Neurospora sp. Mediated Synthesis of Naringenin for the Production of Bioactive Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Neurospora Isolates
2.2. Fungus Slide Culture and Staining and Molecular DNA Sequencing Identification
2.3. Screening of Naringinase-Producing Neurospora Strain
2.4. Production of Naringinase Enzyme by Isolated Neurospora sp.
2.5. Biotransformation of Naringin to Naringenin by Neurospora sp.
2.6. Identification and Confirmation of Biotransformed Naringenin by Thin Layer Chromatography (TLC), 1H NMR and UV-Vis Spectroscopy
2.6.1. Thin Layer Chromatography (TLC) and 1H NMR
2.6.2. Habelt and Pittner Spectrophotometric Method
2.7. Preparation of Naringenin-Nano Silver (Ag) and Gold (Au) Conjugates and Their Characterization
2.8. Combinational Antibacterial Studies of Naringenin and Naringenin-Nano Ag and Au Conjugates
2.9. In Vitro Nematicidal Activity
3. Results
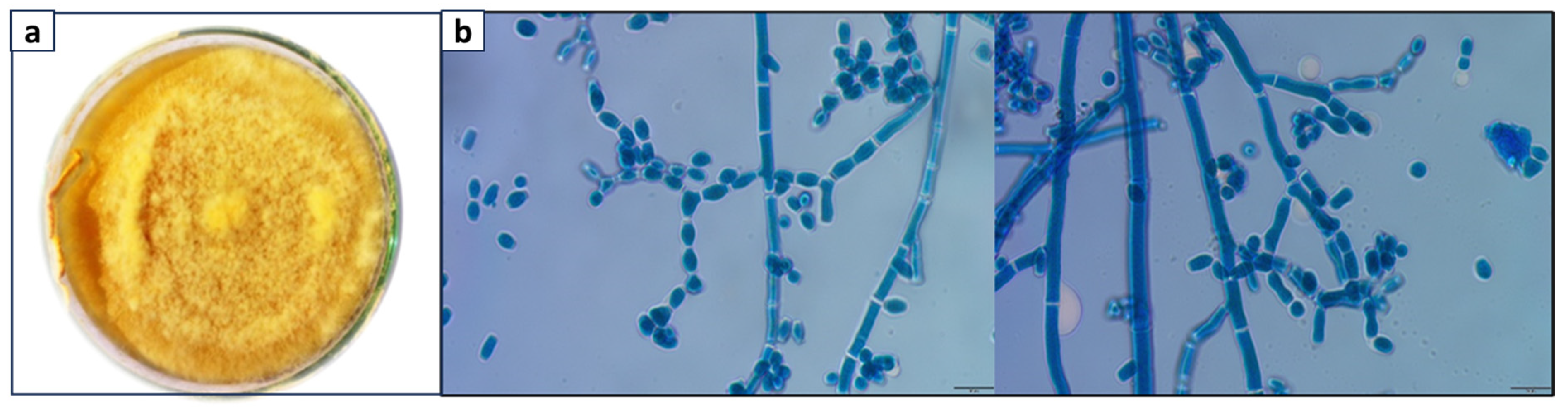
3.1. Identification of the Naringinase-Producing Fungus Isolate
3.2. Neurospora sp. Mediated Biotransformation of Naringin to Naringenin
3.3. Thin Layer Chromatography and NMR Analysis
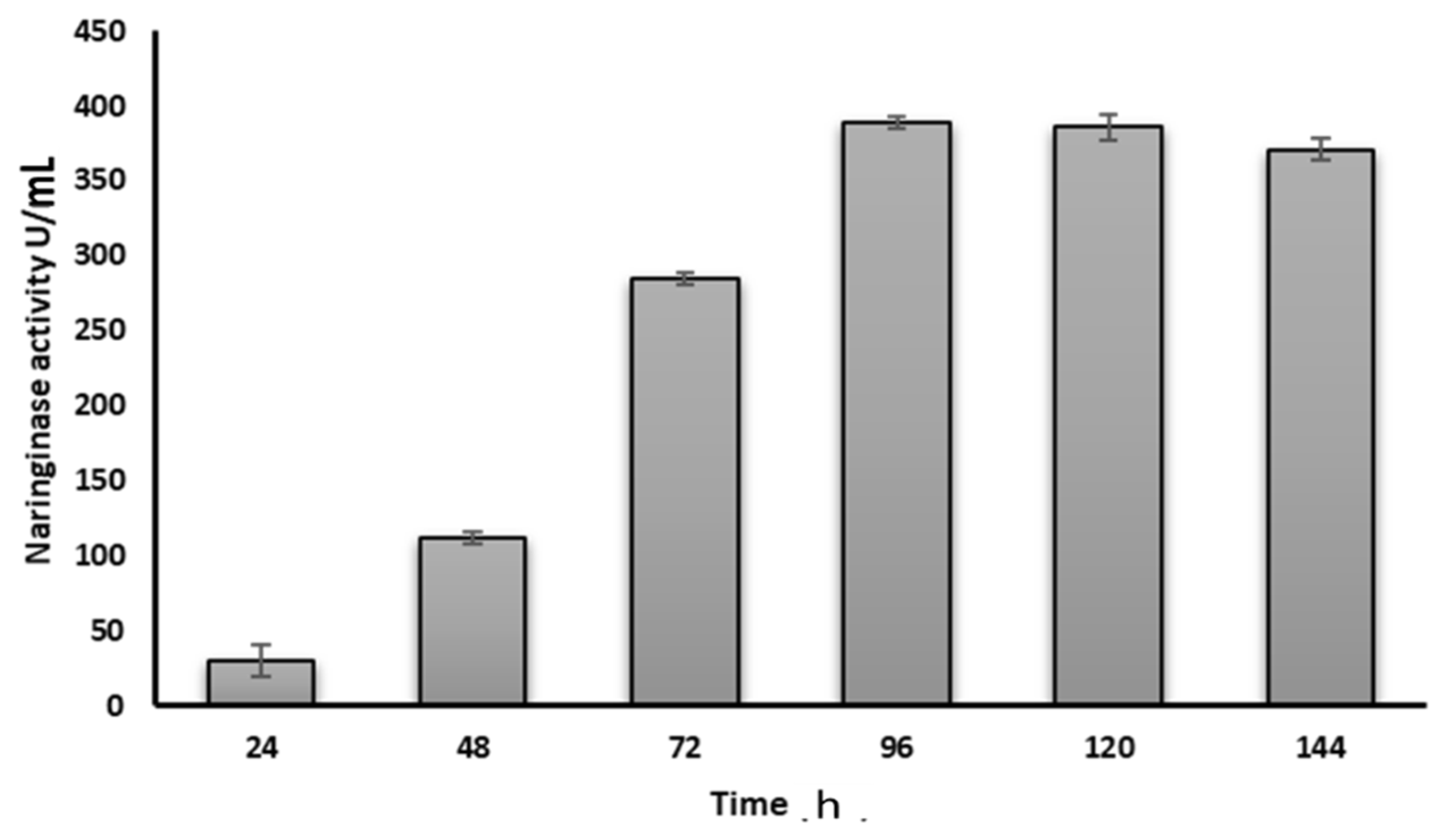
3.4. Production of Naringinase Enzyme by Neurospora sp. Utilizing Orange Peels
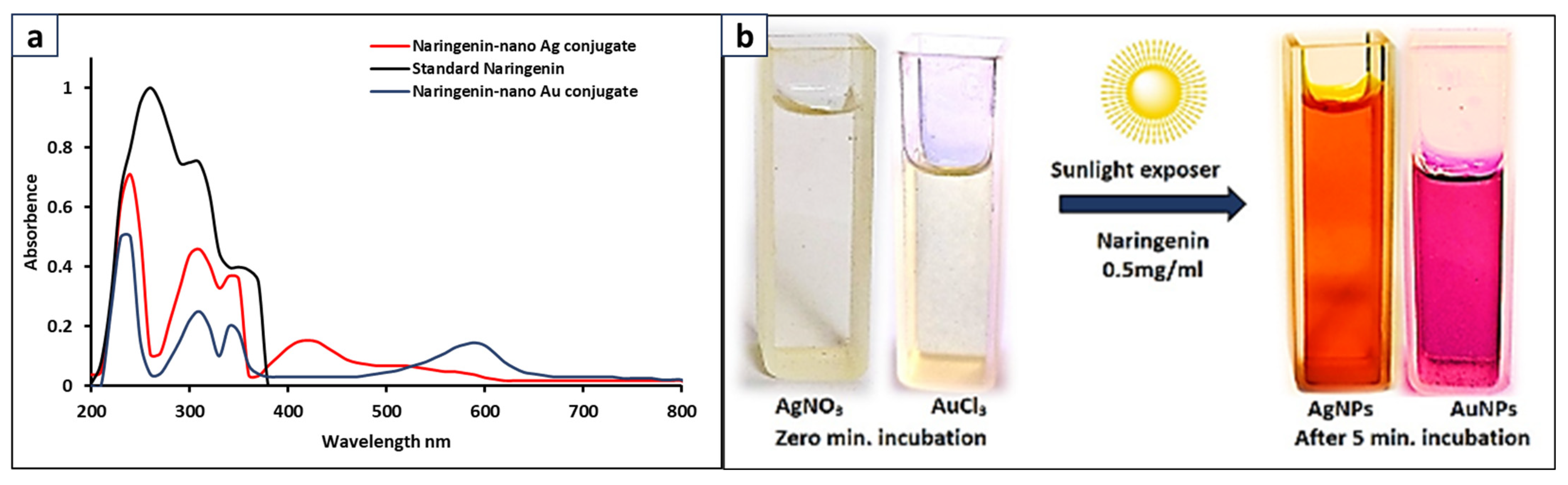
3.5. Synthesis of Naringenin-Nano Ag and Au Conjugates
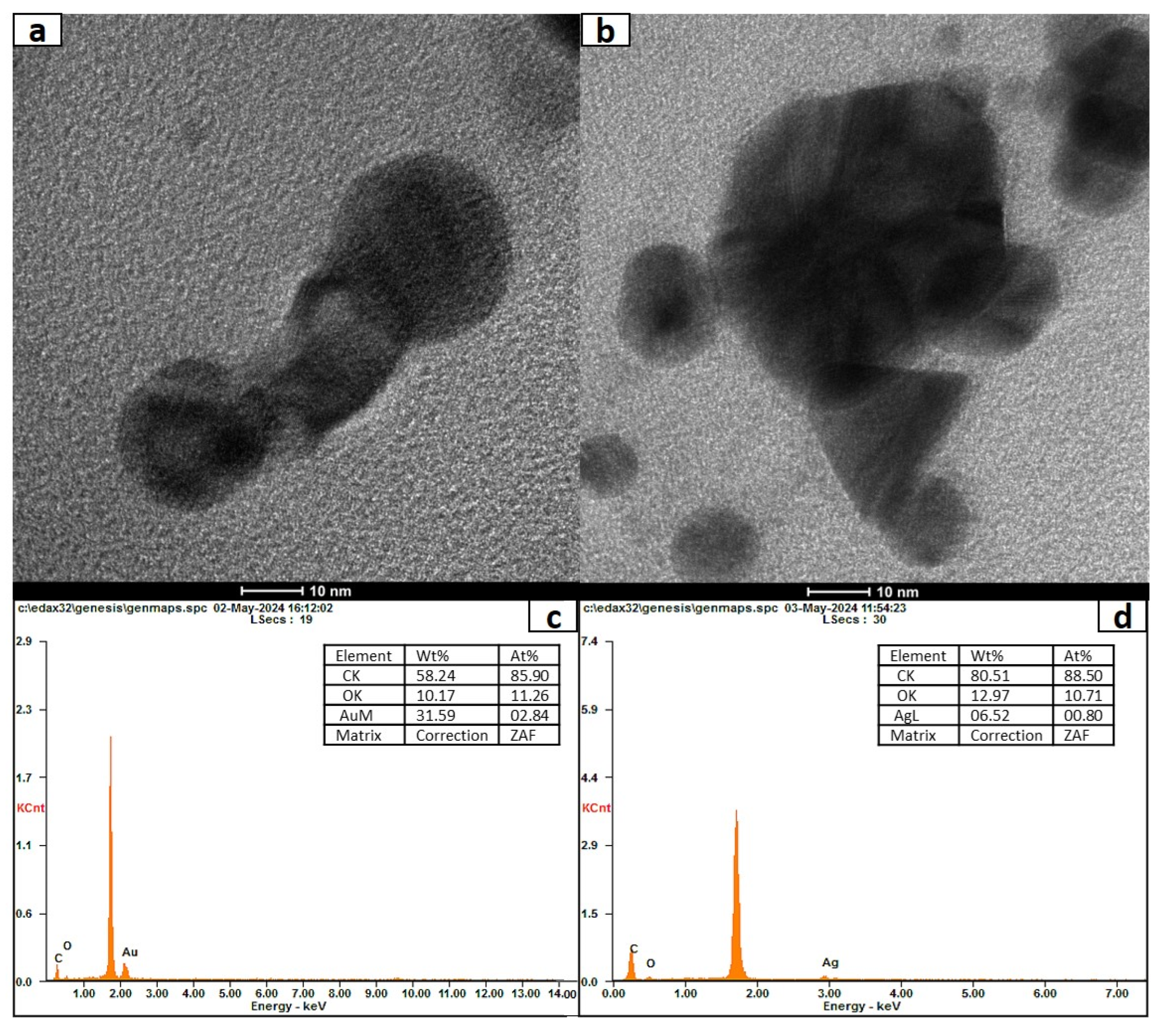
3.6. Characterization of Nanoconjugates of Ag and Au with Naringenin
