Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Pericardia Preparation
2.2. Hybrid Membrane Fabrication
2.3. Tissue Sterilization
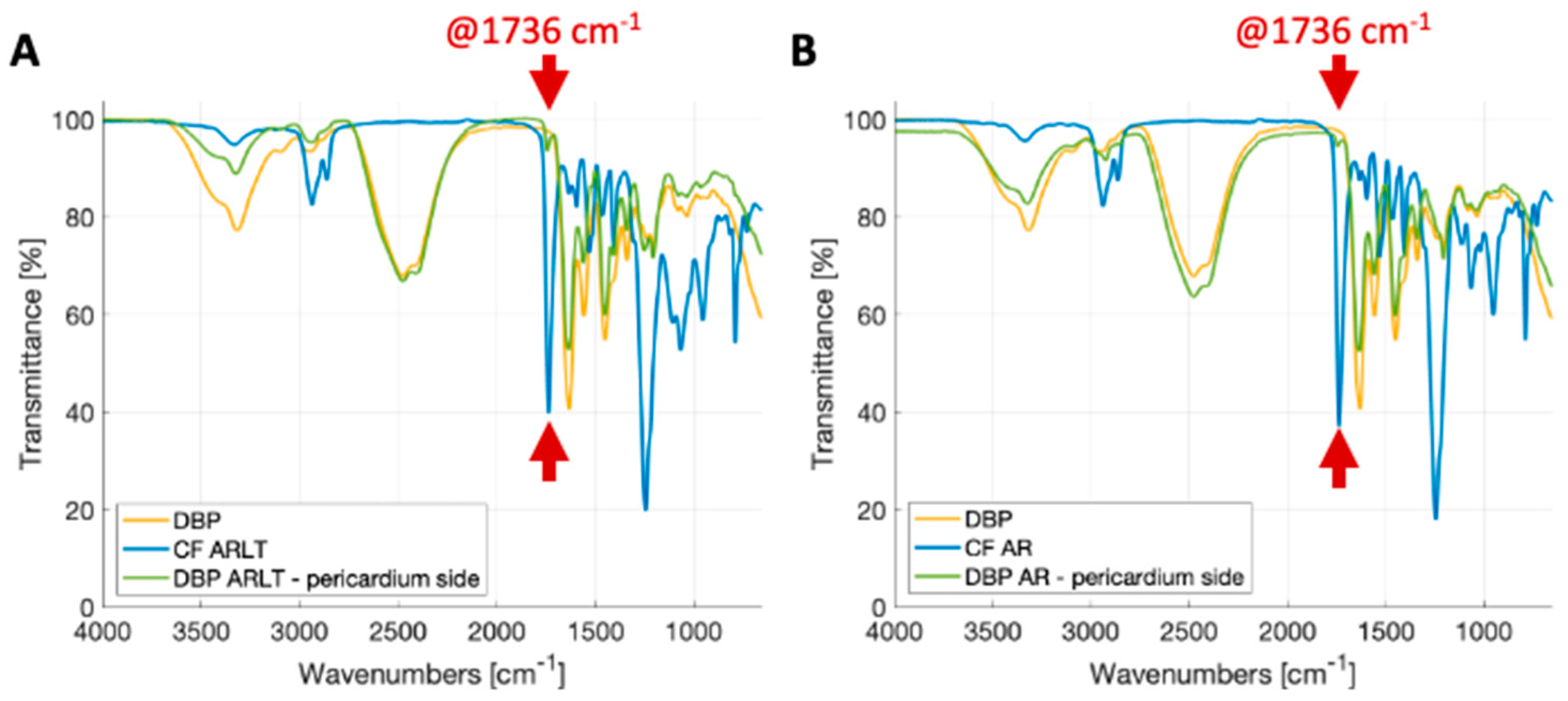
2.4. ATR-FTIR Analysis
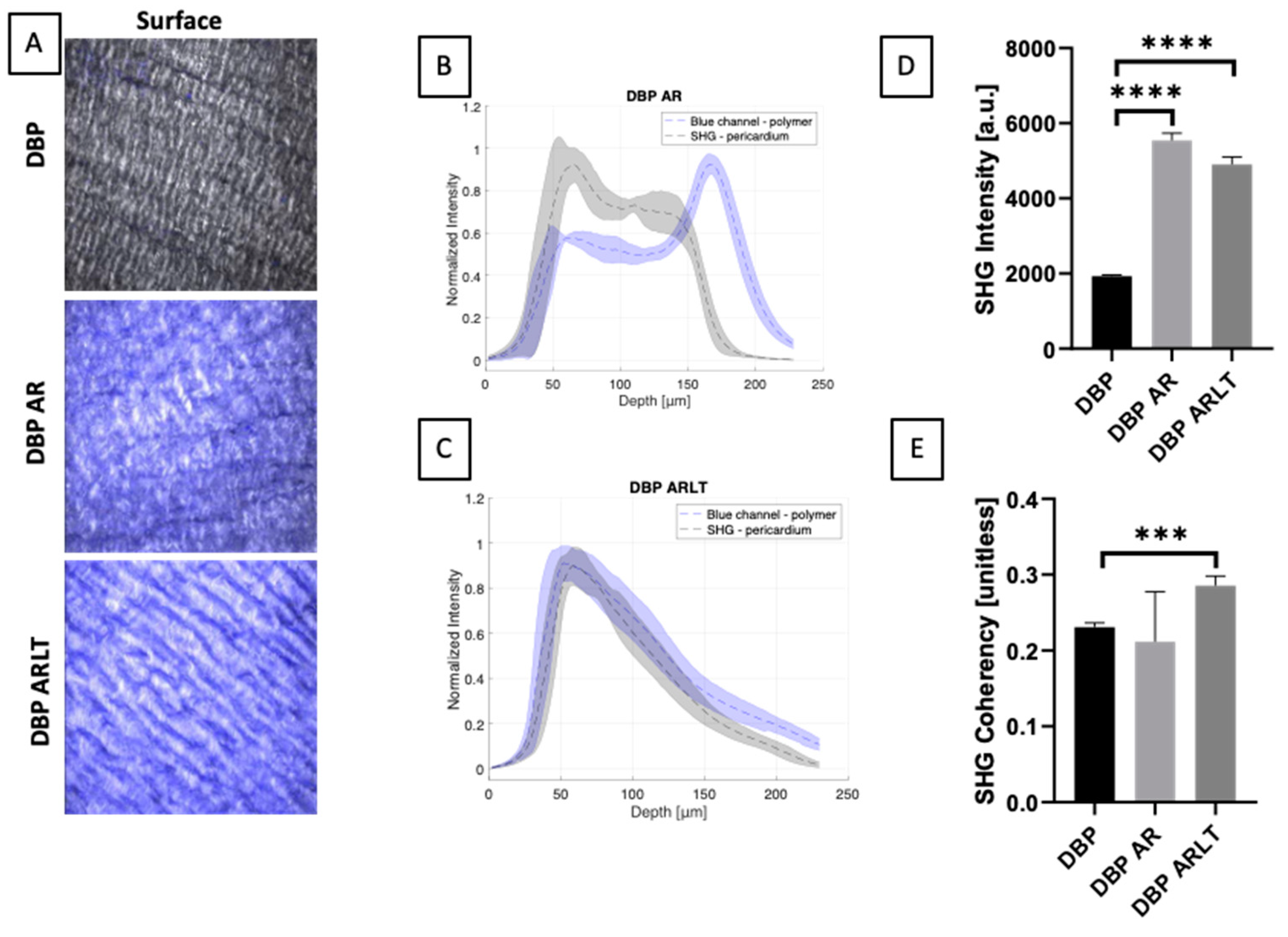
2.5. Two-Photon Microscopy
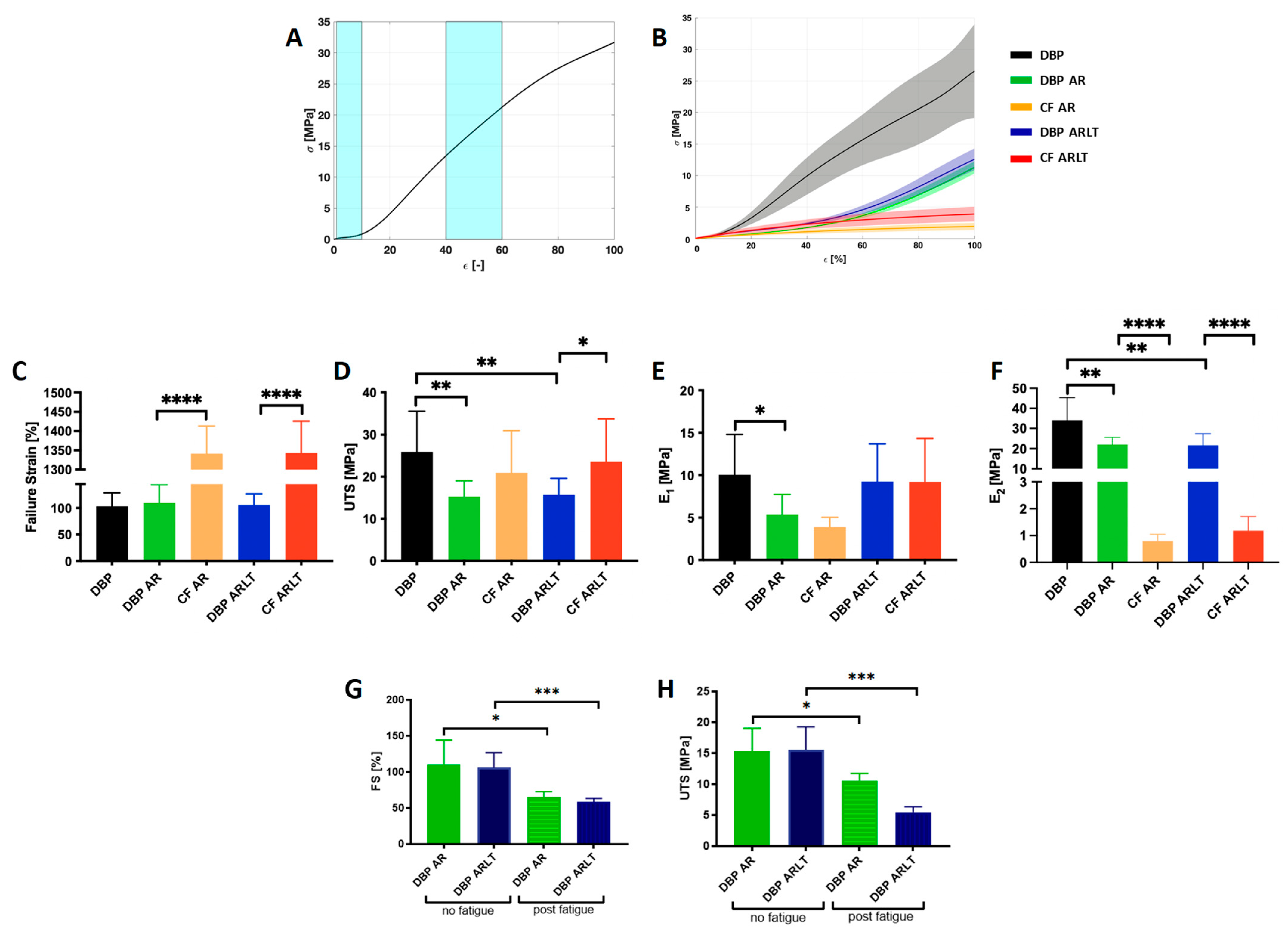
2.6. Mechanical Characterization
2.7. Sterility Test
2.8. Cytotoxicity Assay
3. Results
3.1. Membrane Aspect and Polymer Penetration
3.2. Mechanical Characterization
3.3. Materials Sterilization and Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casarin, M.; Todesco, M.; Fontanella, C.G.; Morlacco, A.; Dal Moro, F.; Bagno, A. Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields. Processes 2023, 11, 2013. [Google Scholar] [CrossRef]
- Chowdhury, S.; Pal, B.; Datta, P. Composite Biomaterials for Bone Grafting and Other Biomedical Applications. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 697–716. ISBN 978-0-12-823291-0. [Google Scholar]
- Hashmi, S. (Ed.) Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-823291-0. [Google Scholar]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.-K.; Na, K.; Keun Han, D. Advanced Hybrid Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Zhao, X.; Wang, Z. Fabrication and Applications of Bioactive Chitosan-Based Organic-Inorganic Hybrid Materials: A Review. Carbohydr. Polym. 2021, 267, 118179. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Liu, Y.-Q.; Zhang, L.-Y.; Liang, D.; Xu, G.-K. Emergence, Pattern, and Frequency of Spontaneous Waves in Spreading Epithelial Monolayers. Nano Lett. 2024, 24, 3631–3637. [Google Scholar] [CrossRef]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen Coated Electrospun Polycaprolactone (PCL) with Titanium Dioxide (TiO2) from an Environmentally Benign Solvent: Preliminary Physico-Chemical Studies for Skin Substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Kanatani, I.; Kanematsu, A.; Inatsugu, Y.; Imamura, M.; Negoro, H.; Ito, N.; Yamamoto, S.; Tabata, Y.; Ikada, Y.; Ogawa, O. Fabrication of an Optimal Urethral Graft Using Collagen-Sponge Tubes Reinforced with Copoly(L-Lactide/ε-Caprolactone) Fabric. Tissue Eng. 2007, 13, 2933–2940. