New Frontiers in Breast Cancer Imaging: The Rise of AI
Abstract
1. Introduction
2. Imaging Modalities and Their Advances
2.1. Mammography
2.1.1. Technique
2.1.2. Cancer Detection
2.1.3. Prognostic Factors
2.1.4. Risk Stratification
2.2. Ultrasound
2.2.1. Cancer Detection and Diagnosis
2.2.2. Prognostic Factors
2.2.3. Surgical Planning
2.3. MRI
2.3.1. Technique
2.3.2. Cancer Detection
2.3.3. Cancer Diagnosis–Lesion Characterization
2.3.4. Prognostic Factors
2.3.5. Risk Stratification
2.4. Other Relevant AI
Surgical Planning
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Giger, M.L. Clinical Artificial Intelligence Applications: Breast Imaging. Radiol. Clin. N. Am. 2021, 59, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Breast Cancer Facts & Figures 2022–2024; American Cancer Society, Inc.: Atlanta, GA, USA, 2022. [Google Scholar]
- Mango, V.L.; Sun, M.; Wynn, R.T.; Ha, R. Should We Ignore, Follow, or Biopsy? Impact of Artificial Intelligence Decision Support on Breast Ultrasound Lesion Assessment. AJR Am. J. Roentgenol. 2020, 214, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Shin, S.; Yang, S.A.; Park, E.K.; Kim, K.H.; Cho, S.I.; Ock, C.Y.; Kim, S. Artificial Intelligence in Breast Cancer Diagnosis and Personalized Medicine. J. Breast Cancer 2023, 26, 405–435. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.; Aukland, B.; Amir, T.; Sevilimedu, V.; Saphier, N.B.; Mango, V.L. Artificial Intelligence Decision Support for Triple-Negative Breast Cancers on Ultrasound. J. Breast Imaging 2024, 6, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.R.; Monga, N.; Johnson, C.; Hawley, J.R.; Patel, M. Artificial Intelligence Applications in Breast Imaging: Current Status and Future Directions. Diagnostics 2023, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Bahl, M.; Chang, J.M.; Mullen, L.A.; Berg, W.A. Artificial Intelligence for Breast Ultrasound: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, K.A.; Mercaldo, S.; Venkatesh, S.L.; Bahl, M. Prediction of Surgical Upstaging Risk of Ductal Carcinoma In Situ Using Machine Learning Models. J. Breast Imaging 2023, 5, 695–702. [Google Scholar] [CrossRef]
- Hou, R.; Grimm, L.J.; Mazurowski, M.A.; Marks, J.R.; King, L.M.; Maley, C.C.; Lynch, T.; van Oirsouw, M.; Rogers, K.; Stone, N.; et al. Prediction of Upstaging in Ductal Carcinoma in Situ Based on Mammographic Radiomic Features. Radiology 2022, 303, 54–62. [Google Scholar] [CrossRef]
- Harowicz, M.R.; Saha, A.; Grimm, L.J.; Marcom, P.K.; Marks, J.R.; Hwang, E.S.; Mazurowski, M.A. Can algorithmically assessed MRI features predict which patients with a preoperative diagnosis of ductal carcinoma in situ are upstaged to invasive breast cancer? J. Magn. Reson. Imaging 2017, 46, 1332–1340. [Google Scholar] [CrossRef]
- Astley, S.M.; Harkness, E.F.; Sergeant, J.C.; Warwick, J.; Stavrinos, P.; Warren, R.; Wilson, M.; Beetles, U.; Gadde, S.; Lim, Y.; et al. A comparison of five methods of measuring mammographic density: A case-control study. Breast Cancer Res. 2018, 20, 10. [Google Scholar] [CrossRef]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Sheng, D.L.; Chen, J.G.; You, C.; Liu, S.; Xu, H.X.; Chang, C. Artificial intelligence in breast imaging: Potentials and challenges. Phys. Med. Biol. 2023, 68, 23TR01. [Google Scholar] [CrossRef]
