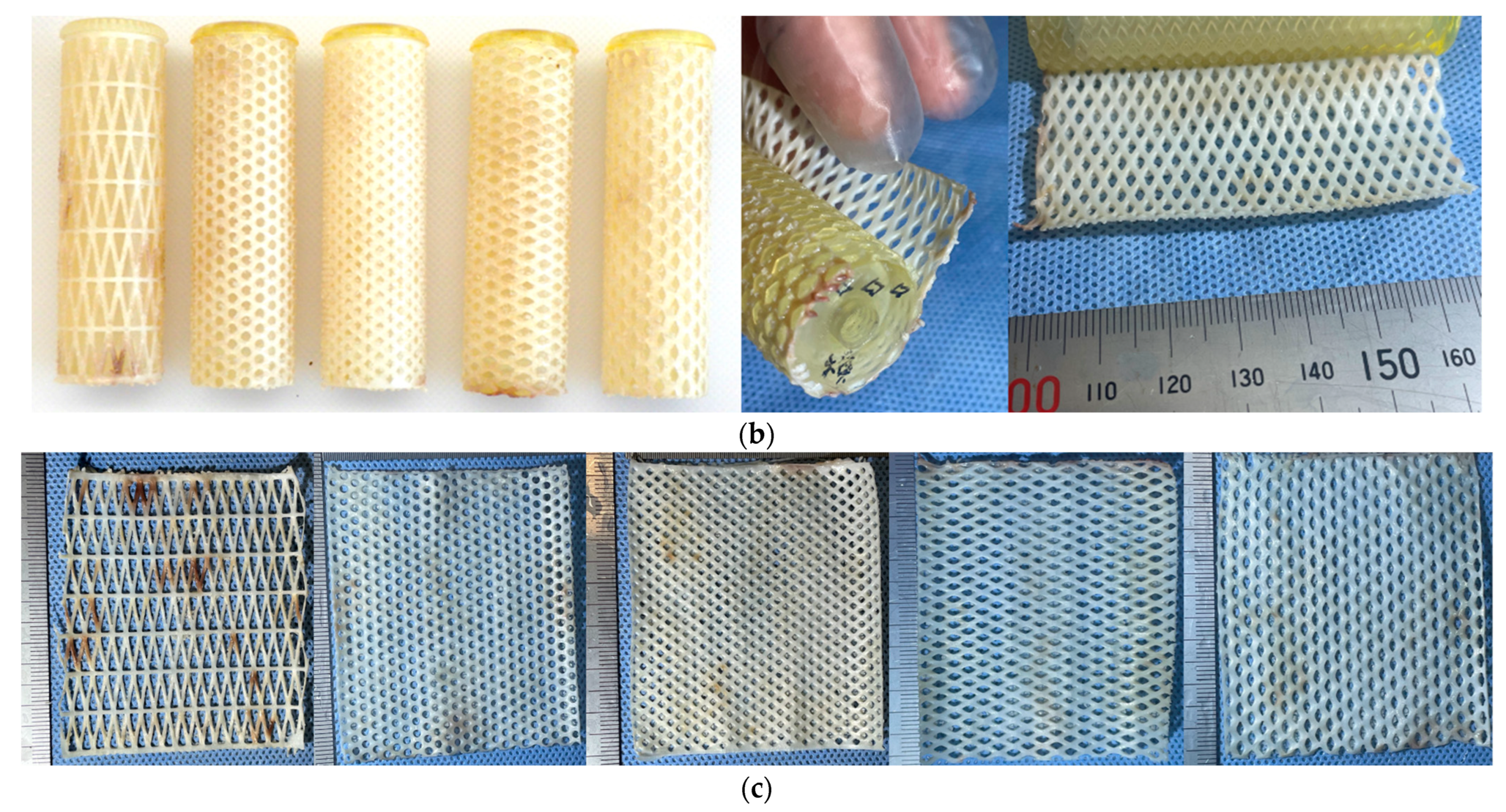
2.6. Net-Shaped iBTA Tissues
2.7. Branch-Shaped iBTA Tissues
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabkin-Aikawa, E.; Mayer, J.E., Jr.; Schoen, F.J. Heart valve regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Hoerstrup, S.P.; Kasimir, M.T.; Seebacher, G.; Weigel, G.; Ullrich, R.; Salzer-Muhar, U.; Rieder, E.; Wolner, E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur. J. Cardio-Thorac. Surg. 2003, 23, 1002–1006. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ishibashi-Ueda, H.; Takamizawa, K. In vivo tissue-engineered small-caliber arterial graft prosthesis consisting of autologous tissue (Biotube). Cell Transplant. 2004, 13, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Kanda, K.; Oie, T.; Okamoto, Y.; Ishibashi-Ueda, H.; Onoyama, M.; Tajikawa, T.; Ohba, K.; Yaku, H.; Nakayama, Y. Architecture of an in vivo-tissue engineered autologous conduit “Biovalve”. J. Biomed. Mater. Res. Part B 2008, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Park, B.Y. The subcutaneous capsules for foreign body in fetal rabbits: Preliminary report. Yonsei Med. J. 2001, 42, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Badid, C.; Mounier, N.; Costa, A.M.A.; Desmouliere, A. Role of myofibroblasts during normal tissue repair and excessive scarring: Interest of their assessment in nephropathies. Histol. Histopathol. 2000, 15, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Higashita, R.; Nakayama, Y.; Shiraishi, Y.; Iwai, R.; Inoue, Y.; Yamada, A.; Terazawa, T.; Tajikawa, T.; Miyazaki, M.; Ohara, M.; et al. Acute phase pilot evaluation of small diameter long iBTA induced vascular graft “Biotube” in a goat model. EJVES. Vasc. Forum 2022, 54, 27–35. [Google Scholar] [CrossRef]
- Nakayama, Y.; Iwai, R.; Terazawa, T.; Tajikawa, T.; Umeno, T.; Kawashima, T.; Nakashima, Y.; Shiraishi, Y.; Yamada, A.; Higashita, R.; et al. Preimplantation evaluation of a small-diameter, long vascular graft (Biotube) for below-knee bypass surgery in goats. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Umeno, T.; Mori, K.; Iwai, R.; Kawashima, T.; Shuto, T.; Nakashima, Y.; Tajikawa, T.; Nakayama, Y.; Miyamoto, S. Carotid artery bypass surgery of in-body tissue architecture-induced small-diameter Biotube in a goat model: A pilot study. Bioengineering 2024, 11, 203. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.M.; Ishibashi-Ueda, H.; Takamizawa, K.; Ando, J.; Kanda, K.; Yaku, H.; Nakayama, Y. In vitro maturation of “biotube” vascular grafts induced by a 2-day pulsatile flow loading. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 320–328. [Google Scholar] [CrossRef]