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, E.-M.; Micol, L.A.; Houis, S.; Wurm, F.M.; Hilborn, J.; Hubbell, J.A.; Frey, P. A Collagen-Poly(Lactic Acid-Co-ɛ-Caprolactone) Hybrid Scaffold for Bladder Tissue Regeneration. Biomaterials 2011, 32, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Ananta, M.; Aulin, C.E.; Hilborn, J.; Aibibu, D.; Houis, S.; Brown, R.A.; Mudera, V. A Poly(Lactic Acid-Co-Caprolactone)–Collagen Hybrid for Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1667–1675. [Google Scholar] [CrossRef]
- Alt, E.; Seliger, C. Antithrombotic Stent Coatings: Hirudin/Iloprost Combination. Semin. Interv. Cardiol. SIIC 1998, 3, 177–183. [Google Scholar]
- Heidenhain, C.; Weichert, W.; Schmidmaier, G.; Wildemann, B.; Hein, M.; Neuhaus, P.; Heise, M. Polymer Coating of Porcine Decellularized and Cross-Linked Aortic Grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 256–263. [Google Scholar] [CrossRef]
- Stamm, C.; Khosravi, A.; Grabow, N.; Schmohl, K.; Treckmann, N.; Drechsel, A.; Nan, M.; Schmitz, K.-P.; Haubold, A.; Steinhoff, G. Biomatrix/Polymer Composite Material for Heart Valve Tissue Engineering. Ann. Thorac. Surg. 2004, 78, 2084–2092, discussion 2092–2093. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Schmohl, K.; Khosravi, A.; Philipp, M.; Scharfschwerdt, M.; Graf, B.; Stamm, C.; Haubold, A.; Schmitz, K.-P.; Steinhoff, G. Mechanical and Structural Properties of a Novel Hybrid Heart Valve Scaffold for Tissue Engineering. Artif. Organs 2004, 28, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Masden, D.L.; Seruya, M.; Higgins, J.P. A Systematic Review of the Outcomes of Distal Upper Extremity Bypass Surgery with Arterial and Venous Conduits. J. Hand Surg. 2012, 37, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-Term Patency of Saphenous Vein and Left Internal Mammary Artery Grafts after Coronary Artery Bypass Surgery. