Evaluating the Role of Breast Ultrasound in Early Detection of Breast Cancer in Low- and Middle-Income Countries: A Comprehensive Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
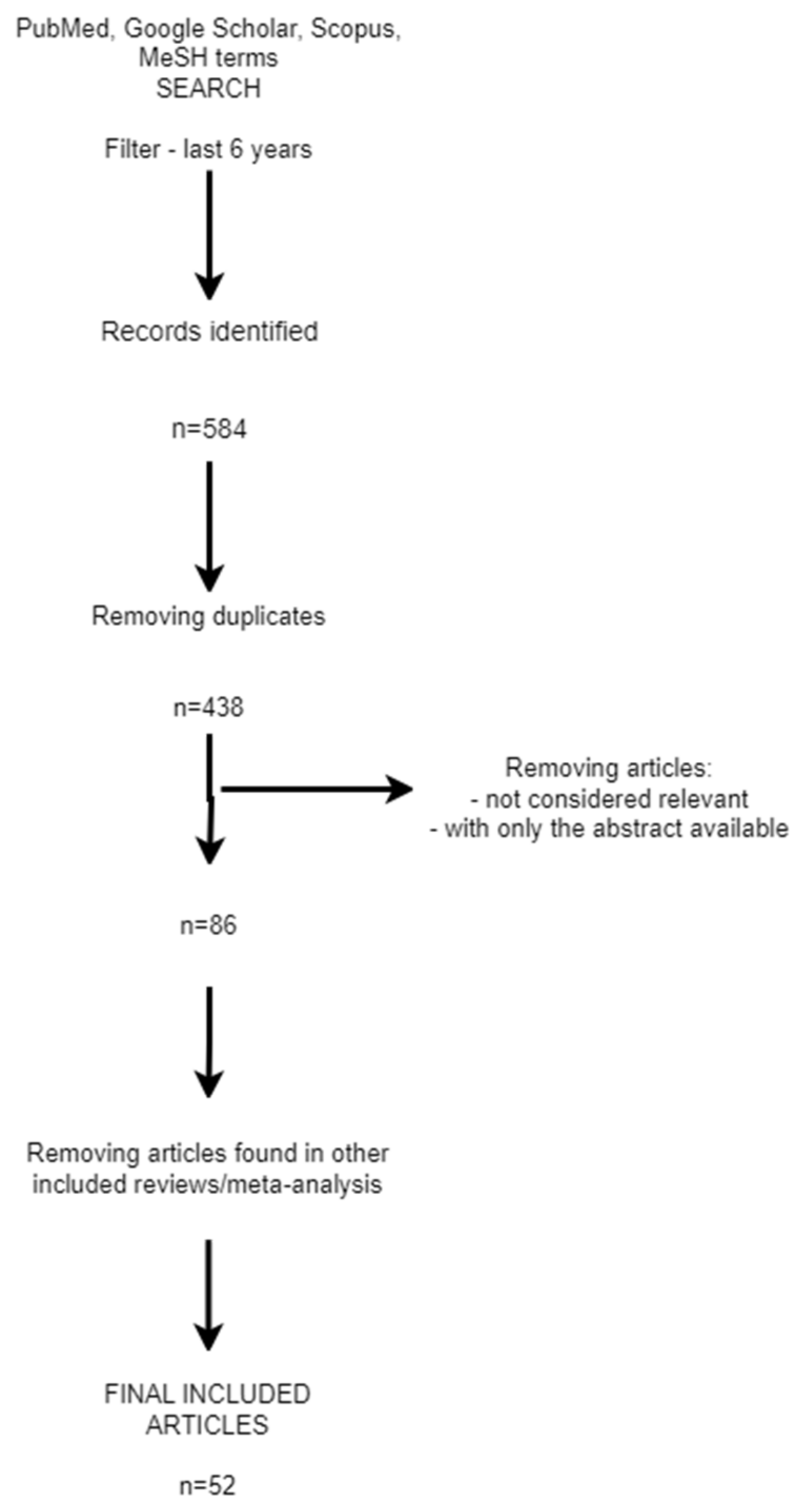
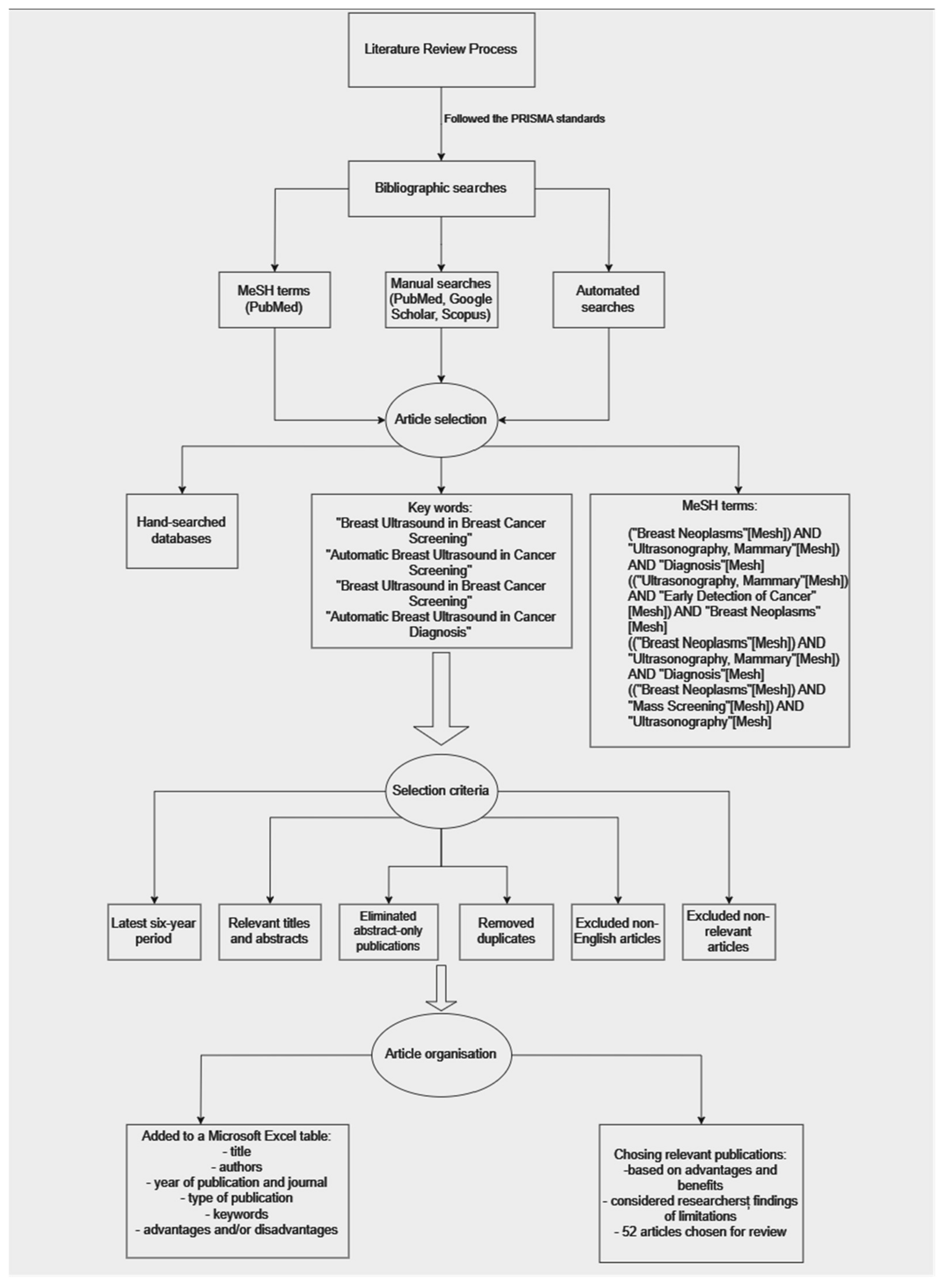
2. Materials and Methods
- ((“Breast Neoplasms”[Mesh]) AND “Ultrasonography, Mammary”[Mesh]) AND “Diagnosis”[Mesh];
- ((“Ultrasonography, Mammary”[Mesh]) AND “Early Detection of Cancer”[Mesh]) AND “Breast Neoplasms”[Mesh];
- ((“Breast Neoplasms”[Mesh]) AND “Ultrasonography, Mammary”[Mesh]) AND “Diagnosis”[Mesh];
- ((“Breast Neoplasms”[Mesh]) AND “Mass Screening”[Mesh]) AND “Ultrasonography”[Mesh].
