Characteristics of Far-Infrared Ray Emitted from Functional Loess Bio-Balls and Its Effect on Improving Blood Flow
Abstract
1. Introduction
2. Materials and Methods
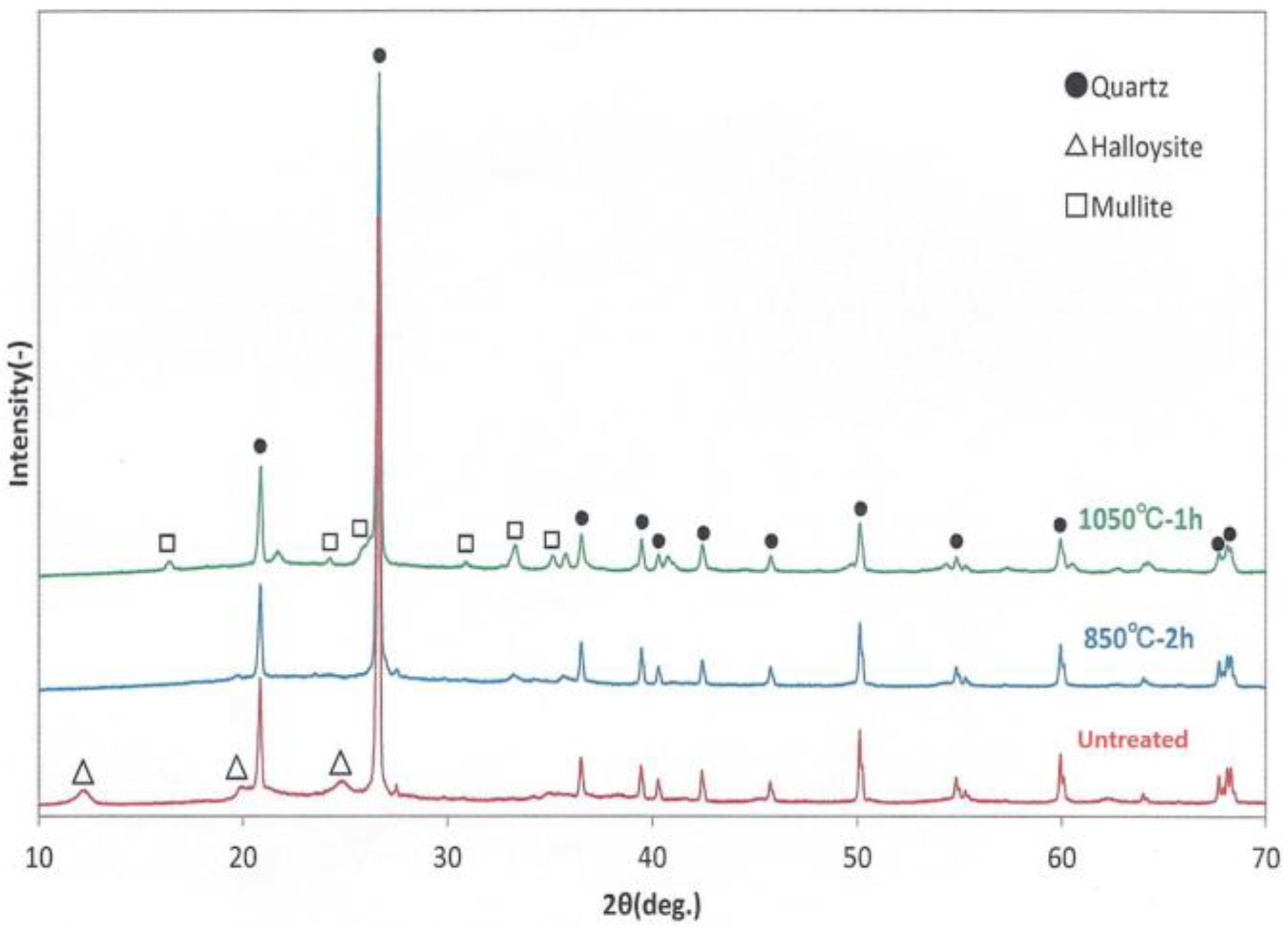
2.1. X-ray Diffraction (XRD)