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Zhang, J.; Liu, Y.; Gao, H.; Xu, G.-K. New Mechanical Markers for Tracking the Progression of Myocardial Infarction. Nano Lett. 2023, 23, 7350–7357. [Google Scholar] [CrossRef] [PubMed]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous Vein Graft Failure After Coronary Artery Bypass Surgery: Pathophysiology, Management, and Future Directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, P.; Post, P.N.; Breslau, P.J.; van Bockel, J.H. Saphenous Vein Versus PTFE for Above-Knee Femoropopliteal Bypass. A Review of the Literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In Vivo Applications of Electrospun Tissue-Engineered Vascular Grafts: A Review. Tissue Eng.-Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Haruguchi, H.; Teraoka, S. Intimal Hyperplasia and Hemodynamic Factors in Arterial Bypass and Arteriovenous Grafts: A Review. J. Artif. Organs 2003, 6, 227–235. [Google Scholar] [CrossRef]
- Sarkar, S.; Salacinski, H.J.; Hamilton, G.; Seifalian, A.M. The Mechanical Properties of Infrainguinal Vascular Bypass Grafts: Their Role in Influencing Patency. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, S.E.; Berry, C.L. Improving Vascular Grafts: The Importance of Mechanical and Haemodynamic Properties. J. Pathol. 2000, 190, 292–299. [Google Scholar] [CrossRef]
- Davies, M.G.; Hagen, P.-O. Pathophysiology of Vein Graft Failure: A Review. Eur. J. Vasc. Endovasc. Surg. 1995, 9, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-Term Results of Tissue-Engineered Vascular Grafts in Humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef] [PubMed]
- Haisch, A.; Loch, A.; David, J.; Pruß, A.; Hansen, R.; Sittinger, M. Preparation of a Pure Autologous Biodegradable Fibrin Matrix for Tissue Engineering. Med. Biol. Eng. Comput. 2000, 38, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.M.; Potter, J.; Szycher, M. A Solution Grade Biostable Polyurethane Elastomer: ChronoFlex® AR. J. Biomater. Appl. 1994, 8, 210–236. [Google Scholar] [CrossRef]