- Overall advantages of using breast ultrasound for breast tissue evaluation;
- Advantages of breast ultrasound in breast cancer screening;
- Disadvantages/limitations of breast ultrasound in breast cancer screening;
- Advantages of breast ultrasound in breast cancer diagnosis;
- Disadvantages/limitations of breast ultrasound in breast cancer diagnosis;
- ABUS in breast cancer screening and diagnosis—advantages and limitations.
3. Overall Advantages of Using Breast Ultrasound for Breast Tissue Evaluation
3.1. Absence of Ionizing Radiation
3.2. Suitability for Repeated Screenings
3.3. Favorable Choice for Younger Patients
3.4. Psychological Impact for Patients
4. Advantages of Breast Ultrasound in Breast Cancer Screening
4.1. Enhanced Sensitivity, Particularly in Dense Breasts
4.2. Versatility and Dynamic Real-Time Imaging
4.3. Accessibility and Cost-Effectiveness
4.4. Application in High-Risk Populations and Younger Women
5. Disadvantages/Limitations of Breast Ultrasound in Breast Cancer Screening
5.1. Lower Specificity and Increased False Positive Results
5.2. Operator Dependence and Variability
5.3. Low Ability to Detect Microcalcifications
5.4. Limited Performance in High-Risk or Dense Breast Tissue
6. Advantages of Breast Ultrasound in Breast Cancer Diagnosis
6.1. Characterization of Lesions
6.2. Real-Time Imaging and Guided Biopsies
6.3. No Ionizing Radiation
6.4. Supplementary Imaging in Challenging Cases
7. Disadvantages/Limitations of Breast Ultrasound in Breast Cancer Diagnosis
7.1. Operator Dependence and Variability
7.2. Lower Specificity and Increased False Positives
7.3. Challenges in Evaluating Dense Breast Tissue
8. ABUS in Breast Cancer Screening and Diagnosis—Advantages and Limitations
9. Types of Lesions Found by Screening with Breast Ultrasound
10. Conclusions
11. Limitations of the Study
12. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef]
- Radecka, B.; Litwiniuk, M. Breast Cancer in Young Women. Ginekol. Pol. 2016, 87, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Buzdar, A.U.; Hortobagyi, G.N. Breast Cancer in Men. Ann. Intern. Med. 2002, 137, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Jankovic, D.; Milenkovic, A.; Stojanov, D. Early Diagnosis and Detection of Breast Cancer. Technol. Health Care 2018, 26, 729–759. [Google Scholar] [CrossRef]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Shoemaker, M.L.; White, M.C.; Wu, M.; Weir, H.K.; Romieu, I. Differences in Breast Cancer Incidence among Young Women Aged 20–49 Years by Stage and Tumor Characteristics, Age, Race, and Ethnicity, 2004–2013. Breast Cancer Res. Treat. 2018, 169, 595–606. [Google Scholar] [CrossRef]
- Daly, A.A.; Rolph, R.; Cutress, R.I.; Copson, E.R. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer Targets Ther. 2021, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Memon, Z.A.; Kanwal, N.; Sami, M.; Larik, P.A.; Farooq, M.Z. Risk of Breast Cancer among Young Women and Importance of Early Screening. Asian Pac. J. Cancer Prev. 2015, 16, 7485–7489. [Google Scholar] [CrossRef]
- Zhu, J.W.; Charkhchi, P.; Adekunte, S.; Akbari, M.R. What Is Known about Breast Cancer in Young Women? Cancers 2023, 15, 1917. [Google Scholar] [CrossRef]
- Kamel, D.; Youssef, V.; Hopman, W.M.; Mates, M. Staging Investigations in Asymptomatic Early Breast Cancer Patients at the Cancer Centre of Southeastern Ontario. Curr. Oncol. 2021, 28, 2190–2198. [Google Scholar] [CrossRef]