- Seth, I.; Bulloch, G.; Joseph, K.; Hunter-Smith, D.J.; Rozen, W.M. Use of Artificial Intelligence in the Advancement of Breast Surgery and Implications for Breast Reconstruction: A Narrative Review. J. Clin. Med. 2023, 12, 5143. [Google Scholar] [CrossRef] [PubMed]
- Vegas, M.R.; Martina, L.; Segovia-Gonzalez, M.; Garcia-Garcia, J.F.; Gonzalez-Gonzalez, A.; Mendieta-Baro, A.; Nieto-Gongora, C.; Benito-Duque, P. Vascular anatomy of the breast and its implications in the breast-sharing reconstruction technique. J. Plast. Reconstr. Aesthet. Surg. 2023, 76, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Huang, S.; Rashid, A.; Varghese, B.; Gholamrezanezhad, A. A Narrative Review of the Use of Artificial Intelligence in Breast, Lung, and Prostate Cancer. Life 2023, 13, 2011. [Google Scholar] [CrossRef] [PubMed]
- Pollack, E.B.; Abbey, G.; Durand, M.A.; Elezaby, M.A.; Farooq, A.; Kelil, T.; Jin, M.; Lugossy, A.-M.; Mango, V.L.; Peart, O.; et al. Developing Breast Imaging Services in Low-Resource Settings. Appl. Radiol. 2022, 51, 28–32. [Google Scholar]
- Masud, R.; Al-Rei, M.; Lokker, C. Computer-Aided Detection for Breast Cancer Screening in Clinical Settings: Scoping Review. JMIR Med. Inform. 2019, 7, e12660. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, C.; Gillan, M.G.; Cairns, J.; Wallis, M.G.; Gilbert, F.J. Is computer aided detection (CAD) cost effective in screening mammography? A model based on the CADET II study. BMC Health Serv. Res. 2011, 11, 11. [Google Scholar] [CrossRef]
- Fenton, J.J.; Abraham, L.; Taplin, S.H.; Geller, B.M.; Carney, P.A.; D’Orsi, C.; Elmore, J.G.; Barlow, W.E. Effectiveness of computer-aided detection in community mammography practice. J. Natl. Cancer Inst. 2011, 103, 1152–1161. [Google Scholar] [CrossRef]
- Lehman, C.D.; Wellman, R.D.; Buist, D.S.; Kerlikowske, K.; Tosteson, A.N.; Miglioretti, D.L. Diagnostic Accuracy of Digital Screening Mammography With and Without Computer-Aided Detection. JAMA Intern. Med. 2015, 175, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Czene, K.; Strand, F.; Zackrisson, S.; Lindholm, P.; Lång, K.; Förnvik, D.; Sartor, H.; Mavaddat, N.; Easton, D.; et al. Identification of Women at High Risk of Breast Cancer Who Need Supplemental Screening. Radiology 2020, 297, 327–333. [Google Scholar] [CrossRef] [PubMed]
- van Winkel, S.L.; Rodríguez-Ruiz, A.; Appelman, L.; Gubern-Mérida, A.; Karssemeijer, N.; Teuwen, J.; Wanders, A.J.T.; Sechopoulos, I.; Mann, R.M. Impact of artificial intelligence support on accuracy and reading time in breast tomosynthesis image interpretation: A multi-reader multi-case study. Eur. Radiol. 2021, 31, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Lång, K.; Josefsson, V.; Larsson, A.M.; Larsson, S.; Högberg, C.; Sartor, H.; Hofvind, S.; Andersson, I.; Rosso, A. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): A clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. 2023, 24, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Kizildag Yirgin, I.; Koyluoglu, Y.O.; Seker, M.E.; Ozkan Gurdal, S.; Ozaydin, A.N.; Ozcinar, B.; Cabioğlu, N.; Ozmen, V.; Aribal, E. Diagnostic Performance of AI for Cancers Registered in A Mammography Screening Program: A Retrospective Analysis. Technol. Cancer Res. Treat. 2022, 21, 15330338221075172. [Google Scholar] [CrossRef] [PubMed]
