Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Saturation Solubility
2.3. Three-Dimensional Fabrication of Implantable PLGA Scaffolds
2.4. Characterization of Scaffold Dimensions
2.5. Quantitative Analysis of Ketoprofen Content
2.6. Determination of Surface Roughness and Surface Morphology of Scaffolds
2.7. Mechanical Testing of Scaffolds
2.8. Differential Scanning Calorimetry
2.9. Static Contact Angle Measurement
2.10. In Vitro Release Studies
2.11. Drug Release Kinetics
2.12. Statistical Analysis
3. Results and Discussion
3.1. Saturation Solubility of Ketoprofen
3.2. Three-Dimensional Printing of Porous PLGA Scaffolds
3.3. Surface Morphology and Surface Roughness
3.4. Mechanical Strength of Scaffolds
3.5. Thermal Analysis
3.6. Contact Angle Measurements
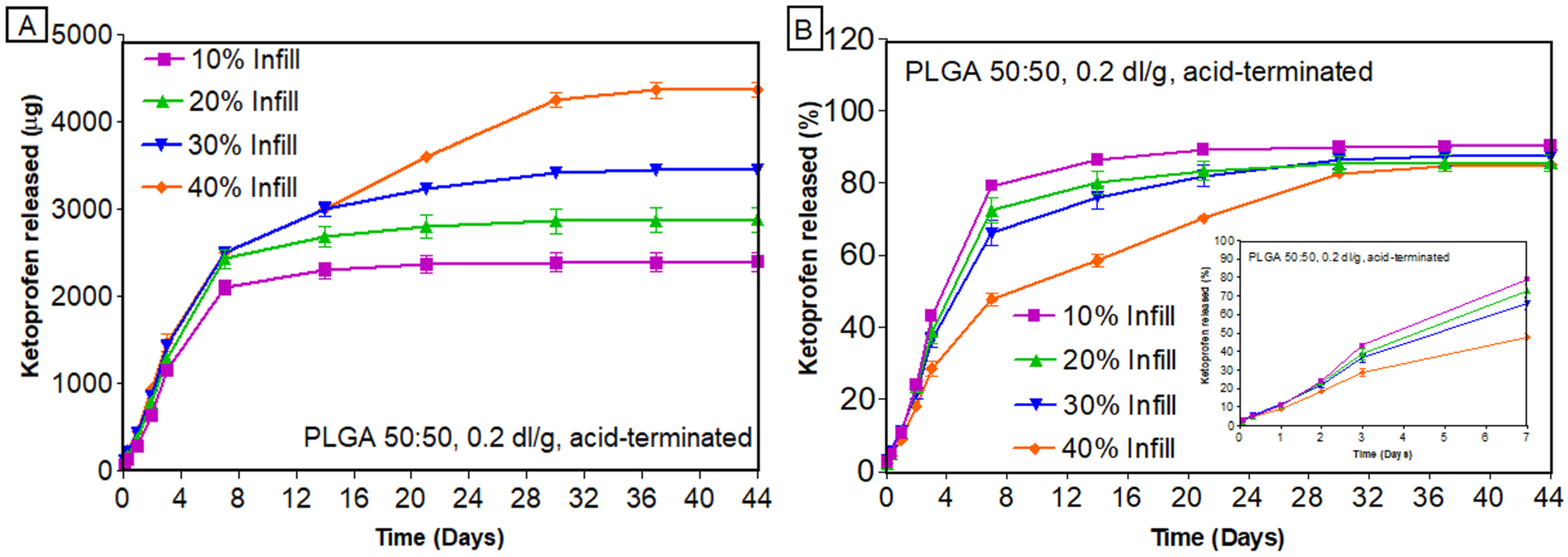
3.7. In Vitro Release of Ketoprofen from PLGA Scaffolds
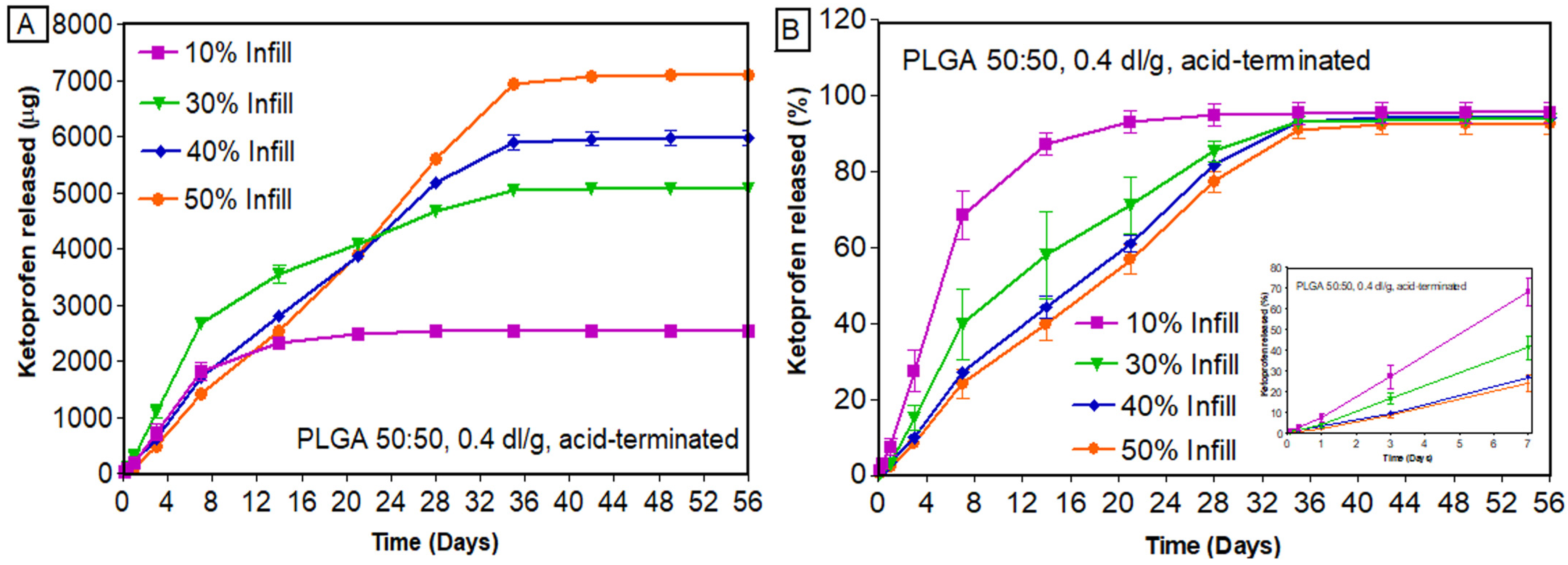
3.7.1. Effect of Infill Density
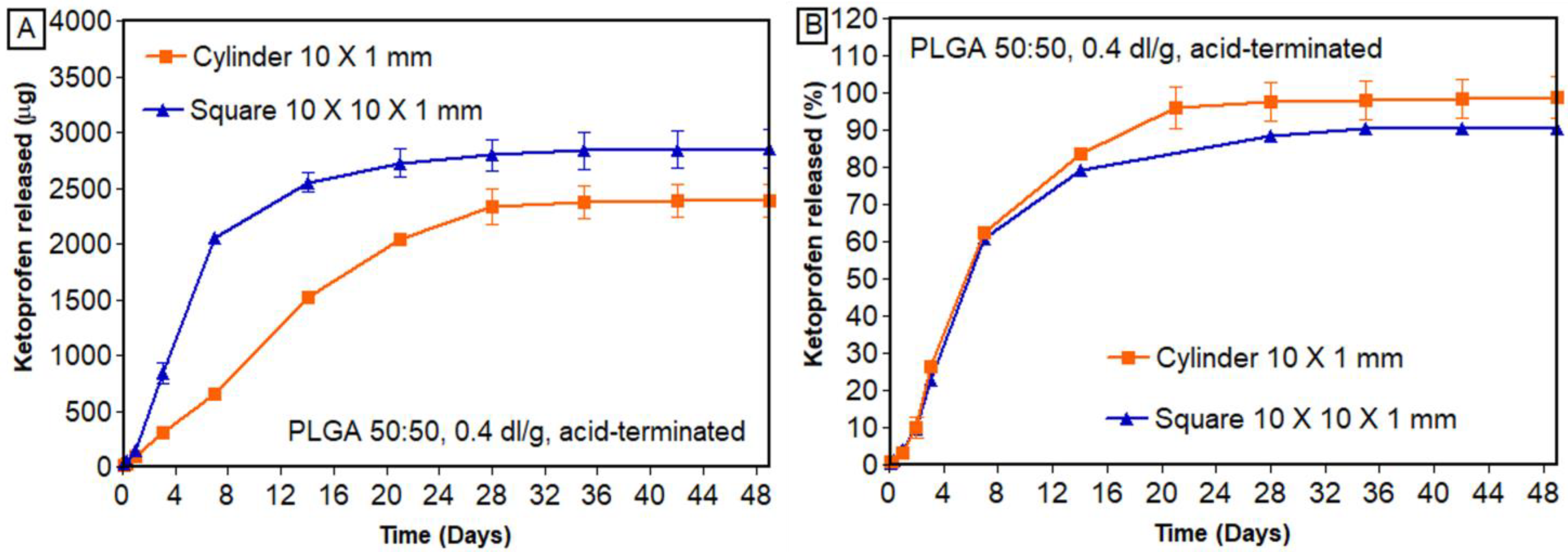
3.7.2. Effect of Geometry
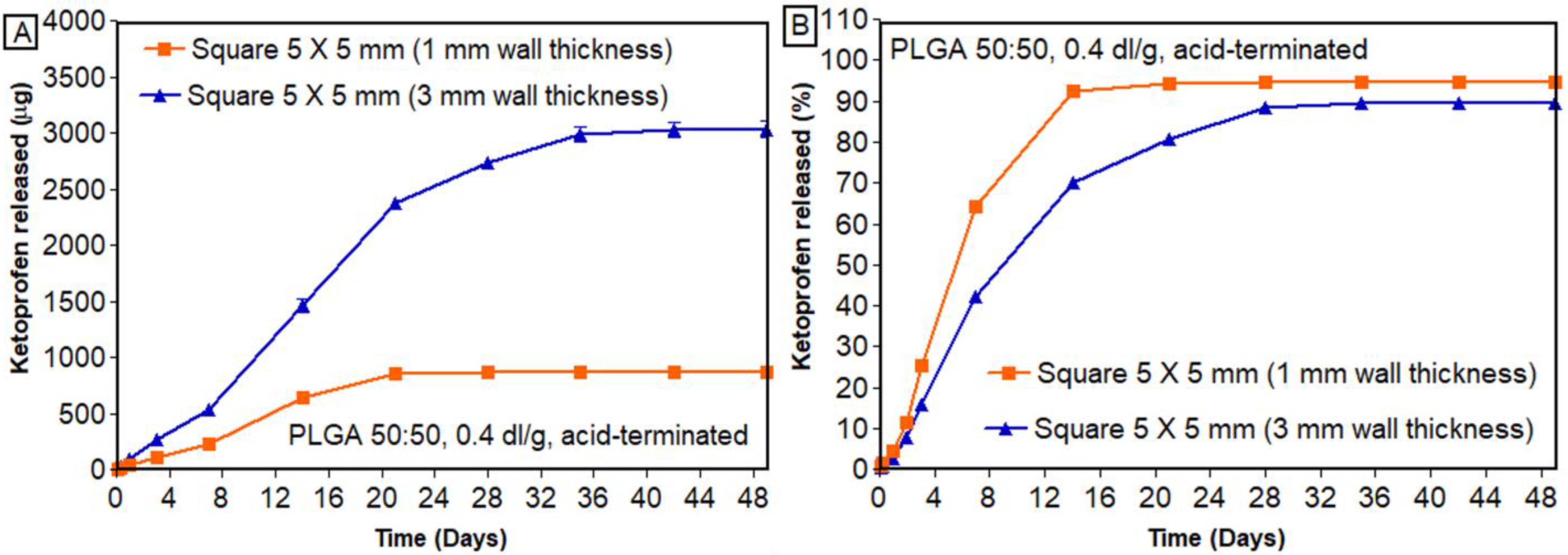
3.7.3. Effect of Wall Thickness
3.7.4. Drug Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolo, P.; Kruth, J.-P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. JBJS 2008, 90 (Suppl. S1), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, D.-W. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron. Eng. 2009, 86, 1447–1450. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Jin, K.; Liu, W.; Qiu, X.; Li, C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng. C 2016, 59, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, G.; Liu, G.; Liu, Y.; Chen, Q.; Ren, L.; Chen, C.; Ding, Z. A biodegradable antibiotic-eluting PLGA nanofiber-loaded deproteinized bone for treatment of infected rabbit bone defects. J. Biomater. Appl. 2016, 31, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Zeng, Y.