- Yamanami, M.; Ishibashi-Ueda, H.; Yamamoto, A.; Iida, H.; Watanabe, T.; Kanda, K.; Yaku, H.; Nakayama, Y. Implantation study of small-caliber “biotube” vascular grafts in a rat model. J. Artif. Organs 2013, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Tsujinaka, T. Acceleration of robust “biotube” vascular graft fabrication by in-body tissue architecture technology using a novel eosin Y-releasing mold. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Furukoshi, M.; Moriwaki, T.; Nakayama, Y. Development of an in vivo tissue-engineered vascular graft with designed wall thickness (biotube type C) based on a novel caged mold. J. Artif. Organs 2016, 19, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ishii, D.; Enmi, J.I.; Iwai, R.; Kurisu, K.; Tatsumi, E.; Nakayama, Y. One year rat study of iBTA-induced “Microbiotube” microvascular grafts with an ultra-small diameter of 0.6 mm. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Kaneko, Y.; Okumura, N.; Terazawa, T. Initial 3-year results of first human use of an in-body tissue-engineered autologous “Biotube” vascular graft for hemodialysis. J. Vasc. Access 2020, 21, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, H.; Mizuno, T.; Moriwaki, T.; Iwai, R.; Ishibashi-Ueda, H.; Yamanami, M.; Kanda, K.; Yaku, H.; Nakayama, Y. Implantation study of a tissue-engineered self-expanding aortic stent graft (bio stent graft) in a beagle model. J. Artif. Organs 2015, 18, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, H.; Mizuno, T.; Moriwaki, T.; Ishibashi-Ueda, H.; Yamanami, M.; Kanda, K.; Yaku, H.; Nakayama, Y. Development of tissue-engineered self-expandable aortic stent grafts (Bio stent grafts) using in-body tissue architecture technology in beagles. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yahata, Y.; Yamanami, M.; Tajikawa, T.; Ohba, K.; Kanda, K.; Yaku, H. A completely autologous valved conduit prepared in the open form of trileaflets (type VI biovalve): Mold design and valve function in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Takewa, Y.; Yamanami, M.; Kishimoto, Y.; Arakawa, M.; Kanda, K.; Matsui, Y.; Oie, T.; Ishibashi-Ueda, H.; Tajikawa, T.; Ohba, K.; et al. In vivo evaluation of an in-body, tissue-engineered, completely autologous valved conduit (biovalve type VI) as an aortic valve in a goat model. J. Artif. Organs 2013, 16, 176–184. [Google Scholar] [CrossRef]
- Nakayama, Y.; Takewa, Y.; Sumikura, H.; Yamanami, M.; Matsui, Y.; Oie, T.; Kishimoto, Y.; Arakawa, M.; Ohmuma, K.; Tajikawa, T.; et al. In-body tissue-engineered aortic valve (Biovalve type VII) architecture based on 3D printer molding. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1–11. [Google Scholar] [CrossRef]
- Fujioka, T.; Tajikawa, T.; Nakayama, Y. Development of left heart simulator considering blood flow dynamics in the ventricle and evaluation of hydrodynamic performance for modeled atrioventricular valve by using the simulator. In Proceedings of the 92nd Annual Meeting of the Kansai Branch of the Japan Society of Mechanical Engineers, Suita, Japan, 13–14 March 2017. [Google Scholar] [CrossRef]
- Nakayama, Y.; Yuasa, K.; Tajikawa, T. First time development of biovalve mitral valve: In vitro performance. Struct. Heart 2019, 3 (Suppl. 1), 138. [Google Scholar] [CrossRef]