- Todesco, M.; Zardin, C.; Iop, L.; Palmosi, T.; Capaldo, P.; Romanato, F.; Gerosa, G.; Bagno, A. Hybrid Membranes for the Production of Blood Contacting Surfaces: Physicochemical, Structural and Biomechanical Characterization. Biomater. Res. 2021, 25, 26. [Google Scholar] [CrossRef]
- Mudigonda, J.; Xu, D.; Amedi, A.; Lane, B.A.; Corporan, D.; Wang, V.; Padala, M. A Biohybrid Material with Extracellular Matrix Core and Polymeric Coating as a Cell Honing Cardiovascular Tissue Substitute. Front. Cardiovasc. Med. 2022, 9, 807255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Eberli, D.; Filho, L.F.; Atala, A.; Yoo, J.J. Composite Scaffolds for the Engineering of Hollow Organs and Tissues. Methods 2009, 47, 109–115. [Google Scholar] [CrossRef]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A Bilayered Hybrid Microfibrous PLGA–Acellular Matrix Scaffold for Hollow Organ Tissue Engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Z.; Hahn, C.; King, M.W.; Guidoin, R. Assessing the Resistance to Calcification of Polyurethane Membranes Used in the Manufacture of Ventricles for a Totally Implantable Artificial Heart. J. Biomed. Mater. Res. 1999, 48, 648–659. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Hahn, C.; Laroche, G.; King, M.W.; Guidoin, R. Totally Implantable Artificial Hearts and Left Ventricular Assist Devices: Selecting Impermeable Polycarbonate Urethane to Manufacture Ventricles. J. Biomed. Mater. Res. 1999, 48, 13–23. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Imran, S.J.; Fortunato, T.M.; Sandrin, D.; Borile, G.; Romanato, F.; Casarin, M.; Giuggioli, G.; Conte, F.; Marchesan, M.; et al. A New Detergent for the Effective Decellularization of Bovine and Porcine Pericardia. Biomimetics 2022, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Cassari, L.; Todesco, M.; Zamuner, A.; Imran, S.J.; Casarin, M.; Sandrin, D.; Ródenas-Rochina, J.; Gomez Ribelles, J.L.; Romanato, F.; Bagno, A.; et al. Covalently Grafted Peptides to Decellularized Pericardium: Modulation of Surface Density. Int. J. Mol. Sci. 2023, 24, 2932. [Google Scholar] [CrossRef] [PubMed]