-T.; Kankala, R.K.; Zhang, S.-S.; Chen, A.-Z.; Wang, S.-B. Characterization and preliminary biological evaluation of 3D-printed porous scaffolds for engineering bone tissues. Materials 2018, 11, 1832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Shuai, C.; Shi, X.; Yang, F.; Tian, H.; Feng, P. Oxygen vacancy boosting Fenton reaction in bone scaffold towards fighting bacterial infection. Int. J. Extrem. Manuf. 2023, 6, 015101. [Google Scholar] [CrossRef]
- Raafat, A.I.; Abd-Allah, W.M. In vitro apatite forming ability and ketoprofen release of radiation synthesized (gelatin-polyvinyl alcohol)/bioglass composite scaffolds for bone tissue regeneration. Polym. Compos. 2018, 39, 606–615. [Google Scholar] [CrossRef]
- Prabaharan, M.; Rodriguez-Perez, M.; De Saja, J.; Mano, J. Preparation and characterization of poly (L-lactic acid)-chitosan hybrid scaffolds with drug release capability. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 81, 427–434. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Ebraheim, N.A.; Jayasuriya, A.C. Investigation of potential injectable polymeric biomaterials for bone regeneration. J. Biomed. Mater. Res. Part A 2013, 101, 2436–2447. [Google Scholar] [CrossRef]
- Ma, P.X.; Choi, J.-W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.C.; Yaszemski, M.J.; Powers, J.M.; Mikos, A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 1996, 7, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Davies, J.E. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 480–487. [Google Scholar] [CrossRef]