- Leibig, C.; Brehmer, M.; Bunk, S.; Byng, D.; Pinker, K.; Umutlu, L. Combining the strengths of radiologists and AI for breast cancer screening: A retrospective analysis. Lancet Digit. Health 2022, 4, e507–e519. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Wylie, E.; Lotter, W.; Lund, H.; Waddell, A.; Madeley, C.; Pereira, G.; Houssami, N. Artificial intelligence (AI) for breast cancer screening: BreastScreen population-based cohort study of cancer detection. EBioMedicine 2023, 90, 104498. [Google Scholar] [CrossRef]
- Eriksson, M.; Román, M.; Gräwingholt, A.; Castells, X.; Nitrosi, A.; Pattacini, P.; Heywang-Köbrunner, S.; Rossi, P.G. European validation of an image-derived AI-based short-term risk model for individualized breast cancer screening-a nested case-control study. Lancet Reg. Health Eur. 2024, 37, 100798. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Teuwen, J.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Mertelmeier, T.; et al. Can we reduce the workload of mammographic screening by automatic identification of normal exams with artificial intelligence? A feasibility study. Eur. Radiol. 2019, 29, 4825–4832. [Google Scholar] [CrossRef]
- Yala, A.; Schuster, T.; Miles, R.; Barzilay, R.; Lehman, C. A Deep Learning Model to Triage Screening Mammograms: A Simulation Study. Radiology 2019, 293, 38–46. [Google Scholar] [CrossRef]
- Morrish, O.W.; Tucker, L.; Black, R.; Willsher, P.; Duffy, S.W.; Gilbert, F.J. Mammographic breast density: Comparison of methods for quantitative evaluation. Radiology 2015, 275, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Alonzo-Proulx, O.; Mawdsley, G.E.; Patrie, J.T.; Yaffe, M.J.; Harvey, J.A. Reliability of automated breast density measurements. Radiology 2015, 275, 366–376. [Google Scholar] [CrossRef] [PubMed]
- García, E.; Diaz, O.; Martí, R.; Diez, Y.; Gubern-Mérida, A.; Sentís, M.; Martí, J.; Oliver, A. Local breast density assessment using reacquired mammographic images. Eur. J. Radiol. 2017, 93, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Virnig, B.A.; Tuttle, T.M.; Shamliyan, T.; Kane, R.L. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J. Natl. Cancer Inst. 2010, 102, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Mutasa, S.; Chang, P.; Van Sant, E.P.; Nemer, J.; Liu, M.; Karcich, J.; Patel, G.; Jambawalikar, S.; Ha, R. Potential Role of Convolutional Neural Network Based Algorithm in Patient Selection for DCIS Observation Trials Using a Mammogram Dataset. Acad. Radiol. 2020, 27, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Robinson, K.; Lan, L.; Baughan, N.; Chan, C.W.; Embury, M.; Whitman, G.J.; El-Zein, R.; Bedrosian, I.; Giger, M.L. Temporal Machine Learning Analysis of Prior Mammograms for Breast Cancer Risk Prediction. Cancers 2023, 15, 2141. [Google Scholar] [CrossRef]
- Arefan, D.; Mohamed, A.A.; Berg, W.A.; Zuley, M.L.; Sumkin, J.H.; Wu, S. Deep learning modeling using normal mammograms for predicting breast cancer risk. Med. Phys. 2020, 47, 110–118. [Google Scholar] [CrossRef]