3.7. Antibacterial Activity of Synthesized Naringenin-Nano Ag and Au Conjugates
3.8. Nematicidal Activity of Biotransformed Product Naringenin and the Naringenin-Nano Ag and Au Conjugates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, A flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Bojan Magesh, S.; Mohanram Ramkumar, K.; Suryanarayanan, S.; Venkata SubbaRao, M. Antioxidant Potential of Naringenin Helps to Protect Liver Tissue from Streptozotocin-Induced Damage. Rep. Biochem. Mol. Biol. 2018, 7, 76–84. [Google Scholar] [PubMed] [PubMed Central]
- Celiz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Memariani, Z.; Abbas, S.Q.; Ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.H.; Shin, Y.W.; Bae, K.H.; Jeong, T.S.; Kwon, Y.K.; Park, Y.B.; Choi, M.S. Effects of naringin and lovastatin on plasma and hepatic lipids in high-fat and high-cholesterol fed rats. Nutr. Res. 2000, 20, 1007–1015. [Google Scholar] [CrossRef]
- Puri, M.; Banerjee, U.C. Production, purification, and characterization of the debittering enzyme naringinase. Biotechnol. Adv. 2000, 18, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Álvarez, R.; Botas, A.; Albillos, S.M.; Rumbero, A.; Martín, J.F.; Liras, P. Molecular genetics of naringenin biosynthesis, a typical plant secondary metabolite produced by Streptomyces clavuligerus. Microb. Cell Factories 2015, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Li, X.; Liu, D.; Dong, X.T.; Li, F.F.; Wang, E.X.; Li, B.Z.; Yuan, Y.J. Production