Effect of Exposure to Particulate Matter on the Ocular Surface in an Experimental Allergic Eye Disease Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Allergic Sensitization and PM Exposure in Mouse
2.3. Clinical Scoring
2.4. Serum Preparation and Enzyme-Linked Immunosorbent Assay (ELISA) for OVA-Specific IgE in Serum
2.5. Hematoxylin and Eosin Staining (H&E)
2.6. Toluidine Blue Staining
2.7. Immunofluorescence and Immunohistochemistry Staining
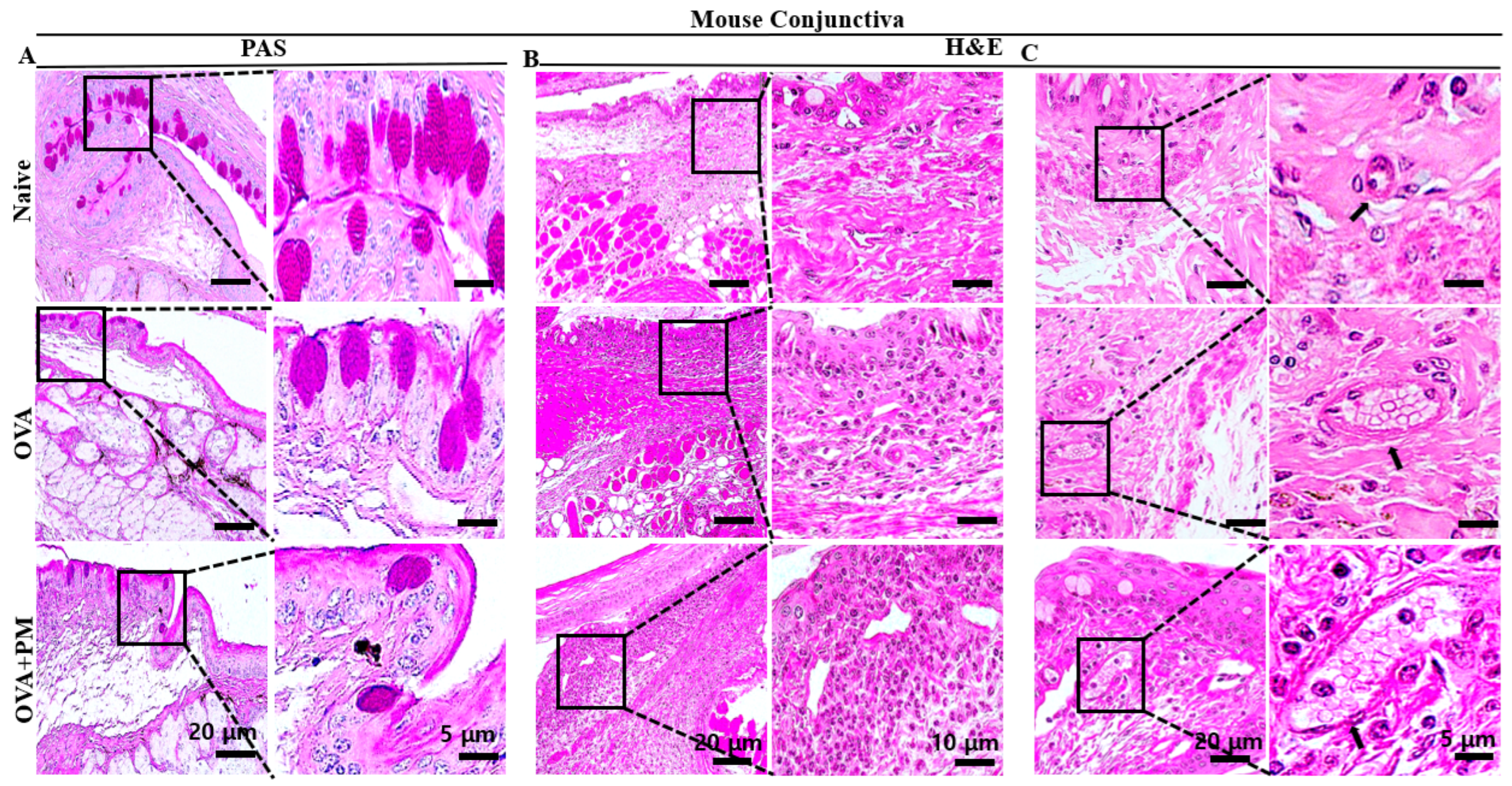
2.8. Periodic Acid-Schiff (PAS) Staining
2.9. TUNEL Assay
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. Effect of PM Exposure on Ocular Surface and Clinical Scores in an AED Mouse Model
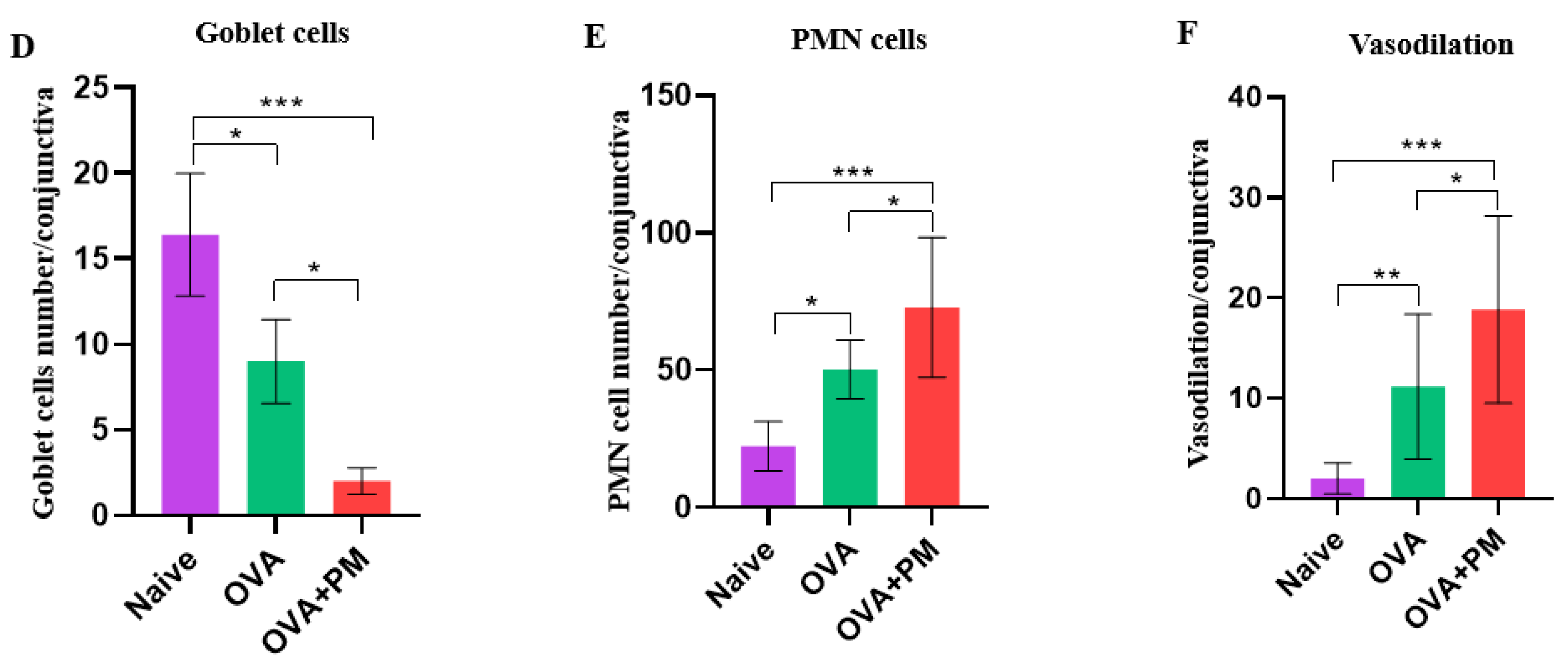
3.2. Effect of PM Exposure on Goblet Cells Numbers in Conjunctiva in an AED Mouse Model
3.3. Effect of PM Exposure on Polymorphonuclear Leukocytes Cell Infiltration in Conjunctiva in an AED Mouse Model
3.4. Effect of PM Exposure on Hemagiectasis in Conjunctiva in an AED Mouse Model
3.5. Effect of PM Exposure on IgE Serum Concentration in an AED Mouse Model
3.6. Effect of PM Exposure on Mast Cell Aggregation in Conjunctiva in an AED Mouse Model
3.7. Effect of PM Exposure on Substance P in Conjunctiva in an AED Mouse Model
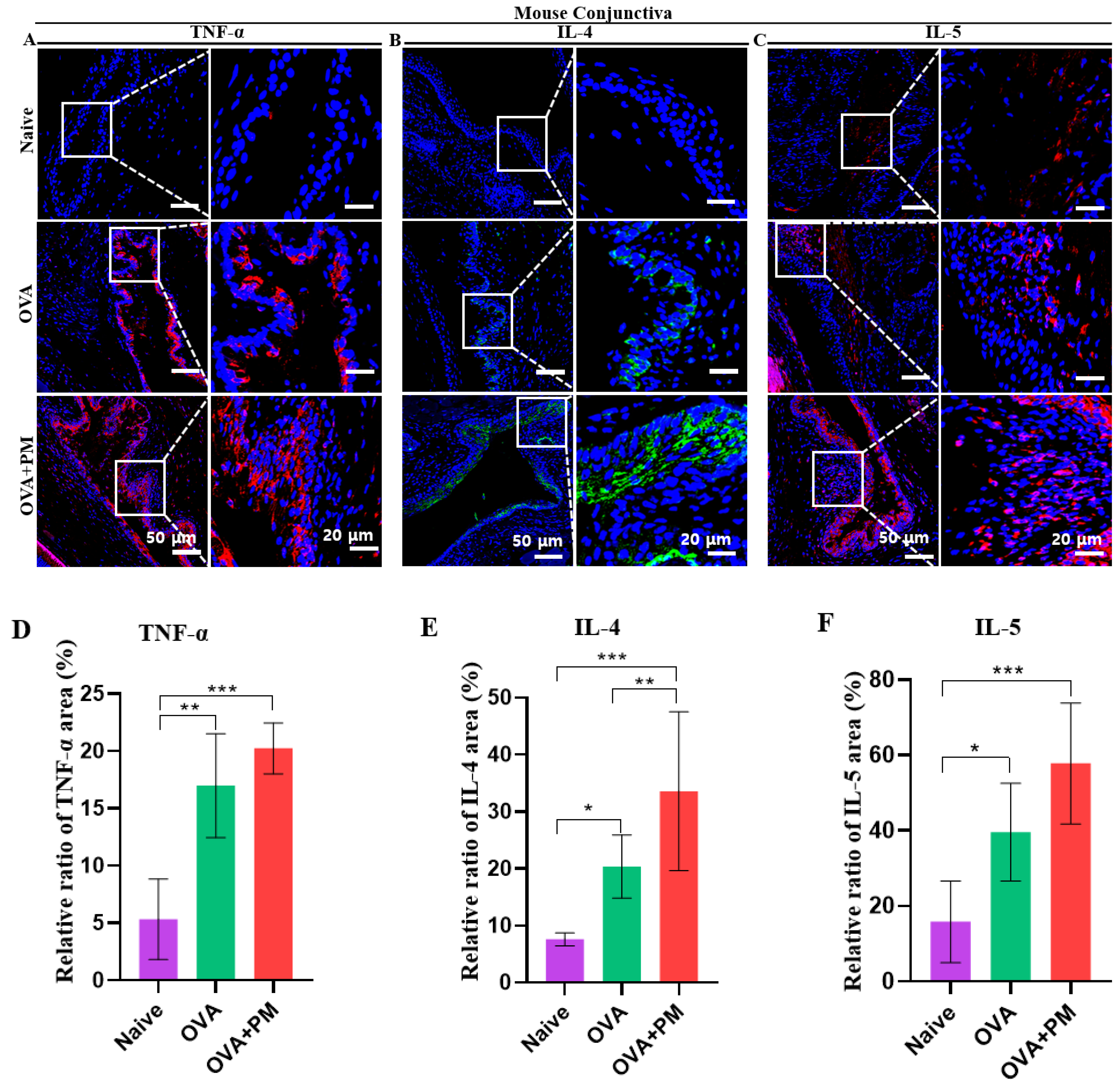
3.8. Effect of PM Exposure on Allergic Inflammatory Cytokines in Conjunctiva and Cornea in an AED Mouse Model
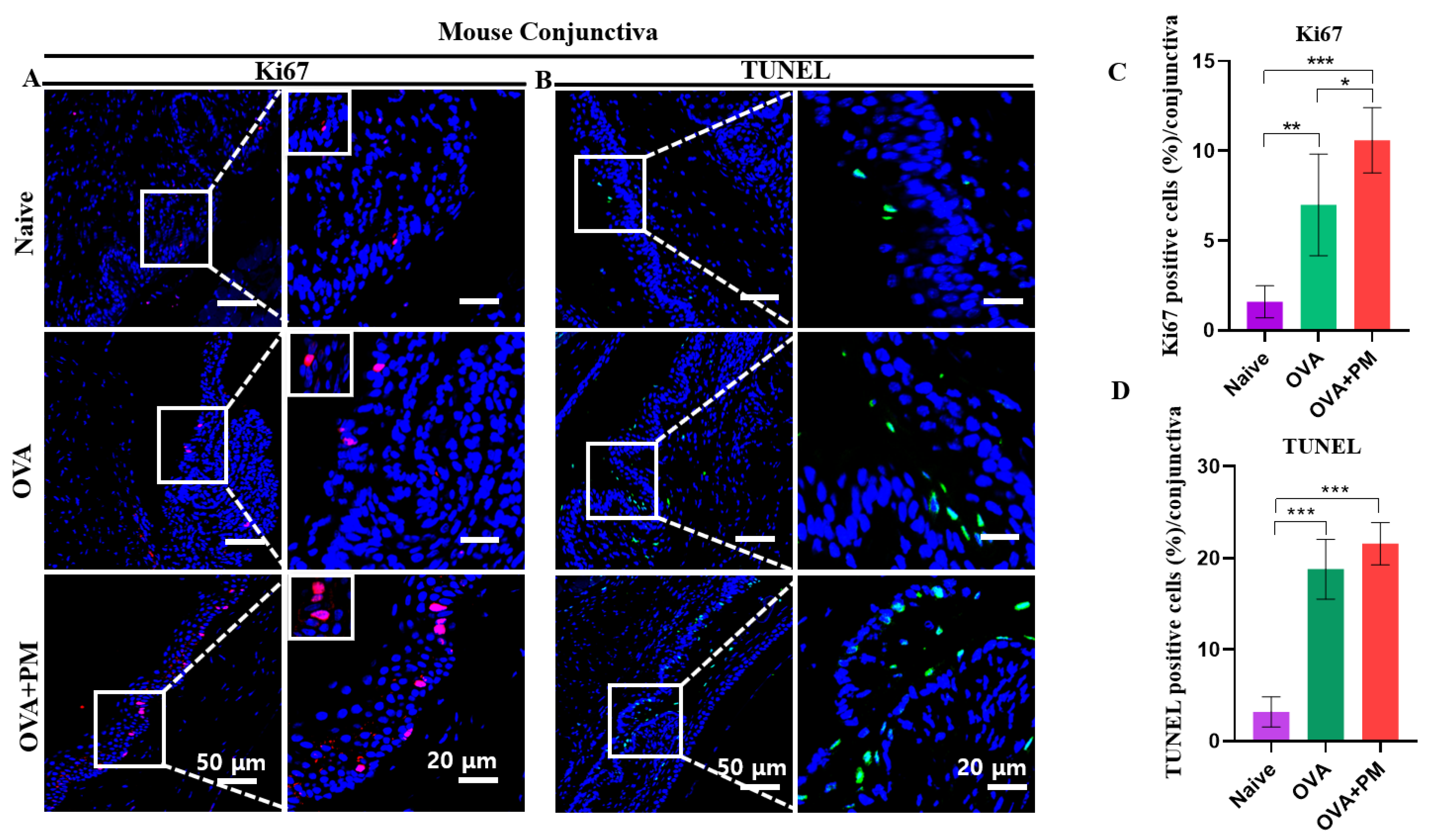
3.9. Effect of PM Exposure on Elevated Cell Proliferation in Conjunctiva and Cornea in an AED Mouse Model
3.10. Effect of PM Exposure on Cellular Apoptosis in Conjunctiva and Cornea in an AED Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | Particulate Matter |
| OVA | Ovalbumin |
| AED | Allergic Eye Disease |
| IgE | Immunoglobulin E |
| PMN | Polymorphonuclear Leukocytes |
| DED | Dry Eye Diseases |
| AC | Allergic Conjunctivitis |
| AKC | Atopic Keratoconjunctivitis |
| VKC | Vernal Keratoconjunctivitis |
| NF-κB | Nuclear Factor Kappa B |
| TNF-α | Tumor Necrosis Factor-Alpha |
| HO-1 | Heme Oxygenase-1 |
| IACUC | Institutional Animal Care and Use Committee |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| Il-4 | Interleukin 4 |