- Eriksson, M.; Czene, K.; Vachon, C.; Conant, E.F.; Hall, P. Long-Term Performance of an Image-Based Short-Term Risk Model for Breast Cancer. J. Clin. Oncol. 2023, 41, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Romanov, S.; Howell, S.; Harkness, E.; Bydder, M.; Evans, D.G.; Squires, S.; Fergie, M.; Astley, S. Artificial Intelligence for Image-Based Breast Cancer Risk Prediction Using Attention. Tomography 2023, 9, 2103–2115. [Google Scholar] [CrossRef]
- Yala, A.; Mikhael, P.G.; Strand, F.; Lin, G.; Smith, K.; Wan, Y.L.; Lamb, L.; Hughes, K.; Lehman, C.; Barzilay, R. Toward robust mammography-based models for breast cancer risk. Sci. Transl. Med. 2021, 13, eaba4373. [Google Scholar] [CrossRef]
- Zhou, B.Y.; Wang, L.F.; Yin, H.H.; Wu, T.F.; Ren, T.T.; Peng, C.; Li, D.X.; Shi, H.; Sun, L.P.; Zhao, C.K.; et al. Decoding the molecular subtypes of breast cancer seen on multimodal ultrasound images using an assembled convolutional neural network model: A prospective and multicentre study. EBioMedicine 2021, 74, 103684. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.; Calabrese, M.; Martinoli, C.; Tagliafico, A.S. Artificial Intelligence in Breast Ultrasound: From Diagnosis to Prognosis-A Rapid Review. Diagnostics 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhao, C.; Zhu, Q.; Zhang, J.; Liu, H.; Li, J.; Jiang, Y. An investigation of the classification accuracy of a deep learning framework-based computer-aided diagnosis system in different pathological types of breast lesions. J. Thorac. Dis. 2019, 11, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G. Future of breast elastography. Ultrasonography 2019, 38, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; López Aldrete, A.L.; Jairaj, A.; Ledesma Parea, J.C.; García, C.Y.; McClennan, R.C.; Cen, S.Y.; Larsen, L.H.; de Lara, M.T.S.; Love, S. Toward AI-supported US Triage of Women with Palpable Breast Lumps in a Low-Resource Setting. Radiology 2023, 307, e223351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Zhou, J.; Shi, Z.T.; Li, N.; Zhou, S.C.; Chang, C. Sonographic Features of Triple-Negative Breast Carcinomas Are Correlated With mRNA-lncRNA Signatures and Risk of Tumor Recurrence. Front. Oncol. 2020, 10, 587422. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Chen, H.; Tang, X.; Chen, B.; Jiang, X.; Liu, L.; Feng, Y.; Liu, L.; Li, L. Ultrasound-Based Radiomics Analysis for Predicting Disease-Free Survival of Invasive Breast Cancer. Front. Oncol. 2021, 11, 621993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pawar, K.; Ekanayake, M.; Pain, C.; Zhong, S.; Egan, G.F. Deep Learning for Image Enhancement and Correction in Magnetic Resonance Imaging-State-of-the-Art and Challenges. J. Digit. Imaging 2023, 36, 204–230. [Google Scholar] [CrossRef]
- Müller-Franzes, G.; Huck, L.; Tayebi Arasteh, S.; Khader, F.; Han, T.; Schulz, V.; Dethlefsen, E.; Kather, J.N.; Nebelung, S.; Nolte, T.; et al. Using Machine Learning to Reduce the Need for Contrast Agents in Breast MRI through Synthetic Images. Radiology 2023, 307, e222211. [Google Scholar] [CrossRef]
- Jiang, Y.; Edwards, A.V.; Newstead, G.M. Artificial Intelligence Applied to Breast MRI for Improved Diagnosis. Radiology 2021, 298, 38–46. [Google Scholar] [CrossRef]