of naringenin from D-xylose with co-culture of E. coli and S. cerevisiae. Eng. Life Sci. 2017, 17, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.D.; Davis, R.H. Evidence for safety of Neurospora species for academic and commercial uses. Appl. Environ. Microbiol. 2000, 66, 5107–5109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.P.; Abraham, A. PVP-coated naringenin nanoparticles for biomedical applications. Chem. Biol. Interact. 2016, 257, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Devel. Ther. 2016, 1, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of naringin and naringenin in different solvents and dissociation constants of naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, J.D.; Mohite, B.V.; Patil, S.V. Naringenin biosynthesis and fabrication of naringenin mediated nano silver conjugate for antimicrobial potential. Nat. Prod. Res. 2022, 37, 3184–3190. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Valadez, R.; Raffaelli, S.; Estevez, M.B.; Pardo, H.; Alborés, S. Biogenic Silver Nanoparticles Conjugated with Nisin: Improving the Antimicrobial and Antibiofilm Properties of Nanomaterials. Chem 2021, 3, 1271–1285. [Google Scholar] [CrossRef]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Pierce, S.; Angeles-Boza, A.M.; Krishna, V.; Rodriguez-Reyes, J.C.F. Enhanced antimicrobial activity of silver nanoparticles conjugated with synthetic peptide by click chemistry. J. Nanoparticle Res. 2020, 22, 90. