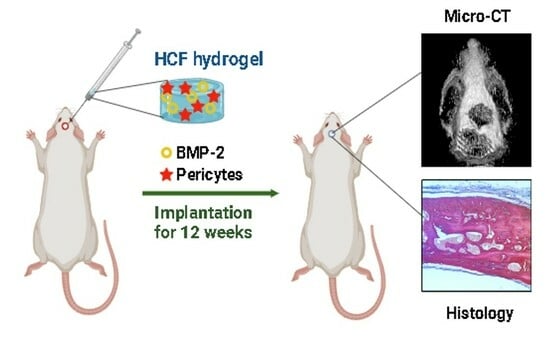
Repair of Rat Calvarial Critical-Sized Defects Using Heparin-Conjugated Fibrin Hydrogel Containing BMP-2 and Adipose-Derived Pericytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture of Rat Adipose-Derived Pericytes
2.3. Preparation of the HCF Hydrogel
2.4. Scanning Electron Microscopy (SEM)
2.5. Measurement of In Vitro BMP-2 Release Kinetics
2.6. Bioactivity of Released BMP-2 In Vitro
2.7. Mineralization Assay
2.8. Osteocalcin and Calcium Assay
2.9. Rat Calvarial Defect Model and Implantation
2.10. Micro-CT Analysis
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
3.1. Characterization of HCF Hydrogel
3.2. BMP-2 Induces Osteogenic Differentiation of ADPs
3.3. Effects of HFC Hydrogel Containing BMP-2 and ADPs on Bone Tissue Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Nishida, J.; Shimamura, T. Methods of Reconstruction for Bone Defect after Tumor Excision: A Review of AlterNatives. Med. Sci. Monit. 2008, 14, RA107–RA113. [Google Scholar]
- Goulet, J.A.; Senunas, L.E.; De Silva, G.L.; Greenfield, M.L. Autogenous Iliac Crest Bone Graft. Complications and Functional Assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Rabitsch, K.; Maurer-Ertl, W.; Pirker-Fruhauf, U.; Wibmer, C.; Leithner, A. Intercalary Reconstructions with Vascularised Fibula and Allograft after Tumour Resection in the Lower Limb. Sarcoma 2013, 2013, 160295. [Google Scholar] [CrossRef]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel Hydroxyapatite Ceramics with an Interconnective Porous Structure Exhibit Superior Osteoconduction In Vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Choi, B.; Kim, S.; Lin, B.; Wu, B.M.; Lee, M. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6, 20110–20121. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable Chitosan-Based Hydrogels for Cartilage Tissue Engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair Shantikumar, V.; Jayakumar, R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled Release by Biodegradable Hydrogels Enhances the Ectopic Bone Formation of Bone Morphogenetic Protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Cheng, Y.; Nada, A.A.; Valmikinathan, C.M.; Lee, P.; Liang, D.; Yu, X.; Kumbar, S.G. In Situ Gelling Polysaccharide-Based Hydrogel for Cell and Drug Delivery in Tissue Engineering. J. Appl. Polym. Sci. 2014, 131, 39934. [Google Scholar] [CrossRef]
- Haifeng, J.; Song, X.; Cheng, H.; Luo, L.; Huang, J.; He, C.; Yin, J.; Zhao, W.; Qiu, L.; Zhao, C. Biocompatible In Situ Polymerization of Multipurpose Polyacrylamide-Based Hydrogels on Skin via Silver Ion Catalyzation. ACS Appl. Mater. Interfaces. 2020, 12, 31079–31089. [Google Scholar]
- James, A.W.; Zara, J.N.; Corselli, M.; Askarinam, A.; Zhou, A.M.; Hourfar, A.; Nguyen, A.; Megerdichian, S.; Asatrian, G.; Pang, S.; et al. An Abundant Perivascular Source of Stem Cells for Bone Tissue Engineering. Stem Cells Transl. Med. 2012, 1, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Péault, B. Perivascular Mesenchymal Progenitors for Bone Regeneration. J. Orthop. Res. 2019, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Zara, J.N.; Zhang, X.; Askarinam, A.; Goyal, R.; Chiang, M.; Yuan, W.; Chang, L.; Corselli, M.; Shen, J.; et al. Perivascular Stem Cells: A Prospectively Purified