- Codari, M.; Schiaffino, S.; Sardanelli, F.; Trimboli, R.M. Artificial Intelligence for Breast MRI in 2008–2018: A Systematic Mapping Review. AJR Am. J. Roentgenol. 2019, 212, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid review: Radiomics and breast cancer. Breast Cancer Res. Treat. 2018, 169, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gong, J.; Wang, S.; Zhu, H.; Zhao, B.; Peng, W. Application of MRI Radiomics-Based Machine Learning Model to Improve Contralateral BI-RADS 4 Lesion Assessment. Front. Oncol. 2020, 10, 531476. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Brooks, J.D.; Bernstein, J.L.; Reiner, A.S.; Pike, M.C.; Morris, E.A. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011, 260, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Dontchos, B.N.; Rahbar, H.; Partridge, S.C.; Korde, L.A.; Lam, D.L.; Scheel, J.R.; Peacock, S.; Lehman, C.D. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated with Breast Cancer Risk? Radiology 2015, 276, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Grimm, L.J.; Ghate, S.V.; Kim, C.E.; Soo, M.S.; Yoon, S.C.; Mazurowski, M.A. Machine learning-based prediction of future breast cancer using algorithmically measured background parenchymal enhancement on high-risk screening MRI. J. Magnet. Reson. Imaging JMRI 2019, 50, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, B.; Dietzel, M.; Baltzer, P.A. MRI Background Parenchymal Enhancement Is Not Associated with Breast Cancer. PLoS ONE 2016, 11, e0158573. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.J.; Saha, A.; Ghate, S.V.; Kim, C.; Soo, M.S.; Yoon, S.C.; Mazurowski, M.A. Relationship between Background Parenchymal Enhancement on High-risk Screening MRI and Future Breast Cancer Risk. Acad. Radiol. 2019, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Mallawaarachchi, I.; Dwivedi, D.K.; Ayyappan, A.P.; Shokar, N.K.; Lakshmanaswamy, R.; Dwivedi, A.K. The Association of Background Parenchymal Enhancement at Breast MRI with Breast Cancer: A Systematic Review and Meta-Analysis. Radiology 2019, 292, 552–561. [Google Scholar] [CrossRef]
- Chen, J.H.; Yu, H.J.; Hsu, C.; Mehta, R.S.; Carpenter, P.M.; Su, M.Y. Background Parenchymal Enhancement of the Contralateral Normal Breast: Association with Tumor Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Transl. Oncol. 2015, 8, 204–209. [Google Scholar] [CrossRef]
- Öztürk, M.; Polat, A.V.; Süllü, Y.; Tomak, L.; Polat, A.K. Background Parenchymal Enhancement and Fibroglandular Tissue Proportion on Breast MRI: Correlation with Hormone Receptor Expression and Molecular Subtypes of Breast Cancer. J. Breast Health 2017, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kato, F.; Oyama-Manabe, N.; Li, R.; Cui, Y.; Tha, K.K.; Yamashita, H.; Kudo, K.; Shirato, H. Identifying Triple-Negative Breast Cancer Using Background Parenchymal Enhancement Heterogeneity on Dynamic Contrast-Enhanced MRI: A Pilot Radiomics Study. PLoS ONE 2015, 10, e0143308. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.A.; Arasu, V.A.; Strand, F.; Li, W.; Onishi, N.; Gibbs, J.; Jones, E.F.; Joe, B.N.; Esserman, L.J.; Newitt, D.C.; et al. Comparison of Segmentation Methods in Assessing Background Parenchymal Enhancement as a Biomarker for Response to Neoadjuvant Therapy. Tomography 2020, 6, 101–110. [Google Scholar] [CrossRef]