| IL-5 | Interleukin-5 |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) deoxyuridine triphosphate(dUTP) nick-end labeling |
References
- Li, J.; Tan, G.; Ding, X.; Wang, Y.; Wu, A.; Yang, Q.; Ye, L.; Shao, Y. A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed. Pharmacother. 2017, 96, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-J.; Chang, H.-H.; Chiang, C.-Y.; Lai, C.-Y.; Hsu, M.-Y.; Wang, K.-R.; Han, H.-H.; Chen, L.-Y.; Lin, D.P.-C. A murine model of acute allergic conjunctivitis induced by continuous exposure to particulate matter 2.5. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.-Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef]
- Saban, D.R.; Hodges, R.R.; Mathew, R.; Reyes, N.J.; Yu, C.; Kaye, R.; Swift, W.; Botten, N.; Serhan, C.N.; Dartt, D.A. Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model. Mucosal Immunol. 2019, 12, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-K.; Zhang, Q.; Qiu, Z.; Chung, K.F. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J. Thorac. Dis. 2015, 7, 23. [Google Scholar] [PubMed]
- Reyes, N.J.; Mathew, R.; Saban, D.R. Induction and characterization of the allergic eye disease mouse model. Type 2 Immun. Methods Protoc. 2018, 1799, 49–57. [Google Scholar]
- Nakazawa, Y.; Oka, M.; Takehana, M. Model for studying anti-allergic drugs for allergic conjunctivitis in animals. Open Med. 2017, 12, 231–238. [Google Scholar] [CrossRef]
- Hwang, M.; Han, S.; Seo, J.-W.; Jeon, K.-J.; Lee, H.S. Traffic-related particulate matter aggravates ocular allergic inflammation by mediating dendritic cell maturation. J. Toxicol. Environ. Health Part A 2021, 84, 661–673. [Google Scholar] [CrossRef]
- Bae, J.-S.; Oh, S.B.; Kim, J.; Kim, H.; Kim, J.H.; Kim, E.-H.; Cho, K.J.; Mo, J.-H. Particulate matter exposure aggravates IL-17-induced eye and nose inflammation in an OVA/Poly (I: C) mouse model. Allergy Asthma Immunol. Res. 2022, 14, 59. [Google Scholar] [CrossRef]
- Wu, J.-Z.; Ge, D.-D.; Zhou, L.-F.; Hou, L.-Y.; Zhou, Y.; Li, Q.-Y. Effects of particulate matter on allergic respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 95–102. [Google Scholar] [CrossRef] [PubMed]