- Karande, T.S.; Ong, J.L.; Agrawal, C.M. Diffusion in musculoskeletal tissue engineering scaffolds: Design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng. 2004, 32, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly (propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Lin, C.Y.; Kikuchi, N.; Hollister, S.J. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J. Biomech. 2004, 37, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhi, W.; Xu, T.; Shi, F.; Duan, K.; Wang, J.; Mu, Y.; Weng, J. Ectopic osteogenesis and angiogenesis regulated by porous architecture of hydroxyapatite scaffolds with similar interconnecting structure in vivo. Regen. Biomater. 2016, 3, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Seuba, J.; Deville, S.; Guizard, C.; Stevenson, A.J. The effect of wall thickness distribution on mechanical reliability and strength in unidirectional porous ceramics. Sci. Technol. Adv. Mater. 2016, 17, 128–135. [Google Scholar] [CrossRef]
- Roy, T.D.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 66, 283–291. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. High-resolution direct 3D printed PLGA scaffolds: Print and shrink. Biofabrication 2014, 7, 015002. [Google Scholar] [CrossRef]
- Lubombo, C.; Huneault, M.A. Effect of infill patterns on the mechanical performance of lightweight 3D-printed cellular PLA parts. Mater. Today Commun. 2018, 17, 214–228. [Google Scholar] [CrossRef]
- Hlinka, M. Non-Destructive Testing for the Influence of Infill Pattern Geometry on Mechanical Stiffness of 3D Printing Materials. Ph.D. Thesis, Florida Atlantic University, Boca Raton, FL, USA, 2020. [Google Scholar]