- Koo, M.M.; Von Wagner, C.; Abel, G.A.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Typical and Atypical Presenting Symptoms of Breast Cancer and Their Associations with Diagnostic Intervals: Evidence from a National Audit of Cancer Diagnosis. Cancer Epidemiol. 2017, 48, 140–146. [Google Scholar] [CrossRef]
- Prusty, R.K.; Begum, S.; Patil, A.; Naik, D.D.; Pimple, S.; Mishra, G. Knowledge of Symptoms and Risk Factors of Breast Cancer among Women: A Community Based Study in a Low Socio-Economic Area of Mumbai, India. BMC Women’s Health 2020, 20, 106. [Google Scholar] [CrossRef]
- Candelaria, R.P.; Hwang, L.; Bouchard, R.R.; Whitman, G.J. Breast Ultrasound: Current Concepts. Semin. Ultrasound CT MRI 2013, 34, 213–225. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Iacob, R.; Manolescu, D.L.; Stoicescu, E.R.; Fabian, A.; Malita, D.; Oancea, C. Breast Cancer—How Can Imaging Help? Healthcare 2022, 10, 1159. [Google Scholar] [CrossRef]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast Cancer Diagnosis: Imaging Techniques and Biochemical Markers. J. Cell. Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef]
- Frija, G.; Salama, D.H.; Kawooya, M.G.; Allen, B. A Paradigm Shift in Point-of-Care Imaging in Low-Income and Middle-Income Countries. eClinicalMedicine 2023, 62, 102114. [Google Scholar] [CrossRef] [PubMed]
- Frija, G.; Blažić, I.; Frush, D.P.; Hierath, M.; Kawooya, M.; Donoso-Bach, L.; Brkljačić, B. How to Improve Access to Medical Imaging in Low- and Middle-Income Countries? eClinicalMedicine 2021, 38, 101034. [Google Scholar] [CrossRef] [PubMed]
- Al Jahed, D.; Dekeyzer, S.; Vanwambeke, K.; Antic, M.; Vanhoenacker, C.; Vanhoenacker, F. Automated Breast Ultrasound (ABUS): A Pictorial Essay of Common Artifacts and Benign and Malignant Pathology. J. Ultrason. 2022, 22, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zanotel, M.; Bednarova, I.; Londero, V.; Linda, A.; Lorenzon, M.; Girometti, R.; Zuiani, C. Automated Breast Ultrasound: Basic Principles and Emerging Clinical Applications. Radiol. Med. 2018, 123, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, E.; Pawlak, M.; Popiela, T.; Rudnicki, W. The Role of ABUS in the Diagnosis of Breast Cancer. J. Ultrason. 2022, 22, 76–85. [Google Scholar] [CrossRef]
- Philadelpho, F.; Calas, M.J.G.; Carneiro, G.D.A.C.; Silveira, I.C.; Vaz, A.B.R.; Nogueira, A.M.C.; Bergmann, A.; Lopes, F.P.P.L. Comparison of Automated Breast Ultrasound and Hand-Held Breast Ultrasound in the Screening of Dense Breasts. Rev. Bras. Ginecol. Obs. 2021, 43, 190–199. [Google Scholar] [CrossRef]
- Nicosia, L.; Ferrari, F.; Bozzini, A.C.; Latronico, A.; Trentin, C.; Meneghetti, L.; Pesapane, F.; Pizzamiglio, M.; Balesetreri, N.; Cassano, E. Automatic Breast Ultrasound: State of the Art and Future Perspectives. Ecancermedicalscience 2020, 14, 1062. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar]
- Evans, A.; Trimboli, R.M.; Athanasiou, A.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Camps Herrero, J.; Clauser, P.; Colin, C.; Cornford, E.; et al. Breast Ultrasound: Recommendations for Information to Women and Referring Physicians by the European Society of Breast Imaging. Insights Imaging 2018, 9, 449–461. [Google Scholar] [CrossRef]