- De Grove, K.; Provoost, S.; Brusselle, G.; Joos, G.; Maes, T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy 2018, 48, 773–786. [Google Scholar] [CrossRef]
- Sompornrattanaphan, M.; Thongngarm, T.; Ratanawatkul, P.; Wongsa, C.; Swigris, J.J. The contribution of particulate matter to respiratory allergy. Asian Pac. J. Allergy Immunol. 2020, 38, 19–28. [Google Scholar] [PubMed]
- Zheng, X.-Y.; Tong, L.; Shen, D.; Yu, J.-E.; Hu, Z.-Q.; Li, Y.-J.; Zhang, L.-J.; Xue, E.-F.; Tang, H.-F. Airborne bacteria enriched PM2.5 enhances the inflammation in an allergic adolescent mouse model induced by ovalbumin. Inflammation 2020, 43, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.R.; Vogel, C.F.; Bein, K.J.; Hughes, H.K.; Smiley-Jewell, S.; Pinkerton, K.E. Ambient particulate matter enhances the pulmonary allergic immune response to house dust mite in a BALB/c mouse model by augmenting Th2-and Th17-immune responses. Physiol. Rep. 2018, 6, e13827. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.A.; Kamal, A.S.; McGee, J.K.; Wood, C.E.; Dye, J.A.; Krantz, Q.T.; Landis, M.S.; Gilmour, M.I.; Gavett, S.H. Differential effects of particulate matter upwind and downwind of an urban freeway in an allergic mouse model. Environ. Sci. Technol. 2015, 49, 3930–3939. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.R.; Bein, K.J.; Smiley-Jewell, S.; Pinkerton, K.E. Fine particulate matter (PM2. 5) enhances allergic sensitization in BALB/c mice. J. Toxicol. Environ. Health Part A 2017, 80, 197–207. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM2. 5 exacerbates oxidative stress and inflammatory response through the Nrf2/NF-κB signaling pathway in OVA-induced allergic rhinitis mouse model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Bonini, S.; Vecchione, A.; Nairn, D.M.; Allansmith, M.R.; Balsano, F. Inflammatory changes in conjunctival scrapings after allergen provocation in humans. J. Allergy Clin. Immunol. 1988, 82, 462–469. [Google Scholar] [CrossRef]
- Fukuda, K.; Ohbayashi, M.; Morohoshi, K.; Zhang, L.; Liu, F.-T.; Ono, S.J. Critical role of IgE-dependent mast cell activation in a murine model of allergic conjunctivitis. J. Allergy Clin. Immunol. 2009, 124, 827–833. e2. [Google Scholar] [CrossRef]