2.2. Infrared Absorption Spectra
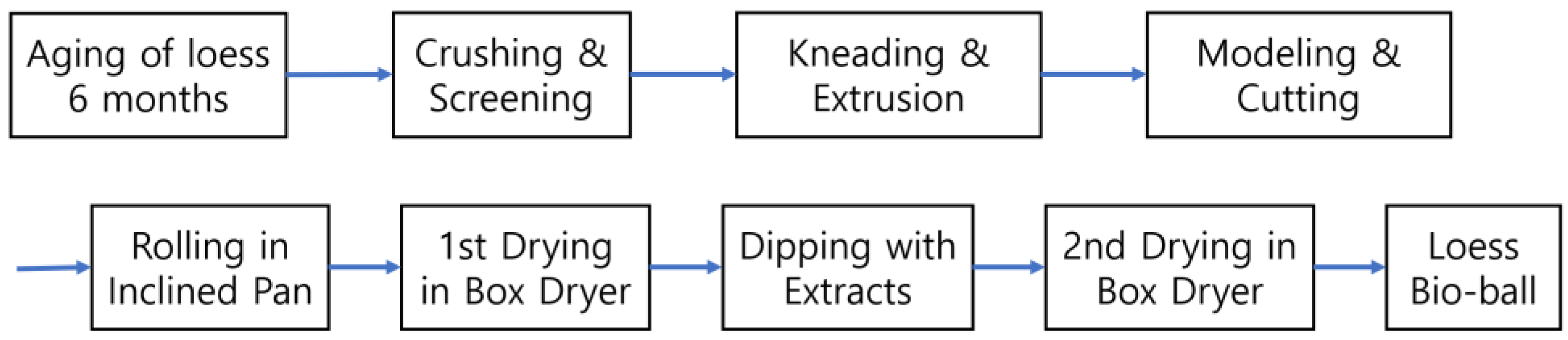
2.3. Low-Temperature Wet-Drying Method
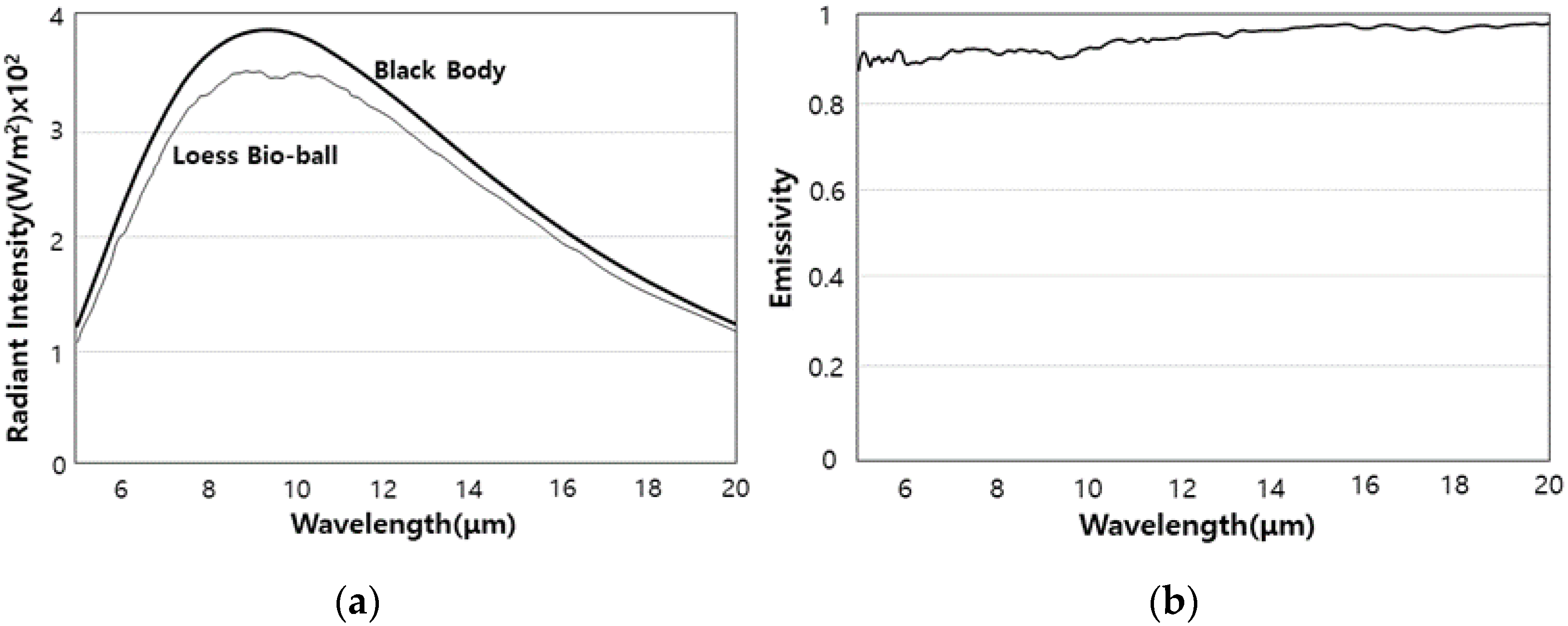
2.4. Radiant Intensity and Emissivity of FIR Emitted from Loess Bio-Balls