- Ranjan, R.; Ayas, C.; Langelaar, M.; van Keulen, F. Topology Optimisation Techniques. In Precision Metal Additive Manufacturing; CRC Press: Boca Raton, FL, USA, 2020; pp. 11–34. [Google Scholar]
- Palaniyappan, S.; Veeman, D.; Narain Kumar, S.; Surendhar, G.J.; Natrayan, L. Effect of printing characteristics for the incorporation of hexagonal-shaped lattice structure on the PLA polymeric material. J. Thermoplast. Compos. Mater. 2023, 36, 2009–2030. [Google Scholar] [CrossRef]
- Fanous, M.; Bitar, M.; Gold, S.; Sobczuk, A.; Hirsch, S.; Ogorka, J.; Imanidis, G. Development of immediate release 3D-printed dosage forms for a poorly water-soluble drug by fused deposition modeling: Study of morphology, solid state and dissolution. Int. J. Pharm. 2021, 599, 120417. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly (D, L-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 767–777. [Google Scholar] [CrossRef]
- Beal, V.E.; Paggi, R.A.; Salmoria, G.V.; Lago, A. Statistical evaluation of laser energy density effect on mechanical properties of polyamide parts manufactured by selective laser sintering. J. Appl. Polym. Sci. 2009, 113, 2910–2919. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M. Selective laser sintering and its application in biomedical engineering. MRS Bull. 2011, 36, 998–1005. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. A simulation work for the influences of aggregation/agglomeration of clay layers on the tensile properties of nanocomposites. JOM 2019, 71, 3989–3995. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Freyman, T.; Yannas, I.; Gibson, L. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Genta, I.; Modena, T.; Conti, B. Effect of porogen on the physico-chemical properties and degradation performance of PLGA scaffolds. Polym. Degrad. Stab. 2010, 95, 694–701. [Google Scholar] [CrossRef]