- Chung, S.-H.; Choi, S.H.; Choi, J.A.; Chuck, R.S.; Joo, C.-K. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol. Vis. 2012, 18, 1966. [Google Scholar] [PubMed]
- McGill, J.; Holgate, S.; Church, M.; Anderson, D.; Bacon, A. Allergic eye disease mechanisms. Br. J. Ophthalmol. 1998, 82, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Metz, D.P.; Hingorani, M.; Calder, V.L.; Buckley, R.J.; Lightman, S.L. T-cell cytokines in chronic allergic eye disease. J. Allergy Clin. Immunol. 1997, 100, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Calder; Jolly; Hingorani; Adamson; Leonardi; Secchi; Buckley; Lightman. Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin. Exp. Allergy 1999, 29, 1214–1222. [Google Scholar] [CrossRef]
- Vitar, R.M.L.; Tau, J.; Reides, C.G.; Berra, A.; Ferreira, S.M.; Llesuy, S.F. Evaluation of oxidative stress markers in human conjunctival epithelial cells exposed to diesel exhaust particles (DEP). Investig. Ophthalmol. Vis. Sci. 2015, 56, 7058–7066. [Google Scholar] [CrossRef] [PubMed]
- Tau, J.; Novaes, P.; Matsuda, M.; Tasat, D.R.; Saldiva, P.H.; Berra, A. Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 2019, 117, 109177. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Choi, H.; Jang, G.; Lee, K.H.; Kim, E.; Kim, K.J.; Jeong, G.-Y.; Kim, J.S.; Na, C.-S.; Kim, S. Long-term exposure to urban particulate matter on the ocular surface and the incidence of deleterious changes in the cornea, conjunctiva and retina in rats. Int. J. Mol. Sci. 2020, 21, 4976. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Reyes, N.; Saban, D.R. Allergic eye disease (AED) progression in the mouse model is associated with quantitative changes in conjunctival innate lymphoid populations. Investig. Ophthalmol. Vis. Sci. 2016, 57, 311. [Google Scholar]
- Magone, M.T.; Chan, C.-C.; Rizzo, L.V.; Kozhich, A.T.; Whitcup, S.M. A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 1998, 87, 75–84. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef]