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.M.; Abdelmoneim, T.S.; Elazzazy, A.M. The impact of silver nanoparticles produced by Bacillus pumilus as antimicrobial and nematicide. Front. Microbiol. 2016, 7, 1746. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, W.A.; Yang, J.; Starr, J.L.; Jo, Y.K. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J. Nematol. 2014, 46, 261. [Google Scholar]
- Thakur, R.K.; Dhirta, B.; Shirkot, P. Studies on effect of gold nanoparticles on Meloidogyne incognita and tomato plants growth and development. BioRxiv 2018. [Google Scholar] [CrossRef]
- Akhter, G.; Khan, A.; Ali, S.G.; Khan, T.A.; Siddiqi, K.S.; Khan, H.M. Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holoparasitic plant. SN Appl. Sci. 2020, 2, 1268. [Google Scholar] [CrossRef]
- Vogel, H.J. Distribution of lysine pathways among fungi: Evolutionary implications. Am. Nat. 1964, 98, 435–446. [Google Scholar] [CrossRef]
- Booth, C. (Ed.) Methods in Microbiology; Academic Press: London, UK, 1971; Volume 4. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Habelt, K.; Pittner, F. A rapid method for the determination of naringin, prunin, and naringenin applied to the assay of naringinase. Anal. Biochem. 1983, 134, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaprakash, M.; Vinoth Kumar, V.; Hemalatha, M.; Melbia, V.; Karthik, P. Solid-state fermentation for the production of debittering enzyme naringinase using Aspergillus niger MTCC 1344. Eng. Life Sci. 2011, 11, 322–325. [Google Scholar] [CrossRef]
- Caraveo, L.; Medina, H.; Rodríguez-Buenfil, I.; Montalvo-Romero, C.; Evangelista-Martínez, Z. A simple plate-assay for screening extracellular naringinase produced by streptomycetes. Microbiol. Methods 2014, 102, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Roitner, M.; Schalkhammer, T.; Pittner, F. Characterisation of naringinase from Aspergillus niger. Monatsh. Chem. 1984, 115, 1255–1267. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- CLDI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020.
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, M.; Kalaivani, R.; Manikandan, S.; Kumaraguru, A.K. Metallic silver nanoparticle: A therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioprocess Biosyst. Eng. 2013, 36, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Kim, J.; Shin, S.; Park, I. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchusxylophilus). Russ. J. Nematol. 2007, 15, 35. [Google Scholar]
- Salunkhe, J.D.; Patil, S.V. Improved naringinase double screen plate assay: Progress towards the perfect screening. Nat. Prod. Res. 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chemical Book. 2017. Available online: https://www.chemicalbook.com/SpectrumEN_67604-48-2_1HNMR.htm (accessed on 18 February 2023).