- Zhang, B.; Nasereddin, J.; McDonagh, T.; von Zeppelin, D.; Gleadall, A.; Alqahtani, F.; Bibb, R.; Belton, P.; Qi, S. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int. J. Pharm. 2021, 604, 120626. [Google Scholar] [CrossRef] [PubMed]
- Korte, C.; Quodbach, J. 3D-printed network structures as controlled-release drug delivery systems: Dose adjustment, API release analysis and prediction. AAPS PharmSciTech 2018, 19, 3333–3342. [Google Scholar] [CrossRef]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111334. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 105–113. [Google Scholar] [CrossRef]
- Oh, S.; Cho, S.; Lee, J. Preparation and characterization of hydrophilic PLGA/Tween 80 films and porous scaffolds. Mol. Cryst. Liq. Cryst. 2004, 418, 229–241. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Manini, G.; Benali, S.; Raquez, J.-M.; Goole, J. Proof of concept of a predictive model of drug release from long-acting implants obtained by fused-deposition modeling. Int. J. Pharm. 2022, 618, 121663. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Pillai, A.R.; Zhang, J.; Zhang, Y.; Kulkarni, V.; Maniruzzaman, M. Novel on-demand 3-dimensional (3-D) printed tablets using fill density as an effective release-controlling tool. Polymers 2020, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- Manini, G.; Deldime, M.; Benali, S.; Raquez, J.-M.; Goole, J. Long-acting implantable dosage forms containing paliperidone palmitate obtained by 3D printing. Int. J. Pharm. 