2.5. Transmitted Energy of FIR Passing through Five Materials
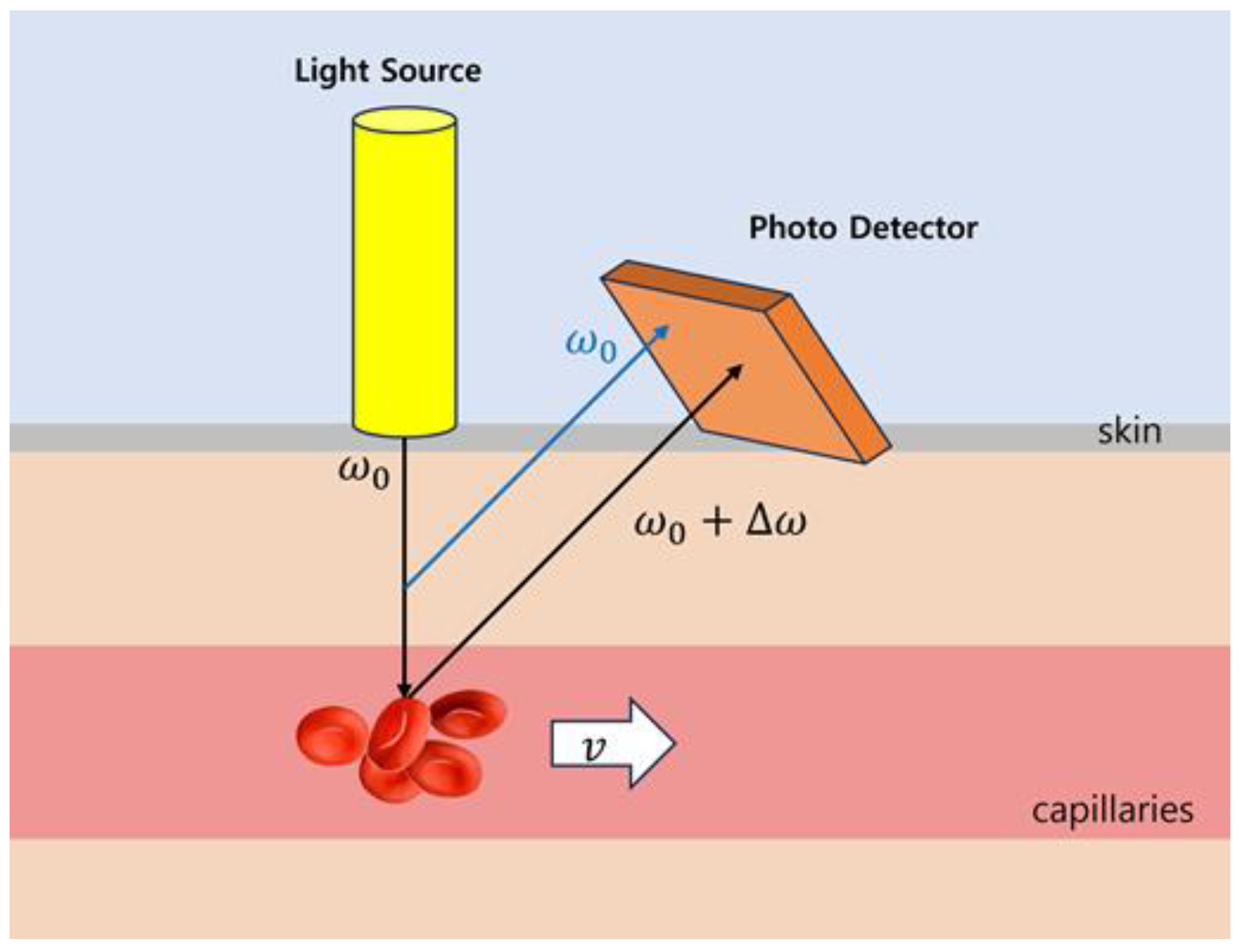
2.6. Blood-Flow Measurement Using Laser Doppler Flowmetry
2.7. Blood-Flow Measurement on Loess Bio-Ball Mat
2.7.1. Participants
2.7.2. Study Design
2.7.3. Statistical Analyses