- Mizuno, T.; Takewa, Y.; Sumikura, H.; Ohnuma, K.; Moriwaki, T.; Yamanami, M.; Oie, T.; Tatsumi, E.; Uechi, M.; Nakayama, Y. Preparation of an autologous heart valve with a stent (stent-biovalve) using the stent eversion method. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Takewa, Y.; Nakayama, Y.; Date, K.; Sumikura, H.; Moriwaki, T.; Nishimura, M.; Tatsumi, E. Sutureless aortic valve replacement using a novel autologous tissue heart valve with stent (stent biovalve): Proof of concept. J. Artif. Organs 2015, 18, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Sumikura, H.; Takewa, Y.; Tatsumi, E.; Nakayama, Y. Development of self-expanding valved stents with autologous tubular leaflet tissues for transcatheter valve implantation. J. Artif. Organs 2015, 18, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Takewa, Y.; Sumikura, H.; Kishimoto, S.; Naito, N.; Iizuka, K.; Akiyama, D.; Iwai, R.; Tatsumi, E.; Nakayama, Y. Implanted in-body tissue-engineered heart valve can adapt the histological structure to the environment. ASAIO J. 2018, 64, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Matsui, Y.; Tajikawa, T.; Sasagawa, T.; Saito, Y.; Sagishima, S.; Mizuno, T.; Mizuno, M.; Harada, K.; Uchida, S.; et al. Successful implantation of autologous valved conduits with self-expanding stent (stent-biovalve) within the pulmonary artery in beagle dogs. J. Vet. Cardiol. 2015, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sumikura, H.; Nakayama, Y.; Ohnuma, K.; Kishimoto, S.; Takewa, Y.; Tatsumi, E. In vitro hydrodynamic evaluation of a biovalve with stent (tubular leaflet type) for transcatheter pulmonary valve implantation. J. Artif. Organs 2015, 18, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Sumikura, H.; Nakayama, Y.; Ohnuma, K.; Takewa, Y.; Tatsumi, E. Development of a stent-biovalve with round-shaped leaflets: In vitro hydrodynamic evaluation for transcatheter pulmonary valve implantation (TPVI). J. Artif. Organs 2016, 19, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Oshima, N.; Tatsumi, E.; Ichii, O.; Nishimura, T. iBTA-induced bovine Biosheet for repair of abdominal wall defects in a beagle model: Proof of concept. Hernia 2018, 22, 1033–1039. [Google Scholar] [CrossRef]
- Furukoshi, M.; Nakayama, Y. Feasibility of In-body tissue architecture (IBTA) in pediatric cardiovascular surgery: Development of regenerative autologous tissues with growth potential. Ped. Cardiol. Card. Surg. 2016, 32, 199–207. [Google Scholar] [CrossRef]
- Satake, R.; Komura, M.; Komura, H.; Komura, H.; Kodaka, T.; Terawaki, K.; Ikebukuro, K.; Komuro, H.; Yonekawa, H.; Hoshi, H.; et al. Patch tracheoplasty in body tissue engineering using collagenous connective tissue membranes (biosheets). J. Pediatr. Surg. 2016, 51, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, S.; Iwai, R.; Nakayama, Y.; Moriwaki, T.; Okuyama, H. Successful tracheal regeneration using biofabricated autologous analogues without artificial supports. Sci. Rep. 2022, 12, 20279. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, T.; Nishimura, T.; Mitani, T.; Ichii, O.; Ikeda, T.; Kosenda, K.; Tatsumi, E.; Nakayama, Y. Wall thickness control in biotubes prepared using type-C mold. J. Artif. Organs 2018, 21, 387–391. [Google Scholar] [CrossRef]