2021, 603, 120702. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Carlier, E.; Marquette, S.; Peerboom, C.; Denis, L.; Benali, S.; Raquez, J.M.; Amighi, K.; Goole, J. Investigation of the parameters used in fused deposition modeling of poly (lactic acid) to optimize 3D printing sessions. Int. J. Pharm. 2019, 565, 367–377. [Google Scholar] [CrossRef]
- Nober, C.; Manini, G.; Carlier, E.; Raquez, J.-M.; Benali, S.; Dubois, P.; Amighi, K.; Goole, J. Feasibility study into the potential use of fused-deposition modeling to manufacture 3D-printed enteric capsules in compounding pharmacies. Int. J. Pharm. 2019, 569, 118581. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Obeid, S.; Madžarević, M.; Ibrić, S. Tailoring amlodipine release from 3D printed tablets: Influence of infill patterns and wall thickness. Int. J. Pharm. 2021, 610, 121261. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Dean, D.; Lu, A.; Mikos, A.G.; Fisher, J.P. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomater. 2011, 7, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Mikos, A.G.; Fisher, J.P.; Jansen, J.A. Strategic directions in tissue engineering. Tissue Eng. 2007, 13, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.-H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M. Evaluating 3D printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv. Mater. 2015, 27, 138. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, G.; Singh, S.; Linda, W.; Zheng, H.; Ramakrishna, S.; Narayan, R. Mechanical reliability and in vitro bioactivity of 3D-printed porous polylactic acid-hydroxyapatite scaffold. J. Mater. Eng. Perform. 2021, 30, 4946–4956. [Google Scholar] [CrossRef]