- Din, S.; Hamid, S.; Yaseen, A.; Yatoo, A.M.; Ali, S.; Shamim, K.; Mahdi, W.A.; Alshehri, S.; Rehman, M.U.; Shah, W.A. Isolation and Characterization of Flavonoid Naringenin and Evaluation of Cytotoxic and Biological Efficacy of Water Lilly (Nymphaea mexicana Zucc.). Plants 2022, 11, 3588. [Google Scholar] [CrossRef] [PubMed]
- Cordenonsi, L.M.; Sponchiado, R.M.; Campanharo, S.C.; Garcia, C.V.; Raffin, R.P.; Schapoval, E.E. Study of flavonoids present in Pomelo (Citrus máxima) by DSC, UV-VIS, IR, 1H and 13C NMR and MS. Drug Anal. Res. Porto Alegre 2017, 1, 31–37. [Google Scholar] [CrossRef]
- Goyal, L.; Kaushal, S.; Dhillon, N.K.; Heena. Nematicidal potential of Citrus reticulata peel essential oil, isolated major compound and its derivatives against Meloidogyne incognita. Arch. Phytopathol. Plant Pract. 2021, 54, 449–467. [Google Scholar] [CrossRef]
- Patil, S.V.; Koli, S.H.; Mohite, B.V.; Patil, R.P.; Patil, R.R.; Borase, H.P.; Patil, V.S. A novel screening method for potential naringinase-producing microorganisms. Biotechnol. Appl. Biochem. 2019, 66, 323–327. [Google Scholar] [PubMed]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Naringinase Biosynthesis by Aspergillus niger on an Optimized Medium Containing Red Grapefruit Albedo. Molecules 2022, 27, 8763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jia, H.; Xi, M.; Li, J.; Yang, L.; Li, X. Characterization of a naringinase from Aspergillus oryzae 11250 and its application in the debitterization of orange juice. Process Biochem. 2017, 62, 114–121. [Google Scholar] [CrossRef]
- Chen, D.; Niu, T.; Cai, H. Optimizing culture medium for debittering constitutive enzyme naringinase production by Aspergillus oryzae JMU316. African J. Biotechnol. 2010, 9, 4970–4978. [Google Scholar]
- Salehi, B.; Fokou, P.V.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Park, G.H.; Eo, H.J.; Lee, J.W.; Kim, M.K.; Lee, J.R.; Lee, M.H.; Koo, J.S.; Jeong, J.B. Anti-proliferative effect of naringenin through p38-dependent downregulation of cyclin D1 in human colorectal cancer cells. Biomol. Ther. 2015, 23, 339. [Google Scholar] [CrossRef] [PubMed]