- Jared Weinfurtner, R.; Mallory, M.A.; Bermudez, D. Repeat Breast Ultrasound Demonstrates Utility with Added Cancer Detection in Patients Following Breast Imaging Second Opinion Recommendations. Breast J. 2022, 2022, 1561455. [Google Scholar] [CrossRef] [PubMed]
- Dan, Q.; Zheng, T.; Liu, L.; Sun, D.; Chen, Y. Ultrasound for Breast Cancer Screening in Resource-Limited Settings: Current Practice and Future Directions. Cancers 2023, 15, 2112. [Google Scholar] [CrossRef] [PubMed]
- Redmond, C.E.; Healy, G.M.; Murphy, C.F.; O’Doherty, A.; Foster, A. The Use of Ultrasonography and Digital Mammography in Women under 40 Years with Symptomatic Breast Cancer: A 7-Year Irish Experience. Ir. J. Med. Sci. 2017, 186, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Mussetto, I.; Gristina, L.; Schiaffino, S.; Tosto, S.; Raviola, E.; Calabrese, M. Breast Ultrasound: Automated or Hand-Held? Exploring Patients’ Experience and Preference. Eur. Radiol. Exp. 2020, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Dai, T.; Zhang, H. Automatic Detection of Benign/Malignant Tumor in Breast Ultrasound Images Using Optimal Features. Curr. Med. Imaging 2023, 19, 1570–1579. [Google Scholar] [CrossRef]
- Yang, J.H.; Huynh, V.; Leonard, L.D.; Kovar, A.; Bronsert, M.; Ludwigson, A.; Wolverton, D.; Hampanda, K.; Christian, N.; Kim, S.P.; et al. Are Diagnostic Delays Associated with Distress in Breast Cancer Patients? Breast Care 2023, 18, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J. Glob. Oncol. 2019, 5, 1–17. [Google Scholar] [CrossRef]
- Aklilu, S.; Bain, C.; Bansil, P.; de Sanjose, S.; Dunstan, J.A.; Castillo, V.; Tsu, V.; Contreras, I.; Balassanian, R.; Hayes Constant, T.K.; et al. Evaluation of Diagnostic Ultrasound Use in a Breast Cancer Detection Strategy in Northern Peru. PLoS ONE 2021, 16, e0252902. [Google Scholar] [CrossRef] [PubMed]
- Cuesta Cuesta, A.B.; Martín Ríos, M.D.; Noguero Meseguer, M.R.; García Velasco, J.A.; De Matías Martínez, M.; Bartolomé Sotillos, S.; Abreu Griego, E. Precisión de la resonancia magnética, ecografía y mamografía en la medida del tamaño tumoral y su correlación con el tamaño histopatológico en el cáncer de mama primario. Cirugía Española 2019, 97, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.O.; Luz, L.A.D.; Chagas, D.C.; Amorim, J.R.; Nery-Júnior, E.D.J.; Alves, A.C.B.R.; Abreu-Neto, F.T.D.; Oliveira, M.D.C.B.; Silva, D.R.C.; Soares-Júnior, J.M.; et al. Evaluation of the Accuracy of Mammography, Ultrasound and Magnetic Resonance Imaging in Suspect Breast Lesions. Clinics 2020, 75, e1805. [Google Scholar] [CrossRef]
- Bisquera, O.C.; Valparaiso, A.P.; Espiritu, N.T.; Ayuste, E.C.; Paloyo, S.R. Diagnostic Validity of Point-of-Care Breast Ultrasound for Females with Palpable Breast Masses. Clin. Breast Cancer 2023, 23, e189–e193. [Google Scholar] [CrossRef]
- Moradpour, M.; Azizinik, F.; Zeidabadi, H.; Ghomi, Z.; Shakki Katouli, F.; Tavakol, E.; Torabi Parizi, S. The Imaging Findings and Diagnostic Value of Radiology Modalities to Assess Breast Malignancy among Women Aged Younger than 30 Years. Acta Radiol. 2023, 64, 2363–2370. [Google Scholar] [CrossRef]