- Bagno, A.; Aguiari, P.; Fiorese, M.; Iop, L.; Spina, M.; Gerosa, G. Native Bovine and Porcine Pericardia Respond to Load with Additive Recruitment of Collagen Fibers: Additive Recruitment of Collagen Fibers. Artif. Organs 2018, 42, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Merigliano, G.; Candela, V.; Iop, L.; Palmosi, T.; Gerosa, G.; Bagno, A. Hybrid Membranes for Blood-Contacting Surfaces: Preliminary Characterization. In Proceedings of the Seventh National Congress of Bioengineering, Trieste, Italy, 10–12 June 2020; Volume 3. [Google Scholar]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A Sterilization Method for Decellularized Xenogeneic Cardiovascular Scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Brauner, J.W.; Flach, C.R.; Mendelsohn, R. A Quantitative Reconstruction of the Amide I Contour in the IR Spectra of Globular Proteins: From Structure to Spectrum. J. Am. Chem. Soc. 2005, 127, 100–109. [Google Scholar] [CrossRef]
- Kurt Oldenburg. LoadSpectra. MATLAB Central File Exchange. 2023. Available online: https://www.mathworks.com/matlabcentral/fileexchange/57904-loadspectra (accessed on 29 August 2023).
- Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal Label-Free Ex Vivo Imaging Using a Dual-Wavelength Microscope with Axial Chromatic Aberration Compensation. J. Biomed. Opt. 2018, 23, 091403. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.I.; Romanato, F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. [Google Scholar] [CrossRef]
- Zouhair, S.; Dal Sasso, E.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef]
- E.P. Commission Council of Europe 2.6.1. European Pharmacopeia 5.0, 2.6—Biological Tests; 2.6.1 Sterility. Eur. Pharmacopoeia 2005, 5.0, 145–149.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices; Part 5, Tests for in Vitro Cytotoxicity. BSI: London, UK, 2009.