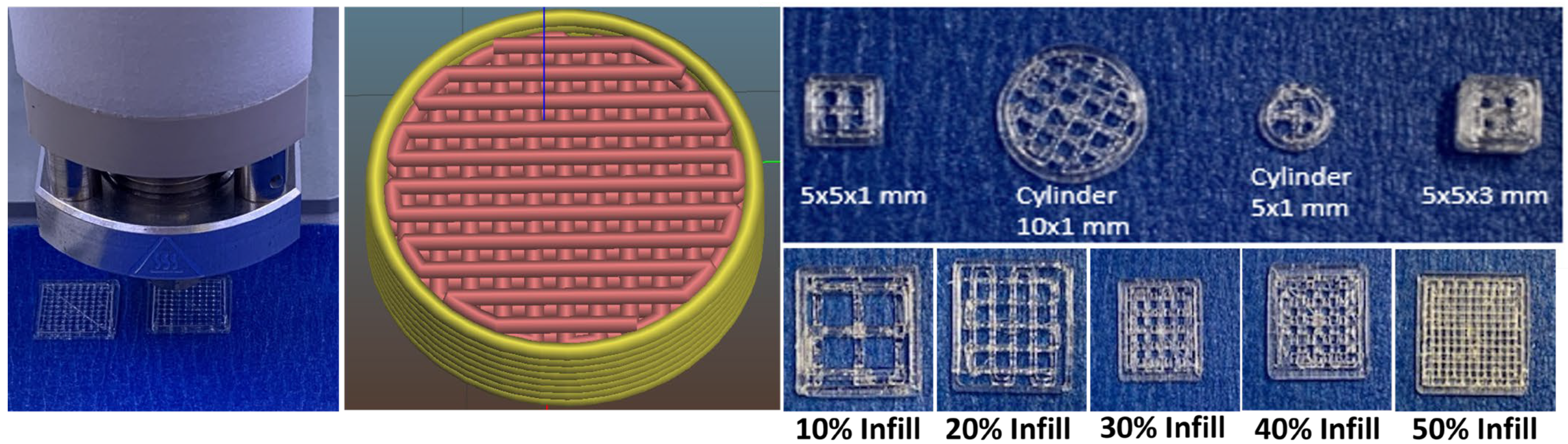
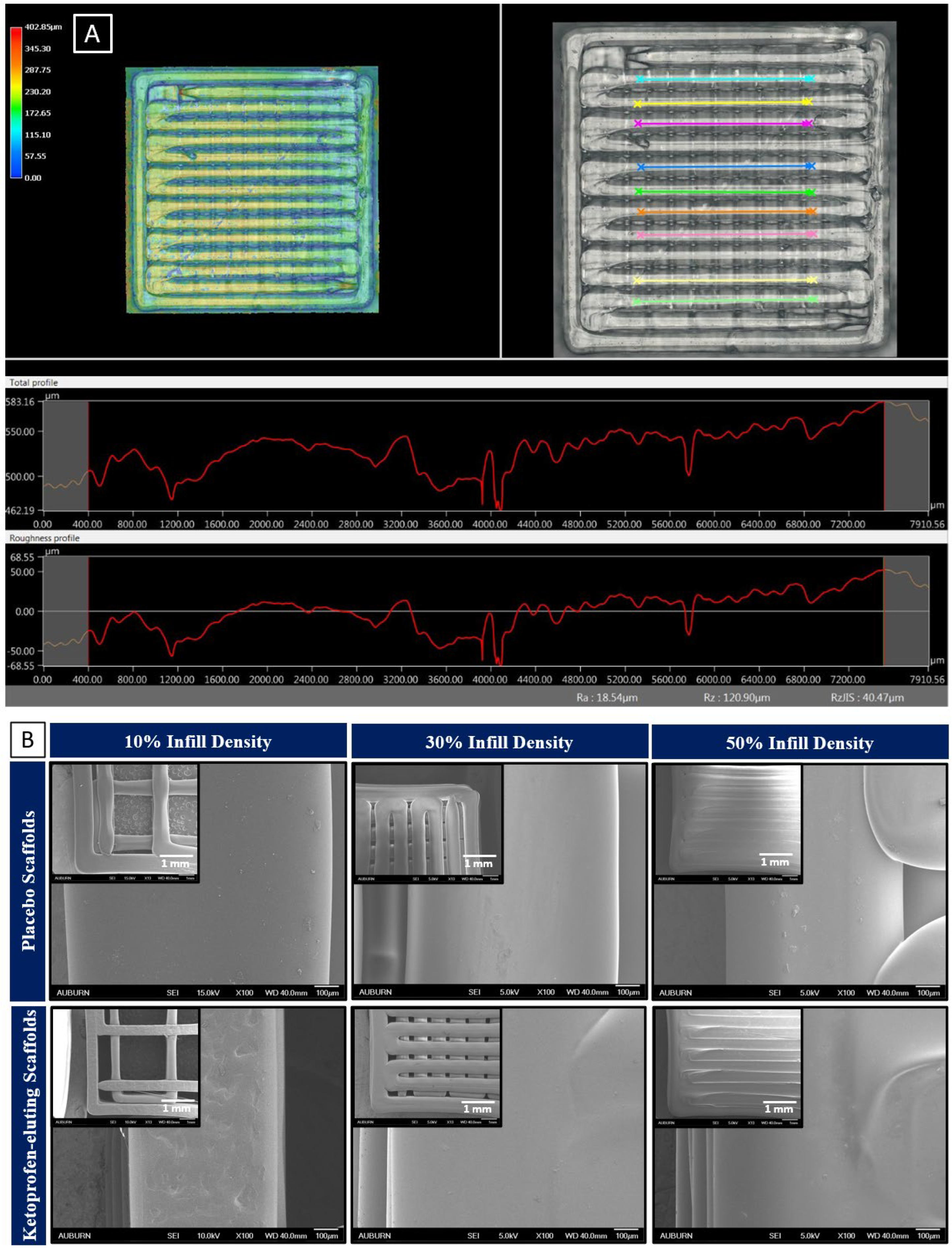



| Printer Parameters | Scaffolds Printed with PLGA 50:50, 0.2 dl/g, Acid-Terminated | Scaffolds Printed with PLGA 50:50, 0.4 dl/g, Acid-Terminated |
|---|---|---|
| Printing temperature (°C) | 90 | 120 |
| Print speed (mm/s) | 2 | 2 |
| Nozzle internal diameter (mm) | 0.4 | 0.4 |
| Print head | Thermoplastic | Thermoplastic |
| Printing technology | Direct powder extrusion | Direct powder extrusion |
| Print bed temperature (°C) | 25 | 25 |
| Pre and post flow (ms) | 50 and 50 | 50 and 50 |
| First layer height (%) | 80 | 80 |
| Printing pressure (kPa) | 200 | 180 |
| Shape | Rectilinear | Rectilinear |
| Infill density (%) | 10, 20, 30, 40 | 10, 30, 40, 50 |
| Dimensions (mm) | 10 × 10 × 1 | 10 × 10 × 1 |
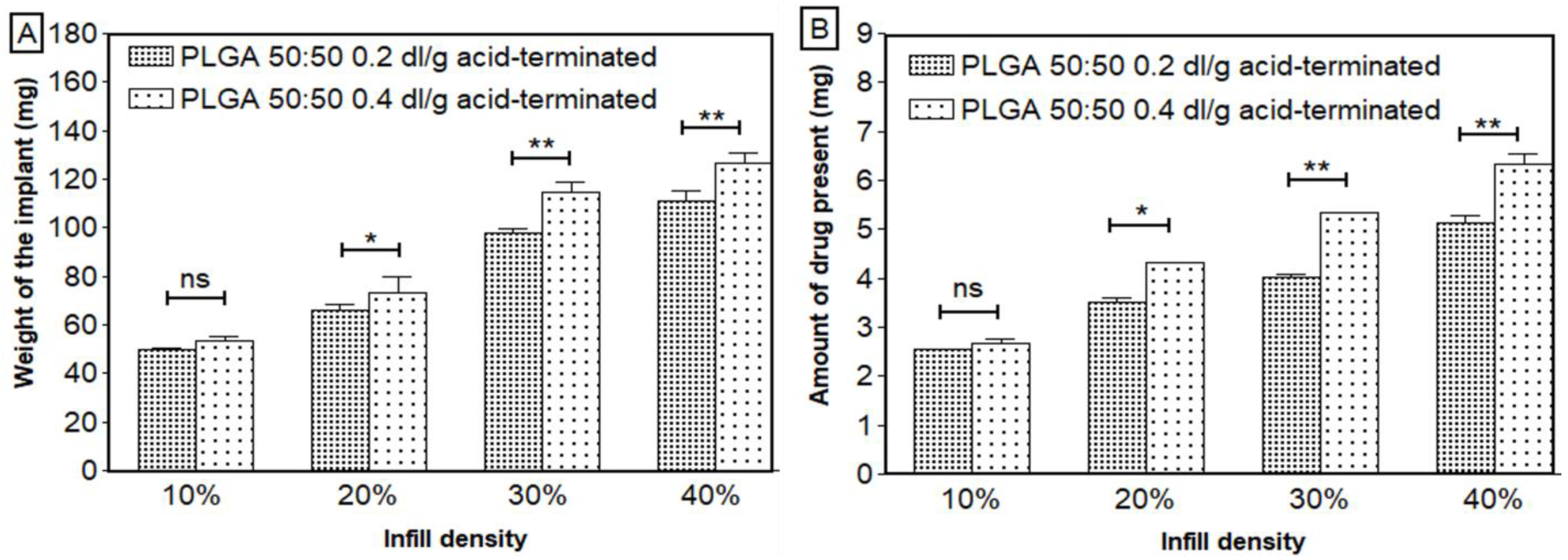
| Weight of the scaffolds (mg) | 48.49–116.55 | 51.47–151.03 |
| Amount of drug loading (mg) | 2.54–5.34 | 2.57–6.69 |
| Scaffold thickness (mm) | 1 | 1 |
| Printing pattern | Crosshatch (0°/90°) | Crosshatch (0°/90°) |
| Batch | Weight of the Scaffold (mg) | Amount of Ketoprofen (mg) | %Assay (Mean ± SD) | |
|---|---|---|---|---|
| Theoretical | Actual | |||
| Batch 1 | 52.55 ± 7.30 | 2.39 ± 0.42 | 2.34 ± 0.32 | 98.76 ± 5.89 |
| Batch 2 | 52.80 ± 2.06 | 2.67 ± 0.10 | 2.68 ± 0.10 | 100.46 ± 3.19 |
| Batch 3 | 48.90 ± 8.34 | 2.91 ± 0.08 | 2.92 ± 0.14 | 100.29 ± 2.98 |
| Factors Studied | Percent Infill | Zero Order (R2) | First Order (R2) | Higuchi Order (R2) | Hixson–Crowell (R2) | Korsmeyer–Peppas * | ||
|---|---|---|---|---|---|---|---|---|
| n | K | R2 | ||||||
| PLGA 50:50, 0.2 dl/g, acid-terminated | 10% | 0.6484 | 0.485 | 0.829 | 0.555 | 0.609 ± 0.025 | 0.288 ± 0.071 | 0.933 ± 0.007 |