- Thigpen, D.; Kappler, A.; Brem, R. The Role of Ultrasound in Screening Dense Breasts—A Review of the Literature and Practical Solutions for Implementation. Diagnostics 2018, 8, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Song, Y.; Chen, C.; Wang, Z.; Li, L.; Liu, M.; Liu, G.; Xu, Y.; Zhou, Y.; et al. Comparison of Ultrasound and Mammography for Early Diagnosis of Breast Cancer among Chinese Women with Suspected Breast Lesions: A Prospective Trial. Thorac. Cancer 2022, 13, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Eng, Y.C.M.; Engoumou, A.M.S.; Awana, A.P.; Onembele, S.P.N.; Ntsama, J.A.M.; Zeh, O.F. Histopathological and Ultrasound Correlation in Women Presenting with Breast Lumps in Yaoundé, Cameroon. Open J. Radiol. 2023, 13, 218–231. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, J.; Chen, Q.; Deng, Y. Comparison of the Sensitivity of Mammography, Ultrasound, Magnetic Resonance Imaging and Combinations of These Imaging Modalities for the Detection of Small (≤2 cm) Breast Cancer. Medicine 2021, 100, e26531. [Google Scholar] [CrossRef]
- Kang, J.; Han, K.; Song, I.; Kim, K.-S.; Jang, W.S.; Kim, M.J.; Yoo, Y. Real-Time Ultrasound Detection of Breast Microcalcifications Using Multifocus Twinkling Artifact Imaging. IEEE Trans. Med. Imaging 2022, 41, 1300–1308. [Google Scholar] [CrossRef]
- Park, C.K.S.; Trumpour, T.; Aziz, A.; Bax, J.S.; Tessier, D.; Gardi, L.; Fenster, A. Cost-Effective, Portable, Patient-Dedicated Three-Dimensional Automated Breast Ultrasound for Point-of-Care Breast Cancer Screening. Sci. Rep. 2023, 13, 14390. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guan, Y.; Liang, D.; Li, D.; He, Y.; Liu, Y. Cost-Effectiveness Evaluation of Risk-Based Breast Cancer Screening in Urban Hebei Province. Sci. Rep. 2023, 13, 3370. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Arao, R.F.; Sprague, B.L.; Kerlikowske, K.; Lehman, C.D.; Smith, R.A.; Henderson, L.M.; Rauscher, G.H.; Miglioretti, D.L. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern. Med. 2019, 179, 658–667. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, D.; Warren, R.; MacKay, J.; Britton, P.; Day, N.E. Screening with Breast Ultrasound in a Population at Moderate Risk Due to Family History. J. Med. Screen. 2001, 8, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.; Calabrese, M.; Martinoli, C.; Tagliafico, A.S. Artificial Intelligence in Breast Ultrasound: From Diagnosis to Prognosis—A Rapid Review. Diagnostics 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Portnow, L.H.; Choridah, L.; Kardinah, K.; Handarini, T.; Pijnappel, R.; Bluekens, A.M.J.; Duijm, L.E.M.; Schoub, P.K.; Smilg, P.S.; Malek, L.; et al. International Interobserver Variability of Breast Density Assessment. J. Am. Coll. Radiol. 2023, 20, 671–684. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.; Morgan, M.P. Microcalcifications in Breast Cancer: From Pathophysiology to Diagnosis and Prognosis. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2018, 1869, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.T.; Helvie, M.A. Breast Cancer Screening in Average and High-Risk Women. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 83, 3–14. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Ren, W.; Chen, M.; Qiao, Y.; Zhao, F. Global Guidelines for Breast Cancer Screening: A Systematic Review. Breast 2022, 64, 85–99. [Google Scholar] [CrossRef]