- Huang, C.-C.; Chen, Y.-J.; Liu, H.-W. Characterization of Composite Nano-Bioscaffolds Based on Collagen and Supercritical Fluids-Assisted Decellularized Fibrous Extracellular Matrix. Polymers 2021, 13, 4326. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C. Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin. Polymers 2021, 13, 3464. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.J.; Veis, A. Fourier Transform Ir Spectroscopy of Collagen and Gelatin Solutions: Deconvolution of the Amide I Band for Conformational Studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Twardowski, J.; Anzenbacher, P.; Masson, M.R. Raman and IR Spectroscopy in Biology and Biochemistry; Ellis Horwood Series in Analytical Chemistry; Ellis Horwood: New York, NY, USA; Polish Scientific Publishers: Warsaw, Poland, 1994; ISBN 978-0-13-751082-5. [Google Scholar]
- Li, X.; Guo, Y.; Ziegler, K.R.; Model, L.S.; Eghbalieh, S.D.D.; Brenes, R.A.; Kim, S.T.; Shu, C.; Dardik, A. Current Usage and Future Directions for the Bovine Pericardial Patch. Ann. Vasc. Surg. 2011, 25, 561–568. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-Diameter Vascular Tissue Engineering. Nat. Rev. Cardiol. 2013, 10, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Chrisikou, I.; Orkoula, M.; Kontoyannis, C. FT-IR/ATR Solid Film Formation: Qualitative and Quantitative Analysis of a Piperacillin-Tazobactam Formulation. Molecules 2020, 25, 6051. [Google Scholar] [CrossRef] [PubMed]