Mesenchymal Stem Cell Population for Bone Tissue Engineering. Stem Cells Transl. Med. 2012, 1, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Askarinam, A.; James, A.W.; Zara, J.N.; Goyal, R.; Corselli, M.; Pan, A.; Liang, P.; Chang, L.; Rackohn, T.; Stoker, D.; et al. Human Perivascular Stem Cells Show Enhanced Osteogenesis and Vasculogenesis with Nel-Like Molecule I Protein. Tissue Eng. Part A 2013, 19, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Tawonsawatruk, T.; West, C.C.; Murray, I.R.; Soo, C.; Péault, B.; Simpson, H. Adipose Derived Pericytes Rescue Fractures from a Failure of Healing—Non-Union. Sci. Rep. 2016, 6, 22779. [Google Scholar] [CrossRef]
- Granjeiro, J.M.; Oliveira, R.C.; Bustos-Valenzuela, J.C.; Sogayar, M.C.; Taga, R. Bone Morphogenetic Proteins: From Structure to Clinical Use. Braz. J. Med. Biol. Res. 2005, 10, 1463–1473. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Frey, S.P.; Sonke, P.; Zellweger, R.; Fracs, F. Bone Morphogenetic Proteins in Clinical Applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef]
- Carter, T.G.; Brar, P.S.; Tolas, A.; Beirne, O.R. Off-Label Use of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) for Reconstruction of Mandibular Bone Defects in Humans. J. Oral. Maxillofac. Surg. 2008, 66, 1417–1425. [Google Scholar] [CrossRef]
- McKay, B.; Sandhu, H.S. Use of Recombinant Human Bone Morphogenetic Protein-2 in Spinal Fusion Applications. Spine 2002, 27, S66–S85. [Google Scholar] [CrossRef]
- Mumcuoglu, D.; Fahmy-Garcia, S.; Ridwan, Y.; Nicke, J.; Farrell, E.; Kluijtmans, S.G.; van Osch, G.J. Injectable BMP-2 Delivery System Based on Collagen-Derived Microspheres and Alginate Induced Bone Formation in a Time- and Dose-Dependent Manner. Eur. Cell Mater. 2018, 26, 242–254. [Google Scholar] [CrossRef]
- Engstrand, T.; Veltheim, R.; Arnander, C.; Docherty-Skogh, A.C.; Westermark, A.; Ohlsson, C.; Adolfsson, L.; Larm, O. A Novel Biodegradable Delivery System for Bone Morphogenetic Protein-2. Plast. Reconstr. Surg. 2008, 121, 1920–1928. [Google Scholar] [CrossRef]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Jeon, J.Y.; Lee, J.H.; Kim, B.S. Heparin-Conjugated Fibrin as an Injectable System for Sustained Delivery of Bone Morphogenetic Protein-2. Tissue Eng. Part A 2010, 4, 1225–1233. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Collen, A.; Smorenburg, S.M.; Peters, E.; Lupu, F.; Koolwijk, P.; Van Noorden, C.; van Hinsbergh, V.W. Unfractionated and Low Molecular Weight Heparin Affect Fibrin Structure and Angiogenesis In Vitro. Cancer Res. 2000, 60, 6196–6200. [Google Scholar]
- Ogay, V.; Kumasheva, V.; Li, Y.; Mukhlis, S.; Sekenova, A.; Olzhayev, F.; Tsoy, A.; Umbayev, B.; Askarova, S.; Shpekov, A.; et al. Improvement of Neurological Function in Rats with Ischemic Stroke by Adipose-Derived Pericytes. Cell Transplant. 2020, 29, 963689720956956. [Google Scholar] [CrossRef]