- Dong, J.M.; Wang, H.X.; Zhong, X.F.; Xu, K.; Bian, J.; Feng, Y.; Chen, L.; Zhang, L.; Wang, X.; Ma, D.J.; et al. Changes in background parenchymal enhancement in HER2-positive breast cancer before and after neoadjuvant chemotherapy: Association with pathologic complete response. Medicine 2018, 97, e12965. [Google Scholar] [CrossRef]
- Wu, S.; Weinstein, S.P.; DeLeo, M.J., 3rd; Conant, E.F.; Chen, J.; Domchek, S.M.; Kontos, D. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: Preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res. BCR 2015, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Zheng, X.; Liu, Z.; Long, W.; Zhang, Y.; Wei, J.; Lu, Y. Automated fibroglandular tissue segmentation in breast MRI using generative adversarial networks. Phys. Med. Biol. 2020, 65, 105006. [Google Scholar] [CrossRef]
- Zhang, M.; Sadinski, M.; Haddad, D.; Bae, M.S.; Martinez, D.; Morris, E.A.; Gibbs, P.; Sutton, E.J. Background Parenchymal Enhancement on Breast MRI as a Prognostic Surrogate: Correlation With Breast Cancer Oncotype Dx Score. Front. Oncol. 2020, 10, 595820. [Google Scholar] [CrossRef] [PubMed]
- Pujara, A.C.; Mikheev, A.; Rusinek, H.; Gao, Y.; Chhor, C.; Pysarenko, K.; Rallapalli, H.; Walczyk, J.; Moccaldi, M.; Babb, J.S.; et al. Comparison between qualitative and quantitative assessment of background parenchymal enhancement on breast MRI. J. Magn. Reson. Imaging 2018, 47, 1685–1691. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, X.; Xiao, Q.; Gu, Y.; Li, Q. Fully automated segmentation of whole breast using dynamic programming in dynamic contrast enhanced MR images. Med. Phys. 2017, 44, 2400–2414. [Google Scholar] [CrossRef]
- Xu, X.; Fu, L.; Chen, Y.; Larsson, R.; Zhang, D.; Suo, S.; Hua, J.; Zhao, J. Breast Region Segmentation being Convolutional Neural Network in Dynamic Contrast Enhanced MRI. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 750–753. [Google Scholar] [CrossRef]
- Borkowski, K.; Rossi, C.; Ciritsis, A.; Marcon, M.; Hejduk, P.; Stieb, S.; Boss, A.; Berger, N. Fully automatic classification of breast MRI background parenchymal enhancement using a transfer learning approach. Medicine 2020, 99, e21243. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.A.; Harris, E.E.; Bailey, L.; Chadha, M.; Dutton, S.C.; Freedman, G.M.; Goyal, S.; Halyard, M.Y.; Horst, K.C.; Novick, K.L.; et al. ACR Appropriateness Criteria® Ductal Carcinoma in Situ. Oncology 2015, 29, 446. [Google Scholar] [PubMed]
- Santiago, L.; Adrada, B.E.; Caudle, A.S.; Clemens, M.W.; Black, D.M.; Arribas, E.M. The role of three-dimensional printing in the surgical management of breast cancer. J. Surg. Oncol. 2019, 120, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Fidvi, S.; Holder, J.; Li, H.; Parnes, G.J.; Shamir, S.B.; Wake, N. Advanced 3D Visualization and 3D Printing in Radiology. Adv. Exp. Med. Biol. 2023, 1406, 103–138. [Google Scholar] [CrossRef] [PubMed]
- Arribas, E.M.; Kelil, T.; Santiago, L.; Ali, A.; Chadalavada, S.C.; Chepelev, L.; Ghodadra, A.; Ionita, C.N.; Lee, J.; Ravi, P.; et al. Radiological Society of North America (RSNA) 3D Printing Special Interest Group (SIG) clinical situations for which 3D printing is considered an appropriate representation or extension of data contained in a medical imaging examination: Breast conditions. 3D Print. Med. 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Rojek, I.; Mikołajewski, D.; Dostatni, E.; Macko, M. AI-Optimized Technological Aspects of the Material Used in 3D Printing Processes for Selected Medical Applications. Materials 2020, 13, 5437. [Google Scholar] [CrossRef] [PubMed]