- Jung, S.J.; Mehta, J.S.; Tong, L. Effects of environment pollution on the ocular surface. Ocul. Surf. 2018, 16, 198–205. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, S.; Seo, J.-W.; Jeon, K.-J. Exposure to traffic-related particulate matter 2.5 triggers Th2-dominant ocular immune response in a murine model. Int. J. Environ. Res. Public Health 2020, 17, 2965. [Google Scholar] [CrossRef]
- Cousins, S.; Rouse, B. Chemical mediators of ocular inflammation. In Ocular Infection and Immunity; Mosby: St Louis, MO, USA, 1996; pp. 50–70. [Google Scholar]
- De Gaulle, I.C. The pathophysiology of ocular allergy: A review. Contact Lens Anterior Eye 2009, 32, 3–15. [Google Scholar]
- Gu, Y.; Hao, S.; Liu, K.; Gao, M.; Lu, B.; Sheng, F.; Zhang, L.; Xu, Y.; Wu, D.; Han, Y. Airborne fine particulate matter (PM 2.5) damages the inner blood–retinal barrier by inducing inflammation and ferroptosis in retinal vascular endothelial cells. Sci. Total Environ. 2022, 838, 156563. [Google Scholar] [CrossRef]
- Upaphong, P.; Thonusin, C.; Wanichthanaolan, O.; Chattipakorn, N.; Chattipakorn, S.C. Consequences of exposure to particulate matter on the ocular surface: Mechanistic insights from cellular mechanisms to epidemiological findings. Environ. Pollut. 2024, 345, 123488. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Orona, N.; Villalon, L.; Saldiva, P.; Tasat, D.R.; Berra, A. Air particulate matter exacerbates lung response on Sjögren’s Syndrome animals. Exp. Toxicol. Pathol. 2015, 67, 125–131. [Google Scholar] [CrossRef]
- Shkirkova, K.; Lamorie-Foote, K.; Connor, M.; Patel, A.; Barisano, G.; Baertsch, H.; Liu, Q.; Morgan, T.E.; Sioutas, C.; Mack, W.J. Effects of ambient particulate matter on vascular tissue: A review. J. Toxicol. Environ. Health Part B 2020, 23, 319–350. [Google Scholar] [CrossRef] [PubMed]
- Cherng, T.W.; Paffett, M.L.; Jackson-Weaver, O.; Campen, M.J.; Walker, B.R.; Kanagy, N.L. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 2011, 119, 98–103. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.K.; Kim, H.Y.; Kim, T.-i. Effects of exposure to ozone on the ocular surface in an experimental model of allergic conjunctivitis. PLoS ONE 2017, 12, e0169209. [Google Scholar]