- Sarsenova, M.; Raimagambetov, Y.; Issabekova, A.; Karzhauov, M.; Kudaibergen, G.; Akhmetkarimova, Z.; Batpen, A.; Ramankulov, Y.; Ogay, V. Regeneration of Osteochondral Defects by Combined Delivery of Synovium-Derived Mesenchymal Stem Cells, TGF-β1 and BMP-4 in Heparin-Conjugated Fibrin Hydrogel. Polymers 2022, 14, 5343. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Martin-Iglesias, S.; Milian, L.; Sancho-Tello, M.; Salvador-Clavell, R.; Martín de Llano, J.J.; Carda, C.; Mata, M. BMP-2 Enhances Osteogenic Differentiation of Human Adipose-Derived and Dental Pulp Stem Cells in 2D and 3D In Vitro Models. Stem Cells Int. 2022, 2022, 4910399. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Chang, L.; Meyers, C.A.; Zhang, L.; Broderick, K.; Lee, M.; Peault, B.; James, A.W. Relative Contributions of Adipose-Resident CD146+ Pericytes and CD34+ Adventitial Progenitor Cells in Bone Tissue Engineering. NPJ Regen. Med. 2019, 4, 1. [Google Scholar] [CrossRef]
- Chen, C.W.; Montelatici, E.; Crisan, M.; Corselli, M.; Huard, J.; Lazzari, L.; Péault, B. Perivascular Multi-Lineage Progenitor Cells in Human Organs: Regenerative Units, Cytokine Sources or Both? Cytokine Growth Factor Rev. 2009, 20, 429–434. [Google Scholar] [CrossRef]
- Tabei, Y.; Lin, W.; Shiomoto, S.; Nakayama, T.; Sonoda, A.; Horie, M. Development of Fibrin Hydrogel-Based In Vitro Bioassay System for Assessment of Skin Permeability to and Pro-inflammatory Activity Mediated by Zinc Ion Released from Nanoparticles. Anal. Bioanal. Chem. 2020, 412, 8269–8282. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-Based and Heparin-Inspired Hydrogels: Size-effect, Gelation and Biomedical Applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mehta, D.; Dasgupta, U.; Bajaj, A. Advances in Engineering of Low Molecular Weight Hydrogels for Chemotherapeutic Applications. Biomed. Mater. 2021, 16, 024102. [Google Scholar] [CrossRef]
- Issabekova, A.; Kudaibergen, G.; Sekenova, A.; Dairov, A.; Sarsenova, M.; Mukhlis, S.; Temirzhan, A.; Baidarbekov, M.; Eskendirova, S.; Ogay, V. The Therapeutic Potential of Pericytes in Bone Tissue Regeneration. Biomedicines 2023, 12, 21. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudaibergen, G.; Mukhlis, S.; Mukhambetova, A.; Issabekova, A.; Sekenova, A.; Sarsenova, M.; Temirzhan, A.; Baidarbekov, M.; Umbayev, B.; Ogay, V. Repair of Rat Calvarial Critical-Sized Defects Using Heparin-Conjugated Fibrin Hydrogel Containing BMP-2 and Adipose-Derived Pericytes. Bioengineering 2024, 11, 437. https://doi.org/10.3390/bioengineering11050437
Kudaibergen G, Mukhlis S, Mukhambetova A, Issabekova A, Sekenova A, Sarsenova M, Temirzhan A, Baidarbekov M, Umbayev B, Ogay V. Repair of Rat Calvarial Critical-Sized Defects Using Heparin-Conjugated Fibrin Hydrogel Containing BMP-2 and Adipose-Derived Pericytes. Bioengineering. 2024; 11(5):437. https://doi.org/10.3390/bioengineering11050437
Chicago/Turabian StyleKudaibergen, Gulshakhar, Sholpan Mukhlis, Ainur Mukhambetova, Assel Issabekova, Aliya Sekenova, Madina Sarsenova, Abay Temirzhan, Murat Baidarbekov, Baurzhan Umbayev, and Vyacheslav Ogay. 2024. "Repair of Rat Calvarial Critical-Sized Defects Using Heparin-Conjugated Fibrin Hydrogel Containing BMP-2 and Adipose-Derived Pericytes" Bioengineering 11, no. 5: 437. https://doi.org/10.3390/bioengineering11050437
APA StyleKudaibergen, G., Mukhlis, S., Mukhambetova, A., Issabekova, A., Sekenova, A., Sarsenova, M., Temirzhan, A., Baidarbekov, M., Umbayev, B., & Ogay, V. (2024). Repair of Rat Calvarial Critical-Sized Defects Using Heparin-Conjugated Fibrin Hydrogel Containing BMP-2 and Adipose-Derived Pericytes. Bioengineering, 11(5), 437. https://doi.org/10.3390/bioengineering11050437