- Mavioso, C.; Araújo, R.J.; Oliveira, H.P.; Anacleto, J.C.; Vasconcelos, M.A.; Pinto, D.; Gouveia, P.F.; Alves, C.; Cardoso, F.; Cardoso, J.S.; et al. Automatic detection of perforators for microsurgical reconstruction. Breast 2020, 50, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Jeon, S.; Heo, C.; Kim, E.K.; Kang, E.; Shin, H.C.; Yang, E.J.; Jeong, J.H. Validating machine learning approaches for prediction of donor related complication in microsurgical breast reconstruction: A retrospective cohort study. Sci. Rep. 2021, 11, 5615. [Google Scholar] [CrossRef]
- Mahmood, T.; Li, J.; Pei, Y.; Akhtar, F. An Automated In-Depth Feature Learning Algorithm for Breast Abnormality Prognosis and Robust Characterization from Mammography Images Using Deep Transfer Learning. Biology 2021, 10, 859. [Google Scholar] [CrossRef]
- Bahl, M. Artificial Intelligence in Clinical Practice: Implementation Considerations and Barriers. J. Breast Imaging 2022, 4, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Omoumi, P.; Ducarouge, A.; Tournier, A.; Harvey, H.; Kahn, C.E., Jr.; Louvet-de Verchère, F.; Pinto Dos Santos, D.; Kober, T.; Richiardi, J. To buy or not to buy-evaluating commercial AI solutions in radiology (the ECLAIR guidelines). Eur. Radiol. 2021, 31, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Agarwal, S.; Coombs, L.; Wald, C.; Dreyer, K. 2020 ACR Data Science Institute Artificial Intelligence Survey. J. Am. Coll. Radiol. 2021, 18, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Ongena, Y.P.; Yakar, D.; Haan, M.; Kwee, T.C. Artificial Intelligence in Screening Mammography: A Population Survey of Women’s Preferences. J. Am. Coll. Radiol. 2021, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, H.; Bidgoli, F.A.; Zhou, B.; Iribarren, C.; Molloi, S.; Baldi, P. Detecting Cardiovascular Disease from Mammograms With Deep Learning. IEEE Trans. Med. Imaging 2017, 36, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Mobini, N.; Codari, M.; Riva, F.; Ienco, M.G.; Capra, D.; Cozzi, A.; Carriero, S.; Spinelli, D.; Trimboli, R.M.; Baselli, G.; et al. Detection and quantification of breast arterial calcifications on mammograms: A deep learning approach. Eur. Radiol. 2023, 33, 6746–6755. [Google Scholar] [CrossRef]
- Dratsch, T.; Chen, X.; Rezazade Mehrizi, M.; Kloeckner, R.; Mähringer-Kunz, A.; Püsken, M.; Baeßler, B.; Sauer, S.; Maintz, D.; Pinto Dos Santos, D. Automation Bias in Mammography: The Impact of Artificial Intelligence BI-RADS Suggestions on Reader Performance. Radiology 2023, 307, e222176. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. 2013 ACR BI-RADS Atlas: Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2014. [Google Scholar]
- Ozcan, B.B.; Wanniarachchi, H.; Mason, R.P.; Dogan, B.E. Current status of optoacoustic breast imaging and future trends in clinical application: Is it ready for prime time? Eur. Radiol. 2024. [Google Scholar] [CrossRef]
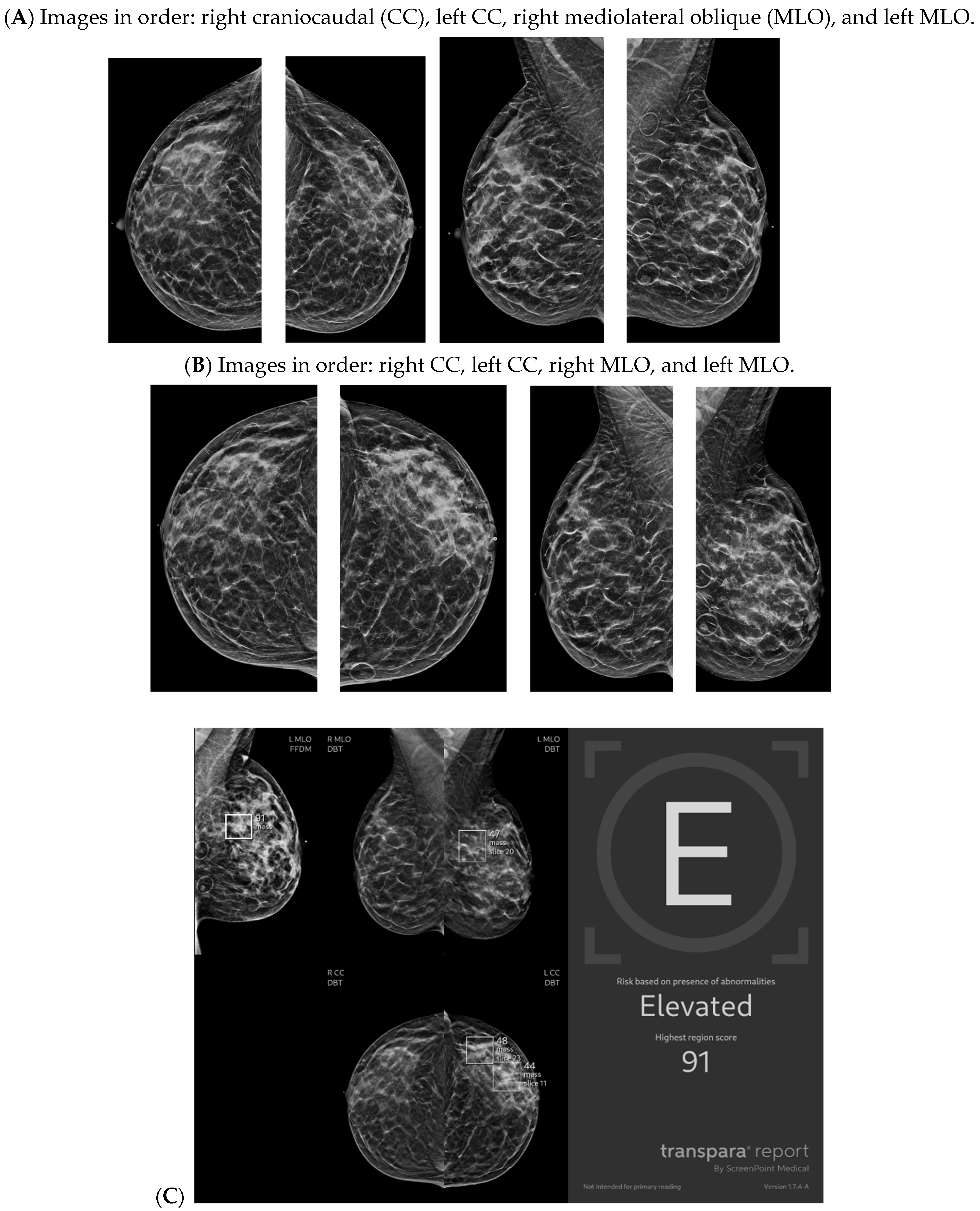
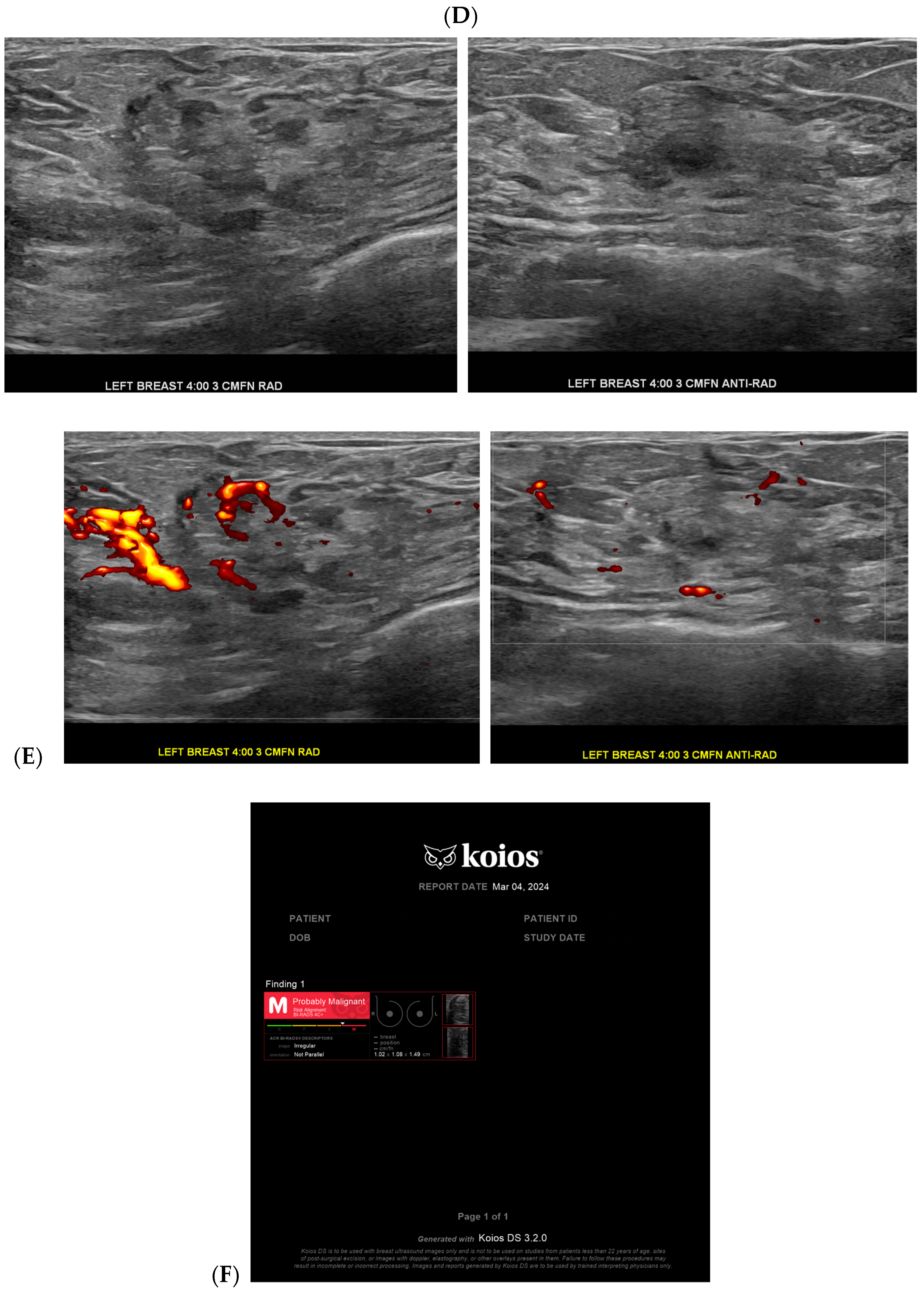
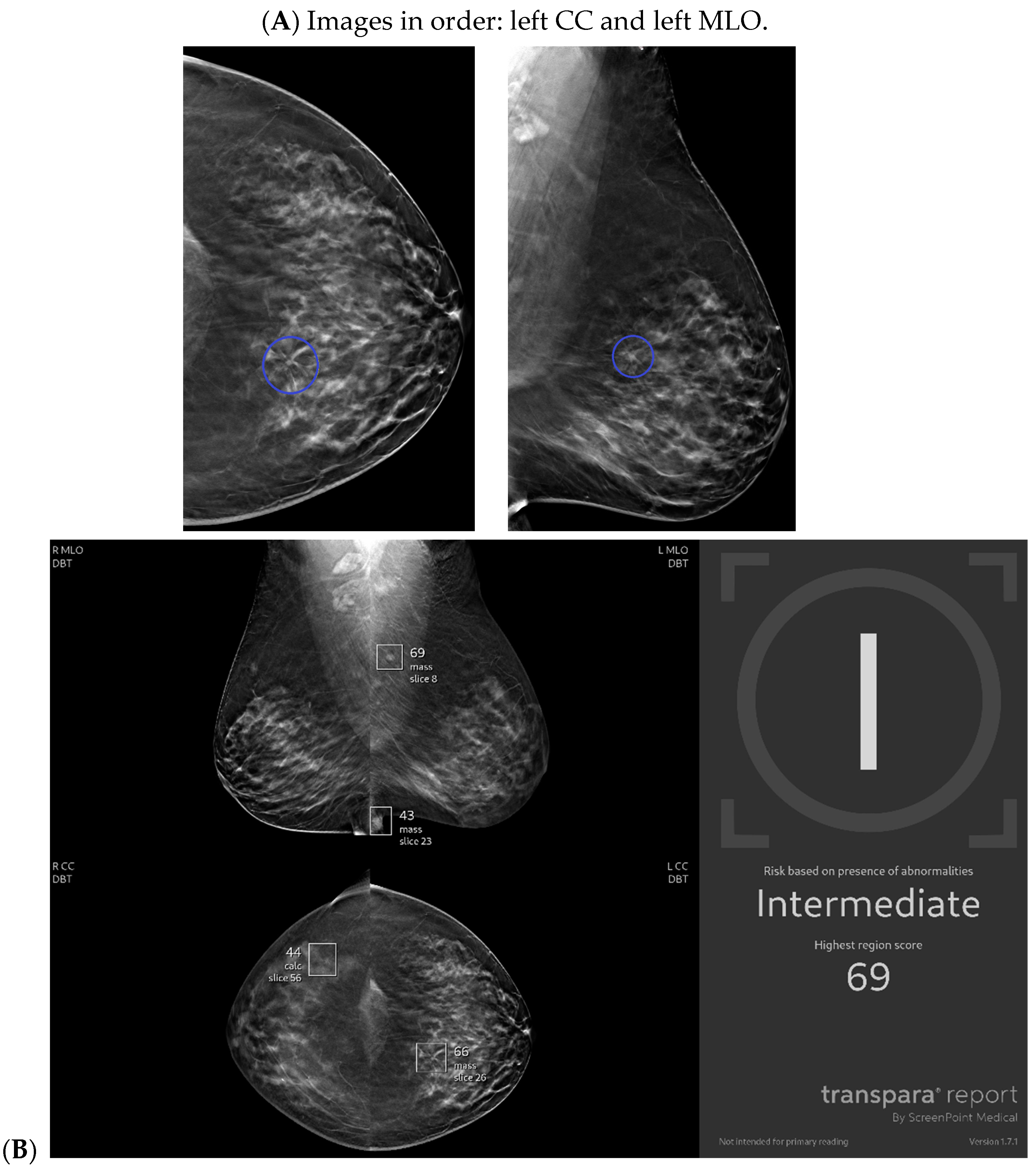