- Sadeghi-Naini, A.; Suraweera, H.; Tran, W.T.; Hadizad, F.; Bruni, G.; Rastegar, R.F.; Curpen, B.; Czarnota, G.J. Breast-Lesion Characterization Using Textural Features of Quantitative Ultrasound Parametric Maps. Sci. Rep. 2017, 7, 13638. [Google Scholar] [CrossRef]
- Alshoabi, S.A.; Alareqi, A.A.; Alhazmi, F.H.; Qurashi, A.A.; Omer, A.M.; Hamid, A.M. Utility of Ultrasound Imaging Features in Diagnosis of Breast Cancer. Cureus 2023, 15, e37691. [Google Scholar] [CrossRef]
- Thomas, R.; Das, S.K.; Balasubramanian, G.; Chandrappa, A. Correlation of Mammography, Ultrasound and Sonoelastographic Findings With Histopathological Diagnosis in Breast Lesions. Cureus 2022, 14, e32318. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.A.; Whaley, D.H.; Lee, C.U. Ultrasound-Guided Breast Biopsies: Basic and New Techniques. J. Ultrasound Med. 2021, 40, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, Y.; Yang, F.; Lin, Y.; Xu, S.; Yin, W.; Zhou, L.; Lu, J. Ultrasound-Guided Breast Biopsy: Improved Accuracy of 10-G Cable-Free Elite Compared With 14-G CCNB. J. Surg. Res. 2020, 247, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, D.; Gao, D.; Lv, X.; Feng, Y.; Zhang, D.; Ye, W. Value of Ultrasound-Guided Biopsy in Evaluating Internal Mammary Lymph Node Metastases in Breast Cancer. Clin. Breast Cancer 2021, 21, 532–538. [Google Scholar] [CrossRef]
- Omidiji, O.A.T.; Campbell, P.C.; Irurhe, N.K.; Atalabi, O.M.; Toyobo, O.O. Breast Cancer Screening in a Resource Poor Country: Ultrasound versus Mammography. Ghana Med. J. 2017, 51, 6–12. [Google Scholar] [CrossRef]
- Burgess, M.T.; Gluskin, J.; Pinker, K. From Bedside to Portable and Wearable: Development of a Conformable Ultrasound Patch for Deep Breast Tissue Imaging. Mol. Oncol. 2023, 17, 1947–1949. [Google Scholar] [CrossRef]
- Rebolj, M.; Assi, V.; Brentnall, A.; Parmar, D.; Duffy, S.W. Addition of Ultrasound to Mammography in the Case of Dense Breast Tissue: Systematic Review and Meta-Analysis. Br. J. Cancer 2018, 118, 1559–1570. [Google Scholar] [CrossRef]
- Faguy, K. Breast Sonography and Mammography: Complementarity and Correlation. Radiol. Technol. 2017, 89, 45M–64M. [Google Scholar]
- Sivarajah, R.T.; Brown, K.; Chetlen, A. “I Can See Clearly Now.” Fundamentals of Breast Ultrasound Optimization. Clin. Imaging 2020, 64, 124–135. [Google Scholar] [CrossRef]
- Afrin, H.; Larson, N.B.; Fatemi, M.; Alizad, A. Deep Learning in Different Ultrasound Methods for Breast Cancer, from Diagnosis to Prognosis: Current Trends, Challenges, and an Analysis. Cancers 2023, 15, 3139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Ding, L.; Liang, X.; Wang, Y.; Greuter, M.J.W.; De Bock, G.H.; Lu, W. Is Ultrasound an Accurate Alternative for Mammography in Breast Cancer Screening in an Asian Population? A Meta-Analysis. Diagnostics 2020, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, S.; Zhang, L.; Sheng, C.; Song, F.; Wang, P.; Huang, Y. Performance of Ultrasonography Screening for Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 499. [Google Scholar] [CrossRef]
- Shen, Y.; Shamout, F.E.; Oliver, J.R.; Witowski, J.; Kannan, K.; Park, J.; Wu, N.; Huddleston, C.; Wolfson, S.; Millet, A.; et al. Artificial Intelligence System Reduces False-Positive Findings in the Interpretation of Breast Ultrasound Exams. Nat. Commun. 2021, 12, 5645. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Vijapura, C.; Patel, M.; De La Cruz, A.; Wahab, R. Breast Cancer in Dense Breasts: Detection Challenges and Supplemental Screening Opportunities. RadioGraphics 2023, 43, e230024. [Google Scholar] [CrossRef] [PubMed]