- Camasão, D.B.; Mantovani, D. The Mechanical Characterization of Blood Vessels and Their Substitutes in the Continuous Quest for Physiological-Relevant Performances. A Critical Review. Mater. Today Bio 2021, 10, 100106. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun Scaffolds for Tissue Engineering of Vascular Grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Hasegawa, M.; Azuma, T. Mechanical Properties of Synthetic Arterial Grafts. J. Biomech. 1979, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Stekelenburg, M.; Rutten, M.C.M.; Snoeckx, L.H.E.H.; Baaijens, F.P.T. Dynamic Straining Combined with Fibrin Gel Cell Seeding Improves Strength of Tissue-Engineered Small-Diameter Vascular Grafts. Tissue Eng. Part A 2009, 15, 1081–1089. [Google Scholar] [CrossRef]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M.; Edwards, A.; Carson, R.J. The Mechanical Behavior of Vascular Grafts: A Review. J. Biomater. Appl. 2001, 15, 241–278. [Google Scholar] [CrossRef]
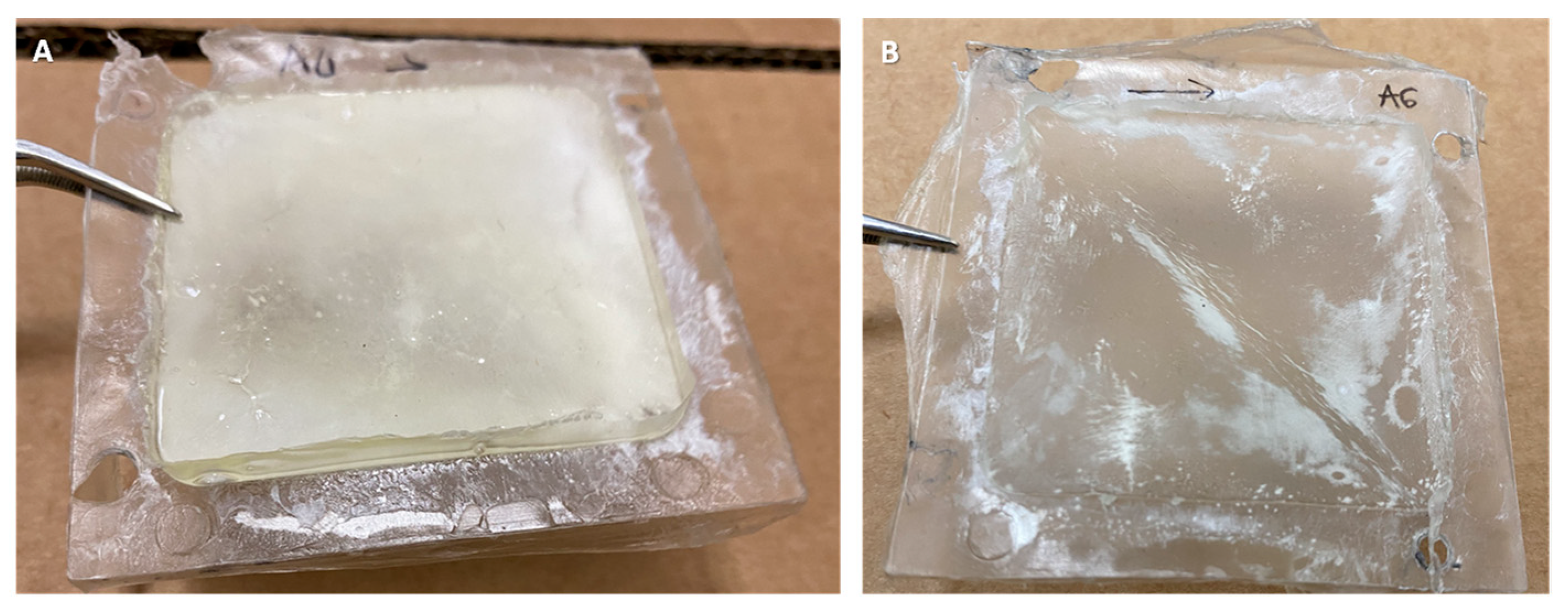
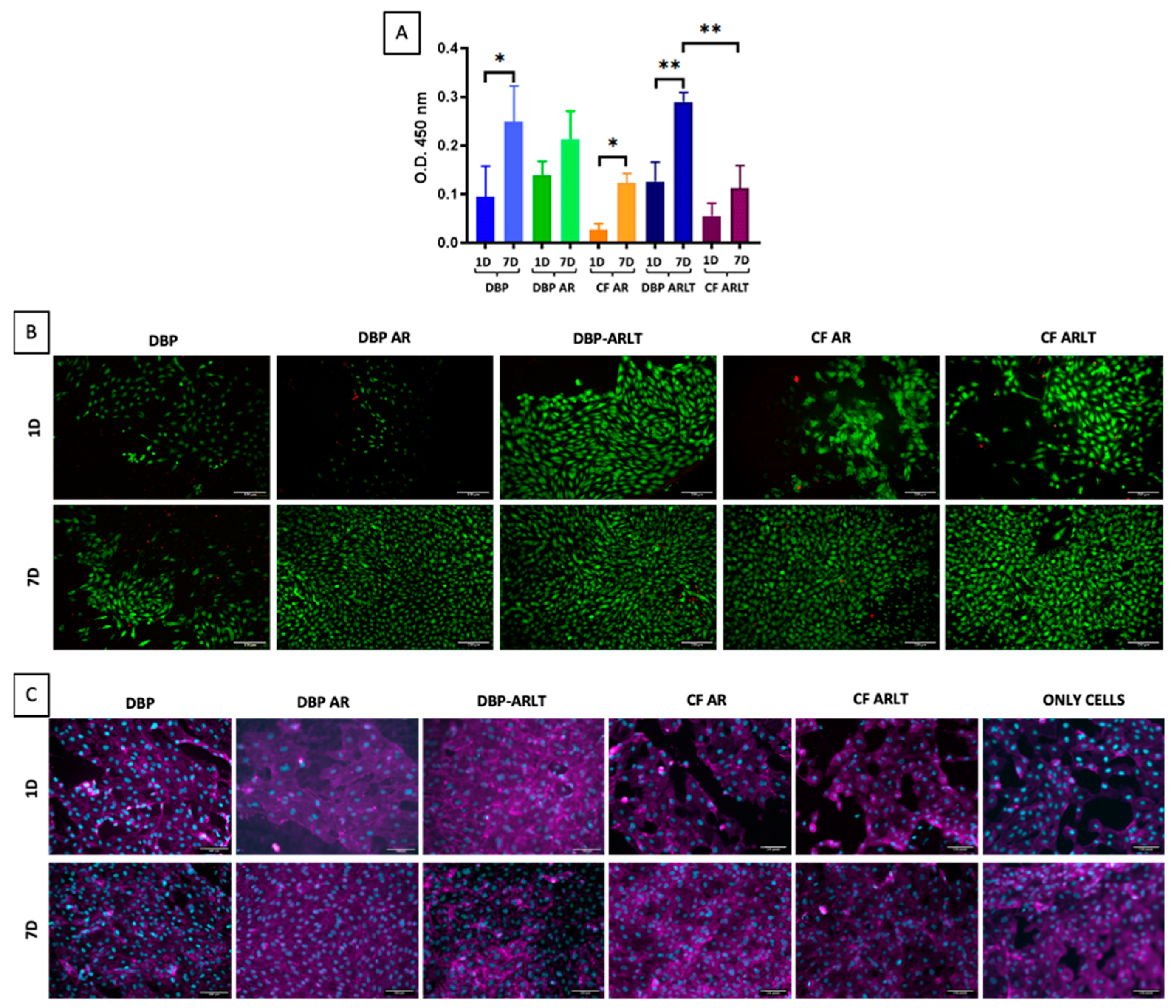
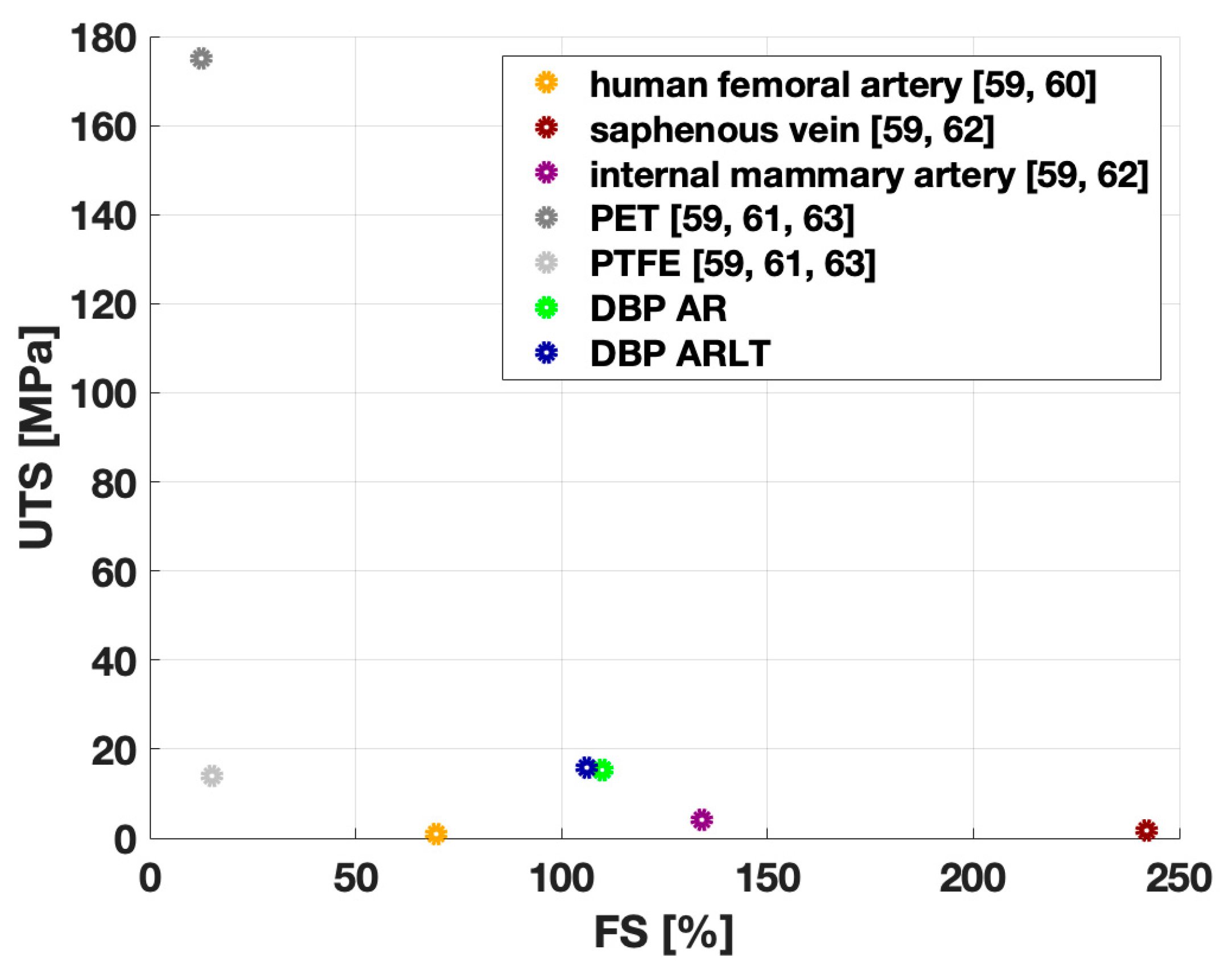
| Sample | CF AR | CF ARLT | DBP | DBP AR | DBP ARLT |