- Sumikura, H.; Nakayama, Y.; Ohnuma, K.; Takewa, Y.; Tatsumi, E. In vitro evaluation of a novel autologous aortic valve (biovalve) with a pulsatile circulation circuit. Artif. Organs 2014, 38, 282–289. [Google Scholar] [CrossRef]
- Shiihara, R.; Tajikawa, T.; Nakayama, Y. Effect of vascular anastomosis on thrombus and neointima formation during vascular bypass surgery with “Biotube” vascular grafts. In Proceedings of the 33rd Japan Society of Mechanical Engineers Conference on Frontiers in Bioengineering, Kobe, Japan, 17–18 December 2022. [Google Scholar] [CrossRef]
- Nakayama, Y.; Furukoshi, M.; Terazawa, T.; Iwai, R. Development of long in vivo tissue-engineered “Biotube” vascular grafts. Biomaterials 2018, 185, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.; Gualerzi, A.; Boschetti, F.; Loy, F.; Cao, G. Decellularized ovine arteries as small-diameter vascular grafts. Biomed. Mater. 2014, 9, 045011. [Google Scholar] [CrossRef]
- Holzapfel, G.A. Biomechanics of Soft Tissue; Academic Press: Burlington, VT, USA, 2001; pp. 1049–1063. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Kurimoto, R.; Nakamichi, R.; Chiba, T.; Matsushima, T.; Fujii, Y.; Sanada, R.; Kato, T.; Shishido, K.; Sakamaki, Y.; et al. Generation of a tendon-like tissue from human iPS cells. J. Tissue Eng. 2022, 13, 20417314221074018. [Google Scholar] [CrossRef]
- Weiss, J.A.; Gardiner, J.C. Computational modeling of ligament mechanics. Crit. Rev. Biomed. Eng. 2001, 29, 303–371. [Google Scholar] [CrossRef]
- Mori, K.; Umeno, T.; Kawashima, T.; Wada, T.; Genda, T.; Arakura, M.; Oda, Y.; Mizoguchi, T.; Iwai, R.; Tajikawa, T.; et al. Breaking the limit of cardiovascular regenerative medicine: Successful 6-month goat implant in world’s first ascending aortic replacement using biotube blood vessels. Bioengineering 2024, 11, 405. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajikawa, T.; Sekido, Y.; Mori, K.; Kawashima, T.; Nakashima, Y.; Miyamoto, S.; Nakayama, Y. Diverse Shape Design and Physical Property Evaluation of In-Body Tissue Architecture-Induced Tissues. Bioengineering 2024, 11, 598. https://doi.org/10.3390/bioengineering11060598
Tajikawa T, Sekido Y, Mori K, Kawashima T, Nakashima Y, Miyamoto S, Nakayama Y. Diverse Shape Design and Physical Property Evaluation of In-Body Tissue Architecture-Induced Tissues. Bioengineering. 2024; 11(6):598. https://doi.org/10.3390/bioengineering11060598
Chicago/Turabian StyleTajikawa, Tsutomu, Yota Sekido, Kazuki Mori, Takayuki Kawashima, Yumiko Nakashima, Shinji Miyamoto, and Yasuhide Nakayama. 2024. "Diverse Shape Design and Physical Property Evaluation of In-Body Tissue Architecture-Induced Tissues" Bioengineering 11, no. 6: 598. https://doi.org/10.3390/bioengineering11060598
APA StyleTajikawa, T., Sekido, Y., Mori, K., Kawashima, T., Nakashima, Y., Miyamoto, S., & Nakayama, Y. (2024). Diverse Shape Design and Physical Property Evaluation of In-Body Tissue Architecture-Induced Tissues. Bioengineering, 11(6), 598. https://doi.org/10.3390/bioengineering11060598