- Leonardi, A. The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr. Allergy Asthma Rep. 2002, 2, 325–331. [Google Scholar] [CrossRef]
- Solomon, A.; Pe’er, J.; Levi-Schaffer, F. Advances in ocular allergy: Basic mechanisms, clinical patterns and new therapies. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, B.; Oortgiesen, M. Neurogenic inflammation and particulate matter (PM) air pollutants. Neurotoxicology 2001, 22, 795–810. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, L.; Shen, M.; Wang, Y.; Wei, Y.; Jeyalatha, V.; Chen, P.; Dong, F.; Wang, G.; Wu, S. Effects of diesel exhaust particles on the condition of mouse ocular surface. Ecotoxicol. Environ. Saf. 2018, 163, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Kang, S.; Anderson, H.; Mills, I.; Walton, H. Epidemiological time series studies of PM2. 5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Hoek, G.; van Den Brandt, P.A.; Goldbohm, R.A.; Fischer, P.; Schouten, L.J.; Jerrett, M.; Hughes, E.; Armstrong, B.; Brunekreef, B. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ. Health Perspect. 2008, 116, 196–202. [Google Scholar] [CrossRef]
- Lyu, D.; Chen, Z.; Almansoob, S.; Chen, H.; Ye, Y.; Song, F.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H. Transcriptomic profiling of human corneal epithelial cells exposed to airborne fine particulate matter (PM 2.5). Ocul. Surf. 2020, 18, 554–564. [Google Scholar] [CrossRef]
- Mimura, T.; Ichinose, T.; Yamagami, S.; Fujishima, H.; Kamei, Y.; Goto, M.; Takada, S.; Matsubara, M. Airborne particulate matter (PM2. 5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 2014, 487, 493–499. [Google Scholar] [CrossRef]
- Li, L.; Xing, C.; Zhou, J.; Niu, L.; Luo, B.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM 2.5) triggers ocular hypertension and glaucoma through pyroptosis. Part. Fibre Toxicol. 2021, 18, 10. [Google Scholar] [CrossRef]
- Park, E.-J.; Chae, J.-B.; Lyu, J.; Yoon, C.; Kim, S.; Yeom, C.; Kim, Y.; Chang, J. Ambient fine particulate matters induce cell death and inflammatory response by influencing mitochondria function in human corneal epithelial cells. Environ. Res. 2017, 159, 595–605. [Google Scholar] [CrossRef]