| 20% | 0.672 | 0.485 | 0.849 | 0.566 | 0.613 ± 0.036 | 0.253 ± 0.077 | 0.941 ± 0.015 | |
| 30% | 0.734 | 0.533 | 0.895 | 0.615 | 0.575 ± 0.051 | 0.340 ± 0.154 | 0.957 ± 0.008 | |
| 40% | 0.860 | 0.595 | 0.970 | 0.706 | 0.607 ± 0.011 | 0.196 ± 0.049 | 0.978 ± 0.007 | |
| PLGA 50:50, 0.4 dl/g, acid-terminated | 10% | 0.660 | 0.495 | 0.916 | 0.567 | 0.705 ± 0.099 | 0.032 ± 0.002 | 0.947 ± 0.024 |
| 30% | 0.839 | 0.566 | 0.898 | 0.687 | 0.810 ± 0.091 | 0.411 ± 0.259 | 0.976 ± 0.011 | |
| 40% | 0.906 | 0.649 | 0.826 | 0.778 | 0.849 ± 0.045 | 0.561 ± 0.138 | 0.990 ± 0.001 | |
| 50% | 0.918 | 0.666 | 0.809 | 0.794 | 0.880 ± 0.052 | 0.678 ± 0.321 | 0.985 ± 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annaji, M.; Mita, N.; Poudel, I.; Boddu, S.H.S.; Fasina, O.; Babu, R.J. Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration. Bioengineering 2024, 11, 259. https://doi.org/10.3390/bioengineering11030259
Annaji M, Mita N, Poudel I, Boddu SHS, Fasina O, Babu RJ. Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration. Bioengineering. 2024; 11(3):259. https://doi.org/10.3390/bioengineering11030259
Chicago/Turabian StyleAnnaji, Manjusha, Nur Mita, Ishwor Poudel, Sai H. S. Boddu, Oladiran Fasina, and R. Jayachandra Babu. 2024. "Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration" Bioengineering 11, no. 3: 259. https://doi.org/10.3390/bioengineering11030259
APA StyleAnnaji, M., Mita, N., Poudel, I., Boddu, S. H. S., Fasina, O., & Babu, R. J. (2024). Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration. Bioengineering, 11(3), 259. https://doi.org/10.3390/bioengineering11030259