- Borase, H.P.; Patil, C.D.; Sauter, I.P.; Rott, M.B.; Patil, S.V. Amoebicidal activity of phytosynthesized silver nanoparticles and their in vitro cytotoxicity to human cells. FEMS Microbiol. Lett. 2013, 345, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Gade, A.; Rai, M. Large-scale synthesis and antibacterial activity of fungal-derived silver nanoparticles. Environ. Chem. Lett. 2016, 15, 427–434. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Składanowski, M.; Wypij, M.; Laskowski, D.; Golińska, P.; Dahm, H.; Rai, M. Silver and gold nanoparticles synthesized from Streptomyces sp. isolated from acid forest soil with special reference to its antibacterial activity against pathogens. J. Clust. Sci. 2017, 28, 59–79. [Google Scholar] [CrossRef]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.B.; Raza Shah, M.; Khan, N.A. Silver nanoparticle conjugation-enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Halawani, E.M.; Hassan, A.M.; El-Rab, S.M.G. Nanoformulation of biogenic cefotaxime-conjugated-silver nanoparticles for enhanced antibacterial efficacy against multidrug-resistant bacteria and anticancer studies. Int. J. Nanomed 2020, 15, 1889. [Google Scholar] [CrossRef]
- Lim, D.; Roh, J.-Y.; Eom, H.-J.; Hyun, J.W.; Choi, J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef]

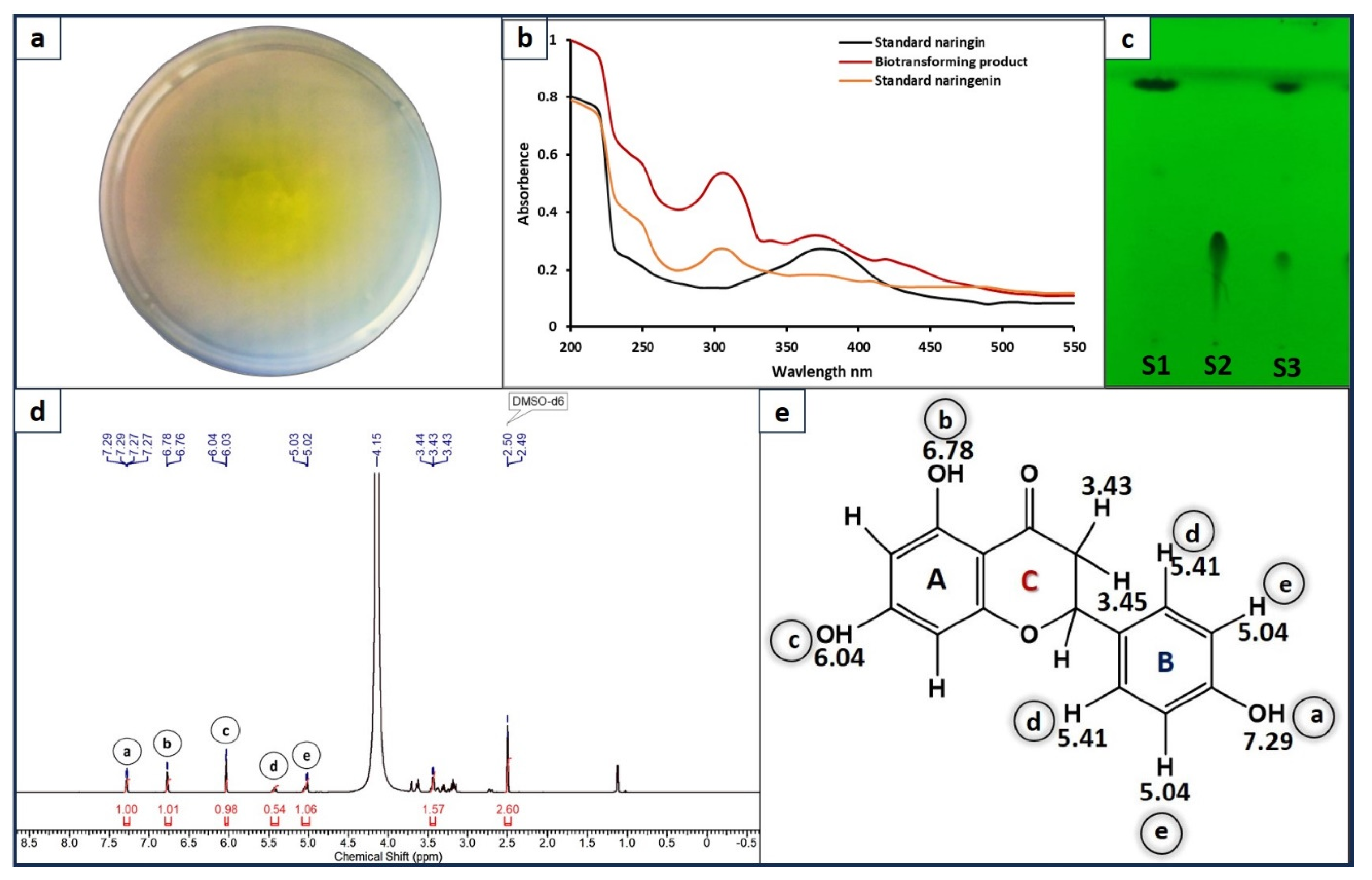

| Peak | Shift (ppm) |
|---|---|
| a | 7.29 |
| b | 6.78 |
| c | 6.04 |
| d | 5.41 |
| e | 5.04 |
| Tested Microorganism | S. aureus | B. subtilis | E. coli | P. aeruginosa |
|---|---|---|---|---|
| ZOI for naringenin (in mm) | 6.8 ± 0.17 | 6.6 ± 0.31 | 6.4 ± 0.19 | 4.7 ± 0.24 |
| ZOI for naringenin-nano Au conjugate (in mm) | 7.2 ± 0.26 | 7.0 ± 0.38 | 7.1 ± 0.29 | 5.0 ± 0.25 |
| ZOI for naringenin–Ag nanoconjugate (in mm) | 14.8 ± 0.35 | 12.8 ± 0.28 | 14.2 ± 0.23 | 14.3 ± 0.21 |
| ZOI for AgNO3 (in mm) | 6.2 | 6.4 | 7.3 | 5.9 |
| ZOI for AuCl3 (in mm) | Nil | Nil | Nil | Nil |