- Steerenberg, P.; Withagen, C.; Van Dalen, W.; Dormans, J.; Cassee, F.; Heisterkamp, S.; Van Loveren, H. Adjuvant activity of ambient particulate matter of different sites, sizes, and seasons in a respiratory allergy mouse model. Toxicol. Appl. Pharmacol. 2004, 200, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Diaz-Sanchez, D.; Li, N. The role of particulate pollutants in pulmonary inflammation and asthma: Evidence for the involvement of organic chemicals and oxidative stress. Curr. Opin. Pulm. Med. 2001, 7, 20–26. [Google Scholar] [CrossRef]
- Fujishima, H.; Satake, Y.; Okada, N.; Kawashima, S.; Matsumoto, K.; Saito, H. Effects of diesel exhaust particles on primary cultured healthy human conjunctival epithelium. Ann. Allergy Asthma Immunol. 2013, 110, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.P.; Lee, H.J.; Lee, D.-U.; Lee, S.K.; Hong, J.-H.; Lee, C.J. Effects of lupenone, lupeol, and taraxerol derived from Adenophora triphylla on the gene expression and production of airway MUC5AC mucin. Tuberc. Respir. Dis. 2015, 78, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Somayajulu, M.; McClellan, S.A.; Wright, R.; Pitchaikannu, A.; Croniger, B.; Zhang, K.; Hazlett, L.D. Airborne Exposure of the Cornea to PM10 Induces Oxidative Stress and Disrupts Nrf2 Mediated Anti-Oxidant Defenses. Int. J. Mol. Sci. 2023, 24, 3911. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kalogeromitros, D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Hines, C. The diverse effects of mast cell mediators. Clin. Rev. Allergy Immunol. 2002, 22, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Rajewsky, K.; Müller, W. Generation and analysis of interleukin-4 deficient mice. Res. Immunol. 1993, 144, 637–638. [Google Scholar] [CrossRef]
- Miyazaki, D.; Tominaga, T.; Yakura, K.; Kuo, C.-H.; Komatsu, N.; Inoue, Y.; Ono, S.J. Conjunctival mast cell as a mediator of eosinophilic response in ocular allergy. Mol. Vis. 2008, 14, 1525. [Google Scholar] [PubMed]
- Sánchez, M.; Fernández Parra, B.; Matheu, V.; Navarro, A.; Ibáñez, M.; Dávila, I.; Dordal, M.; Lluch Bernal, M.; Rondón, C.; Montoro, J. Allergic conjunctivitis. J. Investig. Allergol. Clin. Immunol. 2011, 21 (Suppl. S2), 1–19. [Google Scholar]