- Vourtsis, A.; Berg, W.A. Breast Density Implications and Supplemental Screening. Eur. Radiol. 2019, 29, 1762–1777. [Google Scholar] [CrossRef]
- Boca, I.; Ciurea, A.I.; Ciortea, C.A.; Dudea, S.M. Pros and Cons for Automated Breast Ultrasound (ABUS): A Narrative Review. J. Pers. Med. 2021, 11, 703. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.H.; Moon, W.K. Automated Breast Ultrasound Screening for Dense Breasts. Korean J. Radiol. 2020, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rahmat, K.; Ab Mumin, N.; Ng, W.L.; Mohd Taib, N.A.; Chan, W.Y.; Ramli Hamid, M.T. Automated Breast Ultrasound Provides Comparable Diagnostic Performance in Opportunistic Screening and Diagnostic Assessment. Ultrasound Med. Biol. 2024, 50, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Sharma, R. Benign Lesions on Screening Mammography: Increasing Diagnostic Confidence in a Hitherto Unscreened Population. J. Clin. Diagn. Res. 2017, 11, TC14–TC17. [Google Scholar] [CrossRef]
- Masciadri, N.; Ferranti, C. Benign breast lesions: Ultrasound. J. Ultrasound 2011, 14, 55–65. [Google Scholar] [CrossRef]
- Corvino, A.; Varelli, C.; Catalano, F.; Cocco, G.; Delli Pizzi, A.; Boccatonda, A.; Corvino, F.; Basile, L.; Catalano, O. Use of High-Frequency Transducers in Breast Sonography. J. Pers. Med. 2022, 12, 1960. [Google Scholar] [CrossRef]
- Ibrahim, R.; Rahmat, K.; Fadzli, F.; Rozalli, F.I.; Westerhout, C.J.; Alli, K.; Vijayananthan, A.; Moosa, F. Evaluation of solid breast lesions with power Doppler: Value of penetrating vessels as a predictor of malignancy. Singap. Med. J. 2016, 57, 634–640. [Google Scholar] [CrossRef]
- Busilacchi, P.; Draghi, F.; Preda, L.; Ferranti, C. Has color Doppler a role in the evaluation of mammary lesions? J. Ultrasound 2012, 15, 93–98. [Google Scholar] [CrossRef] [PubMed]
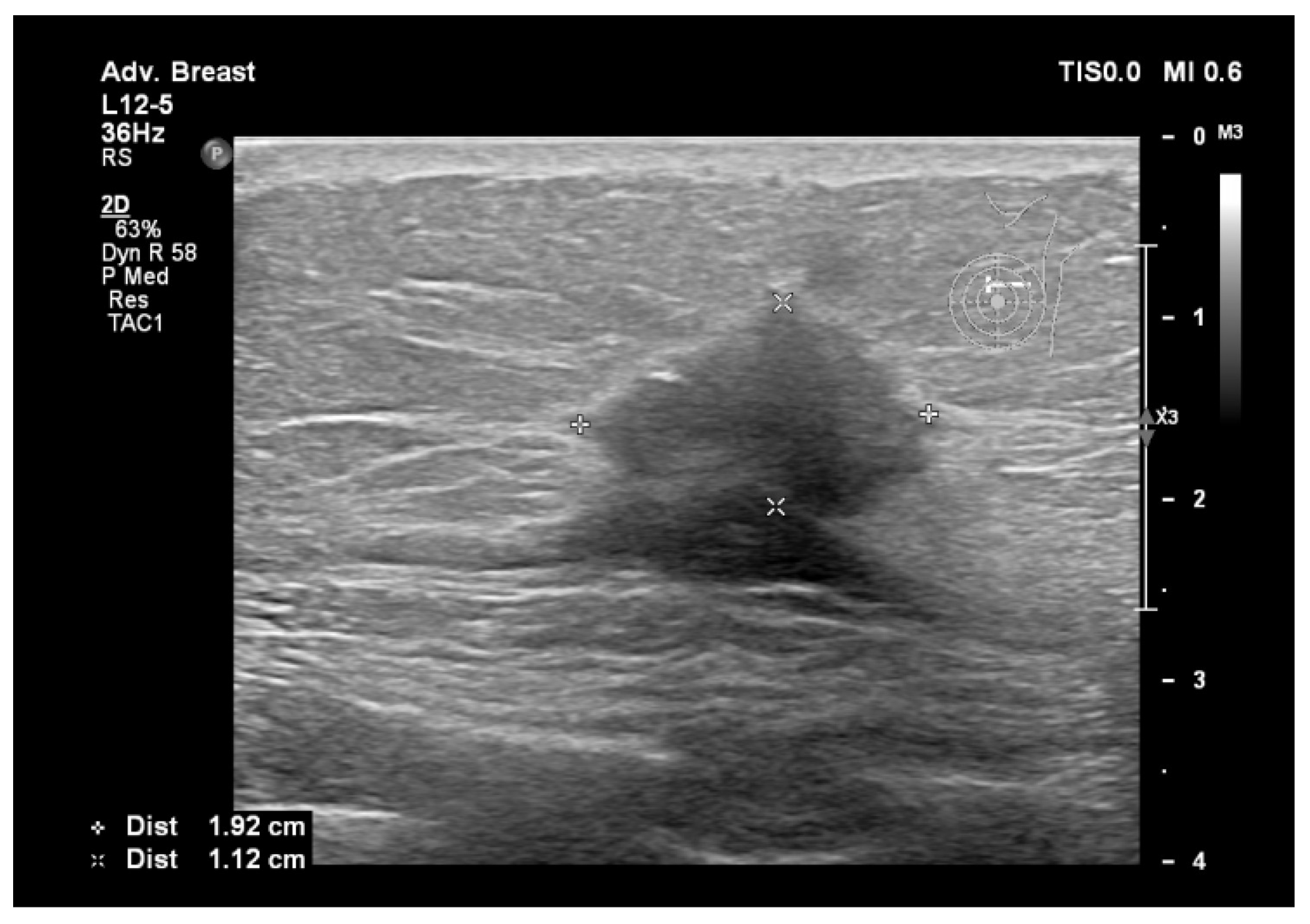
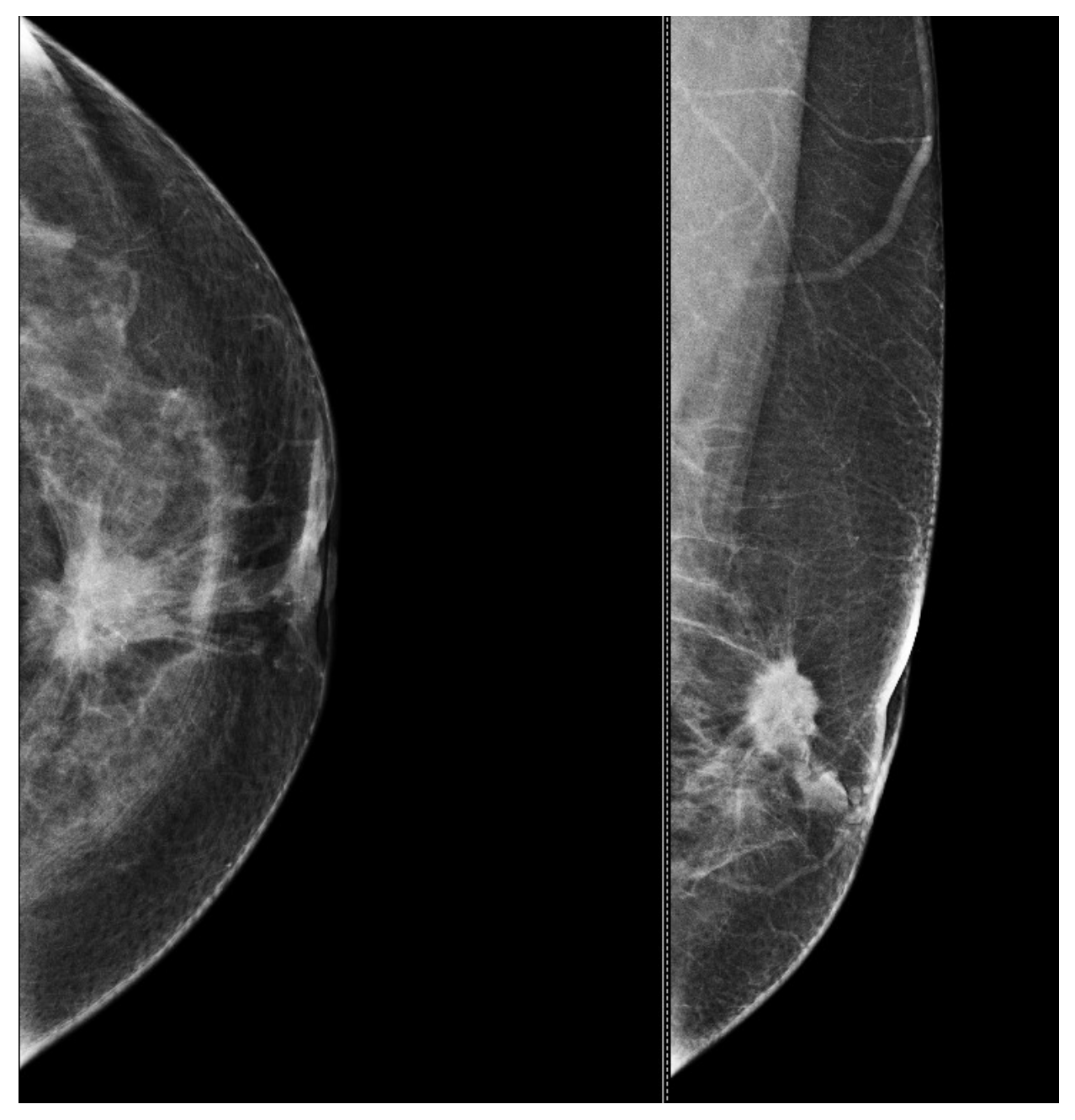
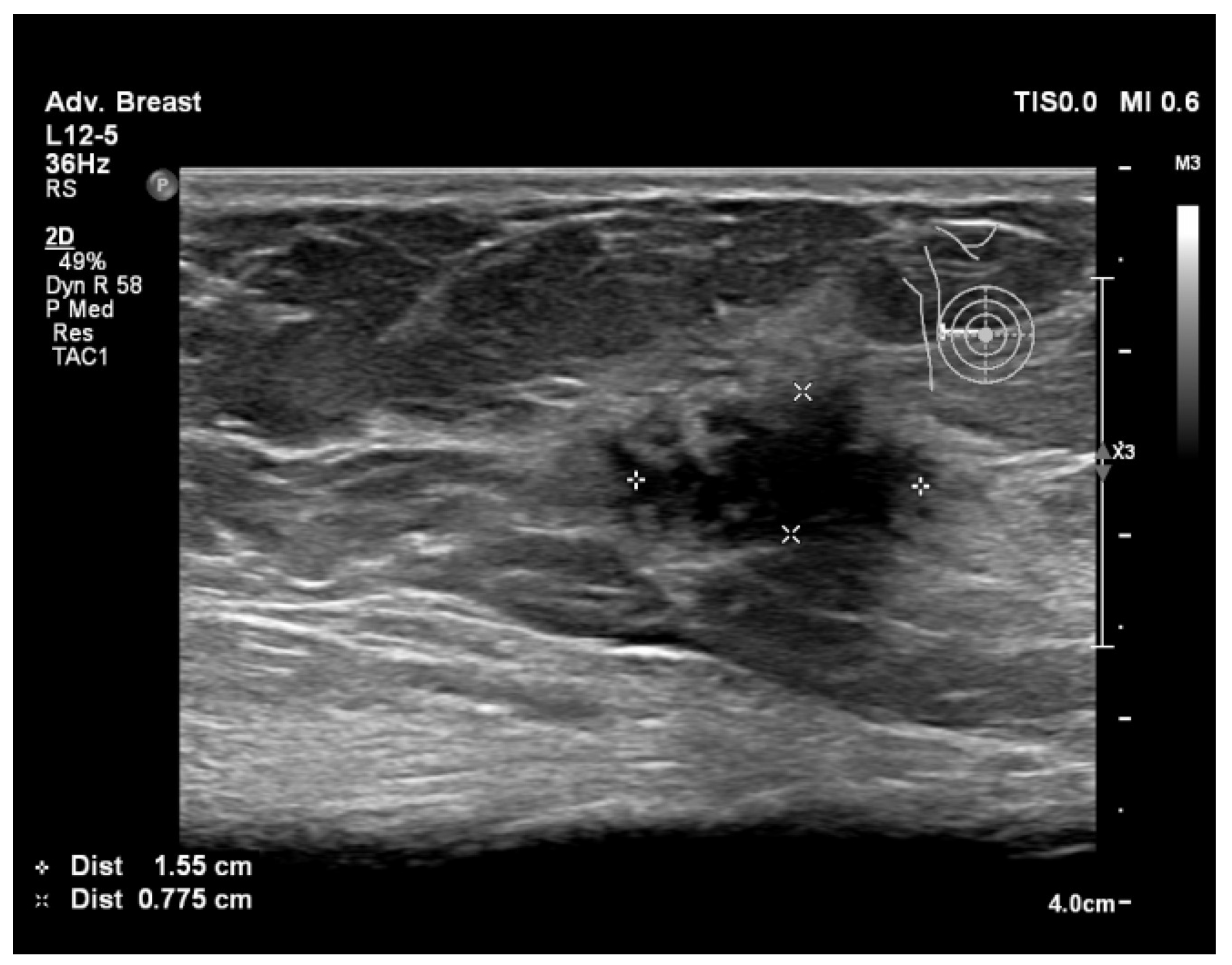
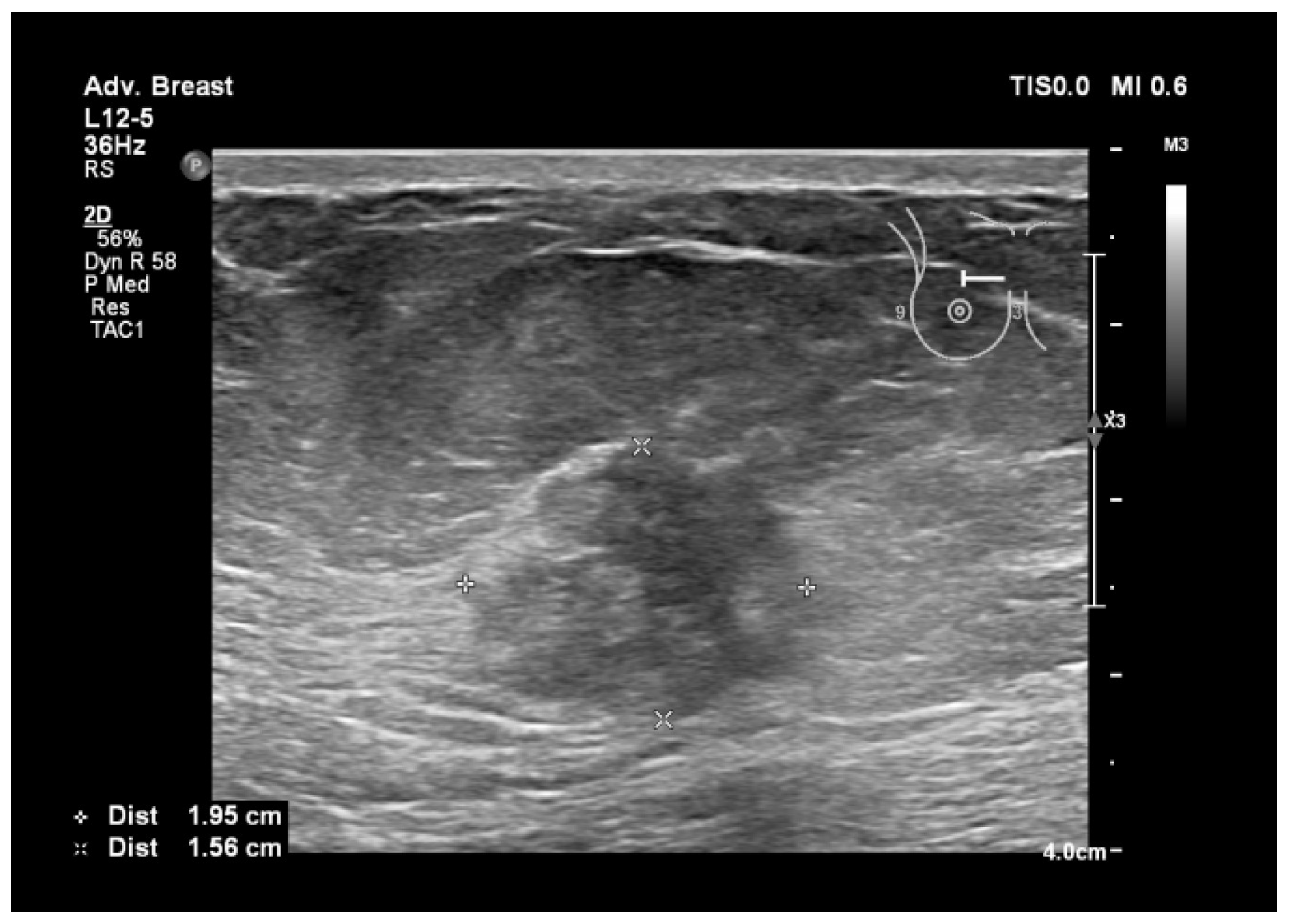
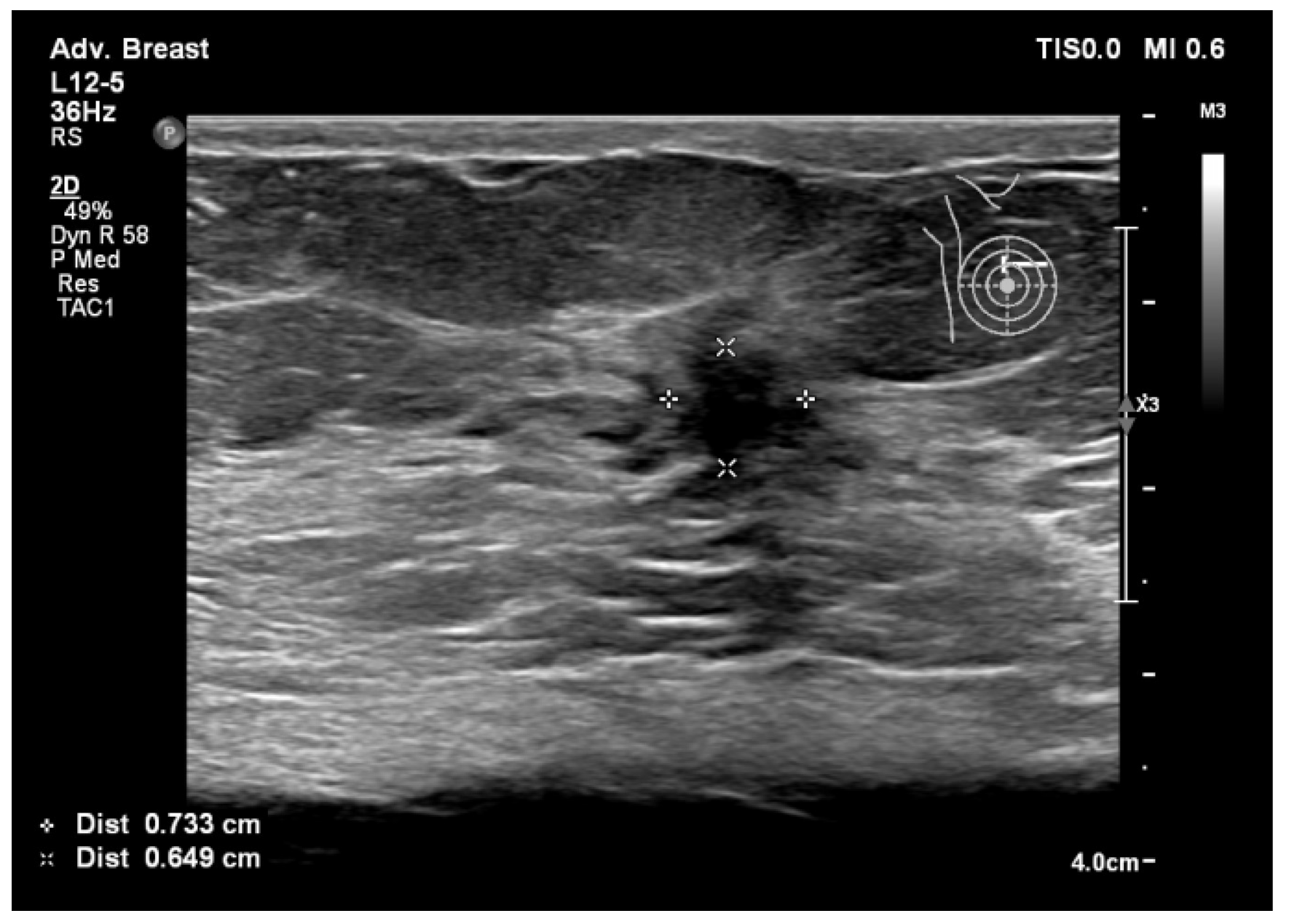
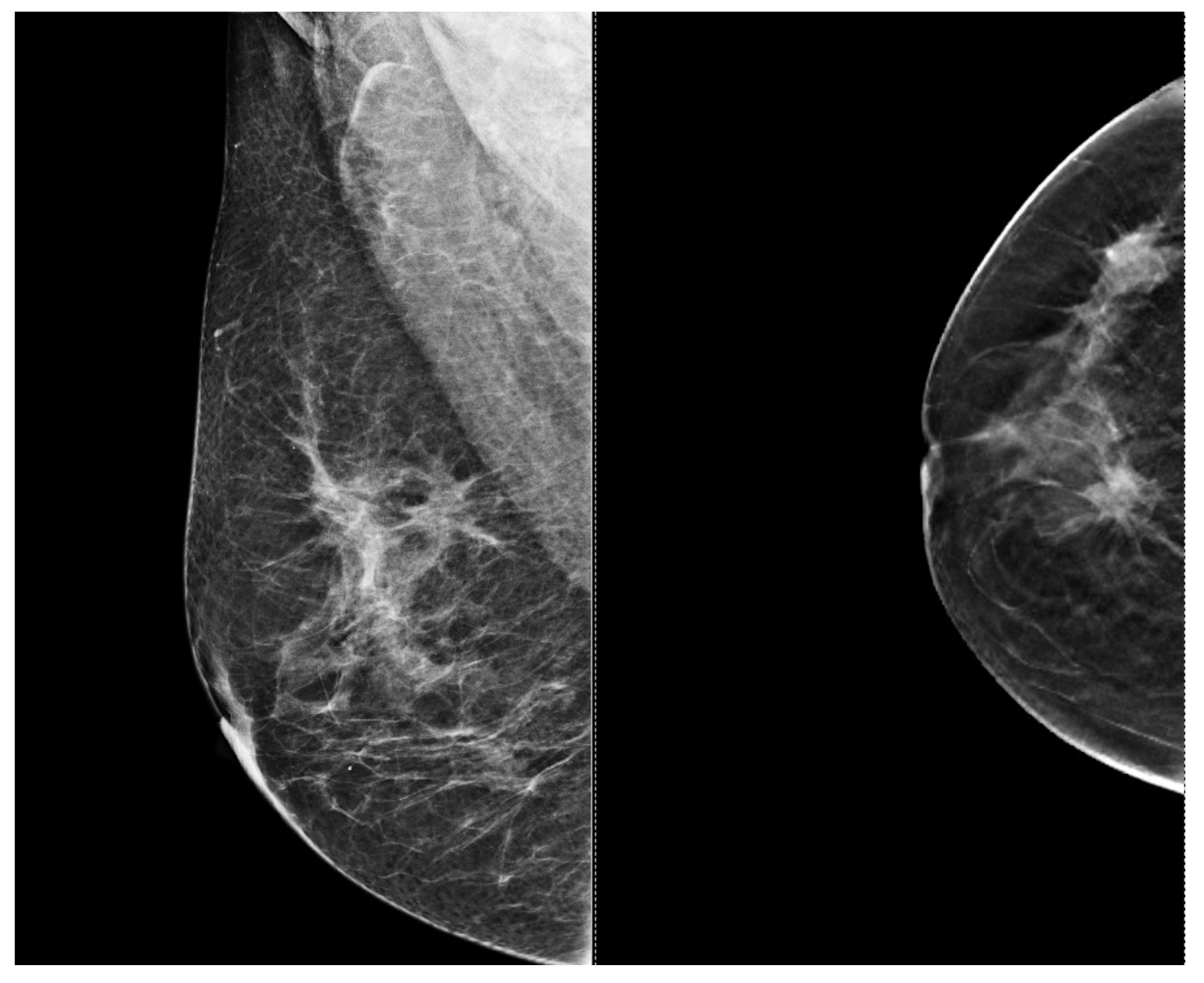
| Aspect | Ultrasound in Breast Cancer Screening and Diagnosis |
|---|---|
| Advantages |
|
| Disadvantages |
|
| Applications |
Considerations: operator-dependent, potential for false positives. |
Considerations: limited microcalcification detection, operator dependence. | |
Considerations: coordination between modalities required. | |
Considerations: may not replace comprehensive exams in all cases. | |
Considerations: limited by equipment and expertise availability. |
| Feature | ABUS | Handheld Ultrasound |
|---|---|---|
| Process of the Technique | Automated scanning providing standardized images | Operator-dependent, manual control over scanning areas |
| Coverage | Comprehensive coverage reducing operator variability | Limited coverage, dependent on operator’s skill and experience |
| Operator Dependence | Reduced operator dependence, minimizing variability | High dependence on operator’s skills, potential variability |
| Detection Accuracy | High sensitivity with systematic approach | Detection accuracy varies based on operator’s proficiency |