| Fold increase in antimicrobial activity (naringenin–Au nanoconjugate) | 0.126 | 0.124 | 0.282 | 0.131 |
| Fold increase in antimicrobial activity (naringenin–Ag nanoconjugate) | 3.737 | 2.761 | 3.922 | 8.251 |
| Test Products | LC50 ± SD (mg L−1) | 95% Fiducial Limits | LC90 ± SD (mg L−1) | 95% Fiducial Limits | Regression Equation |
|---|---|---|---|---|---|
| Naringenin | 88.72 ± 2.53 | 83.71–93.71 | 186.01 ± 6.21 | 174.98–199.62 | Y = 4.78 + 0.320 X |
| Std. (CuSO4) | 41.03 ± 2.51 | 107.35–2.89 | 102.06 ± 113.48 | 35.78–45.69 | Y = 16.0 + 0.242 X |
| AgNO3 | 83.39 ±2.41 | 78.58–88.09 | 174.51 ± 5.35 | 174.94–186.14 | Y = 4.02+ 0.250 X |
| AuCl3 | 120.62 ± 2.56 | 115–125 | 205.34 ± 6.26 | 194.18–219.27 | Y = 3.89 + 0.242 X |
| Naringenin–Au nanoconjugate | 61.43 ± 2.17 | 56.99–65.55 | 132.33 ± 3.33 | 126.25–139.42 | Y = 12.1 + 0.257 X |
| Naringenin–Ag nanoconjugate | 46.23 ± 2.55 | 40.89–50.99 | 118.39 ± 3.17 | 112.60–125.14 | Y = 13.8 + 0.248 X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salunkhe, J.D.; Pulidindi, I.N.; Patil, V.S.; Patil, S.V. Neurospora sp. Mediated Synthesis of Naringenin for the Production of Bioactive Nanomaterials. Bioengineering 2024, 11, 510. https://doi.org/10.3390/bioengineering11050510
Salunkhe JD, Pulidindi IN, Patil VS, Patil SV. Neurospora sp. Mediated Synthesis of Naringenin for the Production of Bioactive Nanomaterials. Bioengineering. 2024; 11(5):510. https://doi.org/10.3390/bioengineering11050510
Chicago/Turabian StyleSalunkhe, Jitendra Dattatray, Indra Neel Pulidindi, Vikas Sambhaji Patil, and Satish Vitthal Patil. 2024. "Neurospora sp. Mediated Synthesis of Naringenin for the Production of Bioactive Nanomaterials" Bioengineering 11, no. 5: 510. https://doi.org/10.3390/bioengineering11050510
APA StyleSalunkhe, J. D., Pulidindi, I. N., Patil, V. S., & Patil, S. V. (2024). Neurospora sp. Mediated Synthesis of Naringenin for the Production of Bioactive Nanomaterials. Bioengineering, 11(5), 510. https://doi.org/10.3390/bioengineering11050510