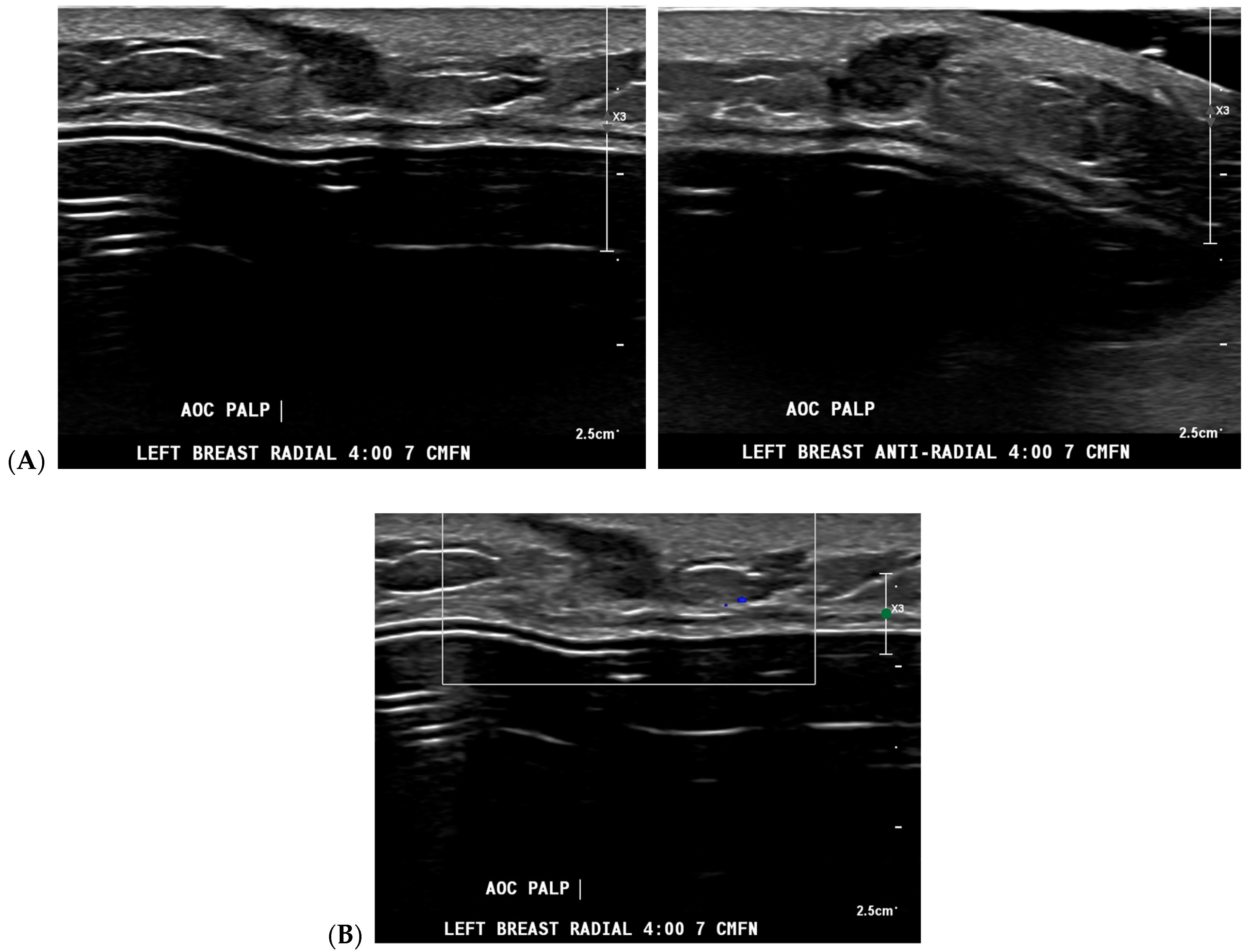

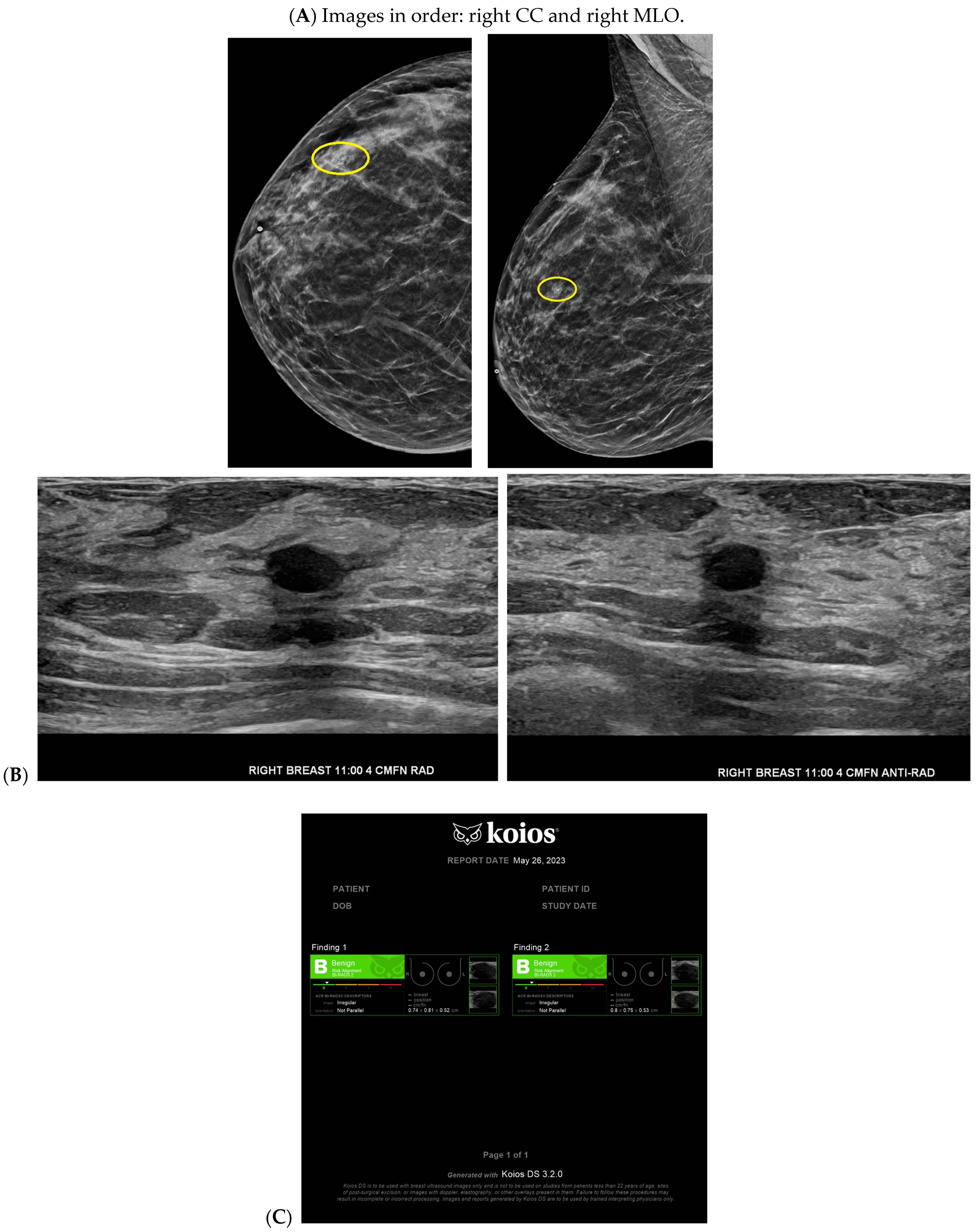
| Topic | References |
|---|---|
| Mammography | |
| Technique | [17,18] |
| Cancer detection | [17,19,20,21,22,23,24,25,26,27,28,29,30,31] |
| Prognosis | [6,12,13,32,33,34] |
| Risk stratification | [10,35,36,37,38,39,40,41] |
| Ultrasound | |
| Cancer detection and diagnosis | [3,5,8,17,42,43,44,45,46] |
| Prognosis | [47,48] |
| Surgical planning | [15] |
| MRI | |
| Technique | [49,50] |
| Cancer detection | [51,52,53] |
| Cancer diagnosis—lesion characterization | [51,54] |
| Prognosis | [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Risk stratification | [11,73,74] |
| Surgical planning | [15,75,76,77,78,79] |
| AI Algorithm | Purpose | Techniques |
|---|---|---|
| Mammography | ||
| Quality | Improve image acquisition by providing real-time feedback regarding position and quality control metrics and aggregate data to help establish trends between staff members | |
| Detection | Detect areas that need to be addressed by a radiologist | Computer-aided detection (CAD) AI, deep convolutional neural networks (CNN) |
| Prognostic factors | Automated estimate of fibroglandular tissue, which is correlated with breast cancer risk | |
| Risk stratification | Predicts the upgrade rate of in situ cancer to invasive malignancy and predicts 5 year risk of developing breast cancer | CNN and radiomics |
| Ultrasound | ||
| Diagnosis | Provides decision support that ultimately improves accurate BI-RADS classification | CNN and an additional algorithm for classification |
| Prognostic factors | Ultrasound features, such as triple-negative breast cancer, used to predict the risk of recurrence | Radiomics analysis |
| Surgical planning | Assess vascular supply of the breast to determine the plausibility of reconstructive techniques | |
| Magnetic Resonance Imaging (MRI) | ||
| Technique | Accelerated image acquisition by improving signal processing and reducing image noise | Artificially filling k-space |
| Diagnosis | Assess radiomic features extracted from contrast-enhanced T1-weighted and T2-weighted images | Machine-based learning |
| Prognostic factors | Quantitative assessment of background parenchymal enhancement, which is a possible risk factor for breast cancer | CNN |
| Risk stratification | Predicts the upgrade rate of in situ cancer to invasive malignancy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamir, S.B.; Sasson, A.L.; Margolies, L.R.; Mendelson, D.S. New Frontiers in Breast Cancer Imaging: The Rise of AI. Bioengineering 2024, 11, 451. https://doi.org/10.3390/bioengineering11050451
Shamir SB, Sasson AL, Margolies LR, Mendelson DS. New Frontiers in Breast Cancer Imaging: The Rise of AI. Bioengineering. 2024; 11(5):451. https://doi.org/10.3390/bioengineering11050451
Chicago/Turabian StyleShamir, Stephanie B., Arielle L. Sasson, Laurie R. Margolies, and David S. Mendelson. 2024. "New Frontiers in Breast Cancer Imaging: The Rise of AI" Bioengineering 11, no. 5: 451. https://doi.org/10.3390/bioengineering11050451
APA StyleShamir, S. B., Sasson, A. L., Margolies, L. R., & Mendelson, D. S. (2024). New Frontiers in Breast Cancer Imaging: The Rise of AI. Bioengineering, 11(5), 451. https://doi.org/10.3390/bioengineering11050451