| Limitations | May generate more false positives, limited microcalcification detection | Operator-dependent, potential coverage and reproducibility limitations, limited microcalcification detection |
| Aspect | Breast Ultrasound | Mammography |
| Advantages |
|
|
| Disadvantages |
|
|
| Research Focus | Sample Characteristics |
|---|---|
| Overall advantages of breast ultrasound for breast tissue evaluation | Individuals requiring regular screenings |
| Those with family history of breast cancer or genetic predisposition | |
| Younger patients, particularly females below 40 years old | |
| Patients seeking comfort and decreased fear during imaging | |
| Advantages of breast ultrasound in breast cancer screening | Women with dense breast tissue |
| Versatile and dynamic real-time imaging | |
| Accessibility and cost-effectiveness | |
| Application in high-risk populations and younger women | |
| Disadvantages/limitations of breast ultrasound in breast cancer screening | Increased false positive results |
| Operator dependence and variability | |
| Limited ability to detect microcalcifications | |
| Limited performance in high-risk or dense breast tissue | |
| Advantages of breast ultrasound in breast cancer diagnosis | Characterization of lesions |
| Real-time imaging and guided biopsies | |
| No ionizing radiation | |
| Supplementary imaging in challenging cases | |
| Disadvantages/limitations of breast ultrasound in breast cancer diagnosis | Operator dependence and variability |
| Lower specificity and increased false positives | |
| Challenges in evaluated dense breast tissue | |
| ABUS in breast cancer screening and diagnosis | Automated and uniform coverage |
| Efficiency and time-saving benefits | |
| High false positive rate | |
| Limited ability to detect microcalcifications | |
| Requires a multimodal approach for comprehensive screening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacob, R.; Iacob, E.R.; Stoicescu, E.R.; Ghenciu, D.M.; Cocolea, D.M.; Constantinescu, A.; Ghenciu, L.A.; Manolescu, D.L. Evaluating the Role of Breast Ultrasound in Early Detection of Breast Cancer in Low- and Middle-Income Countries: A Comprehensive Narrative Review. Bioengineering 2024, 11, 262. https://doi.org/10.3390/bioengineering11030262
Iacob R, Iacob ER, Stoicescu ER, Ghenciu DM, Cocolea DM, Constantinescu A, Ghenciu LA, Manolescu DL. Evaluating the Role of Breast Ultrasound in Early Detection of Breast Cancer in Low- and Middle-Income Countries: A Comprehensive Narrative Review. Bioengineering. 2024; 11(3):262. https://doi.org/10.3390/bioengineering11030262
Chicago/Turabian StyleIacob, Roxana, Emil Radu Iacob, Emil Robert Stoicescu, Delius Mario Ghenciu, Daiana Marina Cocolea, Amalia Constantinescu, Laura Andreea Ghenciu, and Diana Luminita Manolescu. 2024. "Evaluating the Role of Breast Ultrasound in Early Detection of Breast Cancer in Low- and Middle-Income Countries: A Comprehensive Narrative Review" Bioengineering 11, no. 3: 262. https://doi.org/10.3390/bioengineering11030262
APA StyleIacob, R., Iacob, E. R., Stoicescu, E. R., Ghenciu, D. M., Cocolea, D. M., Constantinescu, A., Ghenciu, L. A., & Manolescu, D. L. (2024). Evaluating the Role of Breast Ultrasound in Early Detection of Breast Cancer in Low- and Middle-Income Countries: A Comprehensive Narrative Review. Bioengineering, 11(3), 262. https://doi.org/10.3390/bioengineering11030262