|---|---|---|---|---|---|
| Thickness [mm] | 0.68 ± 0.46 | 0.93 ± 0.54 | 0.29 ± 0.06 | 0.58 ± 0.08 | 0.60 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todesco, M.; Casarin, M.; Sandrin, D.; Astolfi, L.; Romanato, F.; Giuggioli, G.; Conte, F.; Gerosa, G.; Fontanella, C.G.; Bagno, A. Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment. Bioengineering 2024, 11, 436. https://doi.org/10.3390/bioengineering11050436
Todesco M, Casarin M, Sandrin D, Astolfi L, Romanato F, Giuggioli G, Conte F, Gerosa G, Fontanella CG, Bagno A. Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment. Bioengineering. 2024; 11(5):436. https://doi.org/10.3390/bioengineering11050436
Chicago/Turabian StyleTodesco, Martina, Martina Casarin, Deborah Sandrin, Laura Astolfi, Filippo Romanato, Germana Giuggioli, Fabio Conte, Gino Gerosa, Chiara Giulia Fontanella, and Andrea Bagno. 2024. "Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment" Bioengineering 11, no. 5: 436. https://doi.org/10.3390/bioengineering11050436
APA StyleTodesco, M., Casarin, M., Sandrin, D., Astolfi, L., Romanato, F., Giuggioli, G., Conte, F., Gerosa, G., Fontanella, C. G., & Bagno, A. (2024). Hybrid Materials for Vascular Applications: A Preliminary In Vitro Assessment. Bioengineering, 11(5), 436. https://doi.org/10.3390/bioengineering11050436