- Chen, J.; Zhang, J.; Zhao, R.; Jin, J.; Yu, Y.; Li, W.; Wang, W.; Zhou, H.; Su, S.B. Topical application of interleukin-28A attenuates allergic conjunctivitis in an ovalbumin-induced mouse model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Furdova, A.; Czanner, G.; Koller, J.; Vesely, P.; Furda, R.; Pridavkova, Z. Amniotic membrane application in surgical treatment of conjunctival tumors. Sci. Rep. 2023, 13, 2835. [Google Scholar] [CrossRef] [PubMed]
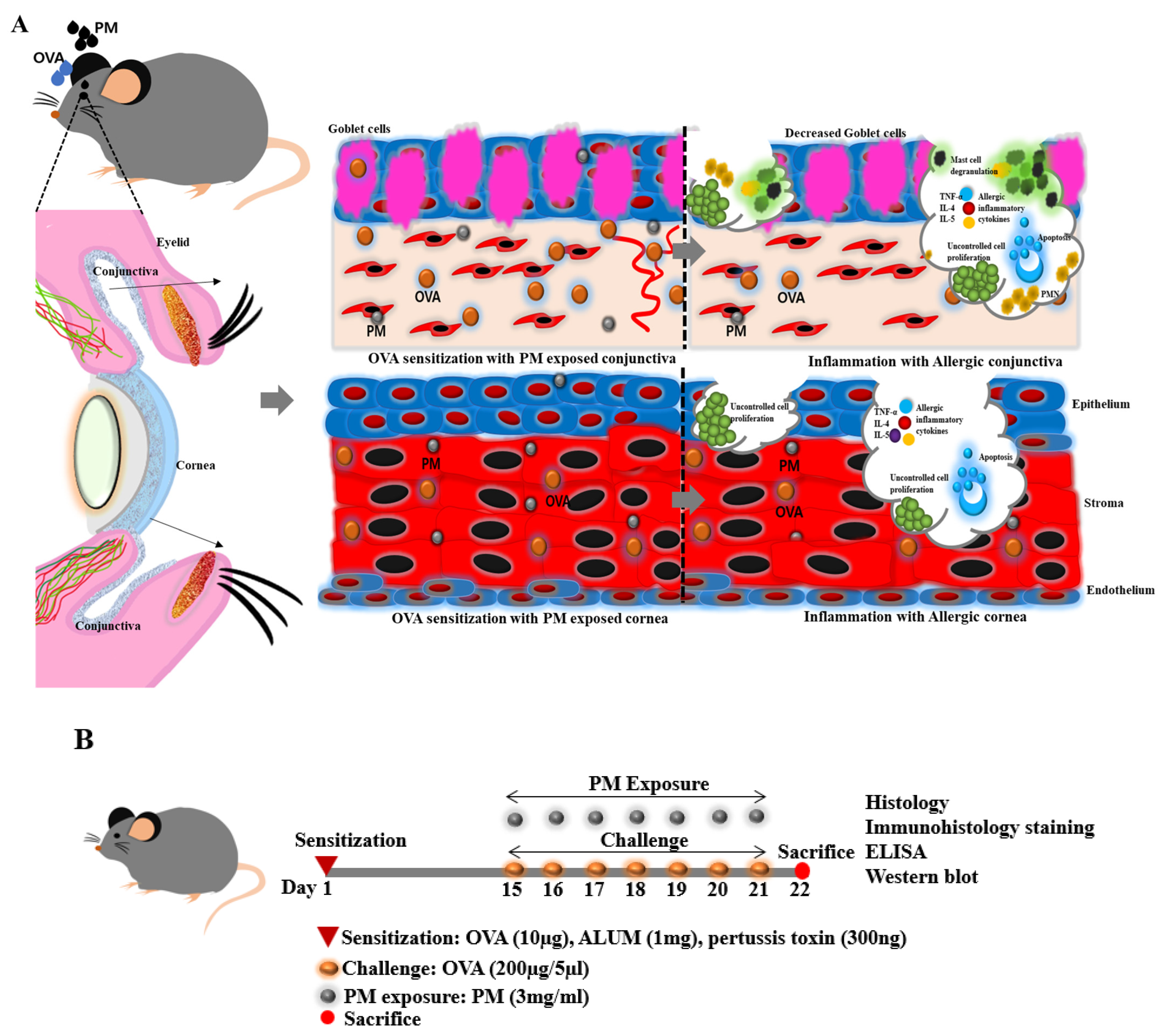
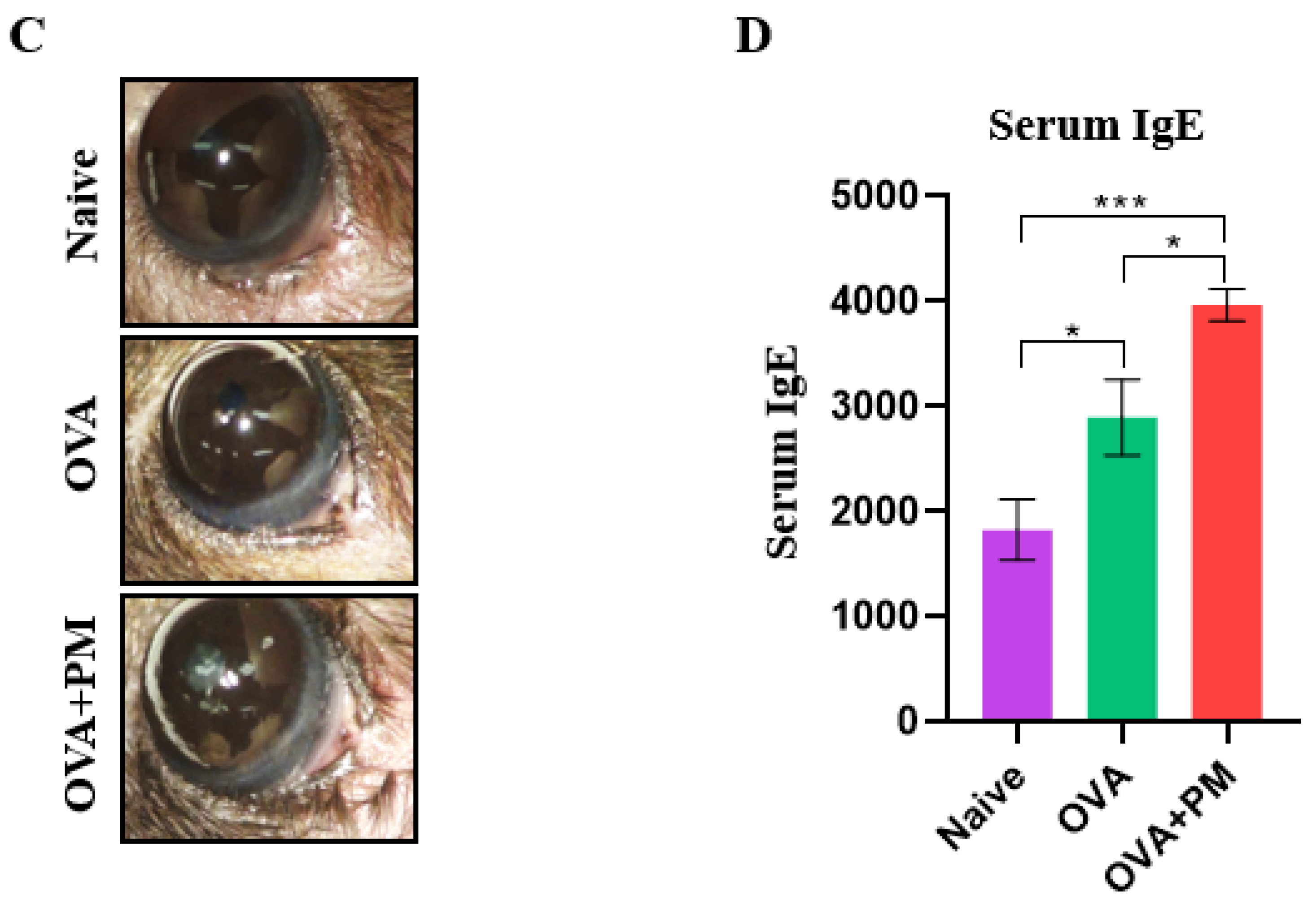


| Score Symptoms | Naive | OVA | OVA + PM |
|---|---|---|---|
| Conjuctival chemosis | 0.05 (0.15) | 1.33 (1.00) | 2.31 (1.10) |
| Conjuctival hyperemia | 0.08 (0.17) | 1.67 (0.71) | 2.54 (1.02) |
| Eyelid edema | 0.10 (0.14) | 1.72 (0.75) | 2.67 (1.41) |
| Tearing and discharge | 0.04 (0.06) | 1.54 (0.99) | 2.56 (1.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhujel, B.; Oh, S.; Hur, W.; Lee, S.; Chung, H.S.; Lee, H.; Park, J.H.; Kim, J.Y. Effect of Exposure to Particulate Matter on the Ocular Surface in an Experimental Allergic Eye Disease Mouse Model. Bioengineering 2024, 11, 498. https://doi.org/10.3390/bioengineering11050498
Bhujel B, Oh S, Hur W, Lee S, Chung HS, Lee H, Park JH, Kim JY. Effect of Exposure to Particulate Matter on the Ocular Surface in an Experimental Allergic Eye Disease Mouse Model. Bioengineering. 2024; 11(5):498. https://doi.org/10.3390/bioengineering11050498
Chicago/Turabian StyleBhujel, Basanta, Seheon Oh, Woojune Hur, Seorin Lee, Ho Seok Chung, Hun Lee, Jin Hyoung Park, and Jae Yong Kim. 2024. "Effect of Exposure to Particulate Matter on the Ocular Surface in an Experimental Allergic Eye Disease Mouse Model" Bioengineering 11, no. 5: 498. https://doi.org/10.3390/bioengineering11050498
APA StyleBhujel, B., Oh, S., Hur, W., Lee, S., Chung, H. S., Lee, H., Park, J. H., & Kim, J. Y. (2024). Effect of Exposure to Particulate Matter on the Ocular Surface in an Experimental Allergic Eye Disease Mouse Model. Bioengineering, 11(5), 498. https://doi.org/10.3390/bioengineering11050498