3. Results and Discussion
3.1. X-ray Diffraction Patterns
3.2. Infrared Absorption Spectra
3.3. Low-Temperature Wet-Drying Method
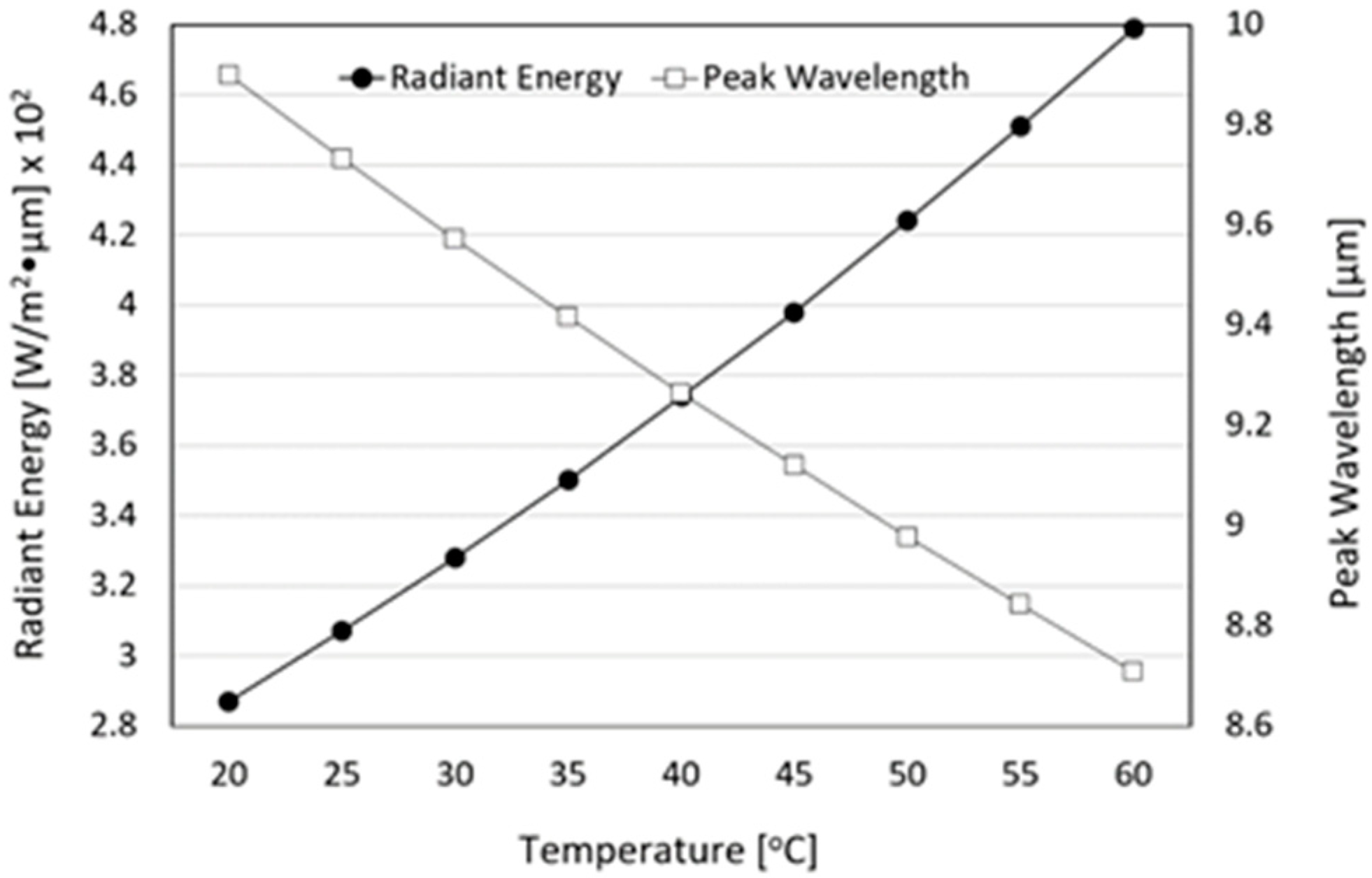
3.4. Radiant Intensity and Emissivity of FIR Emitted from Loess Bio-Balls
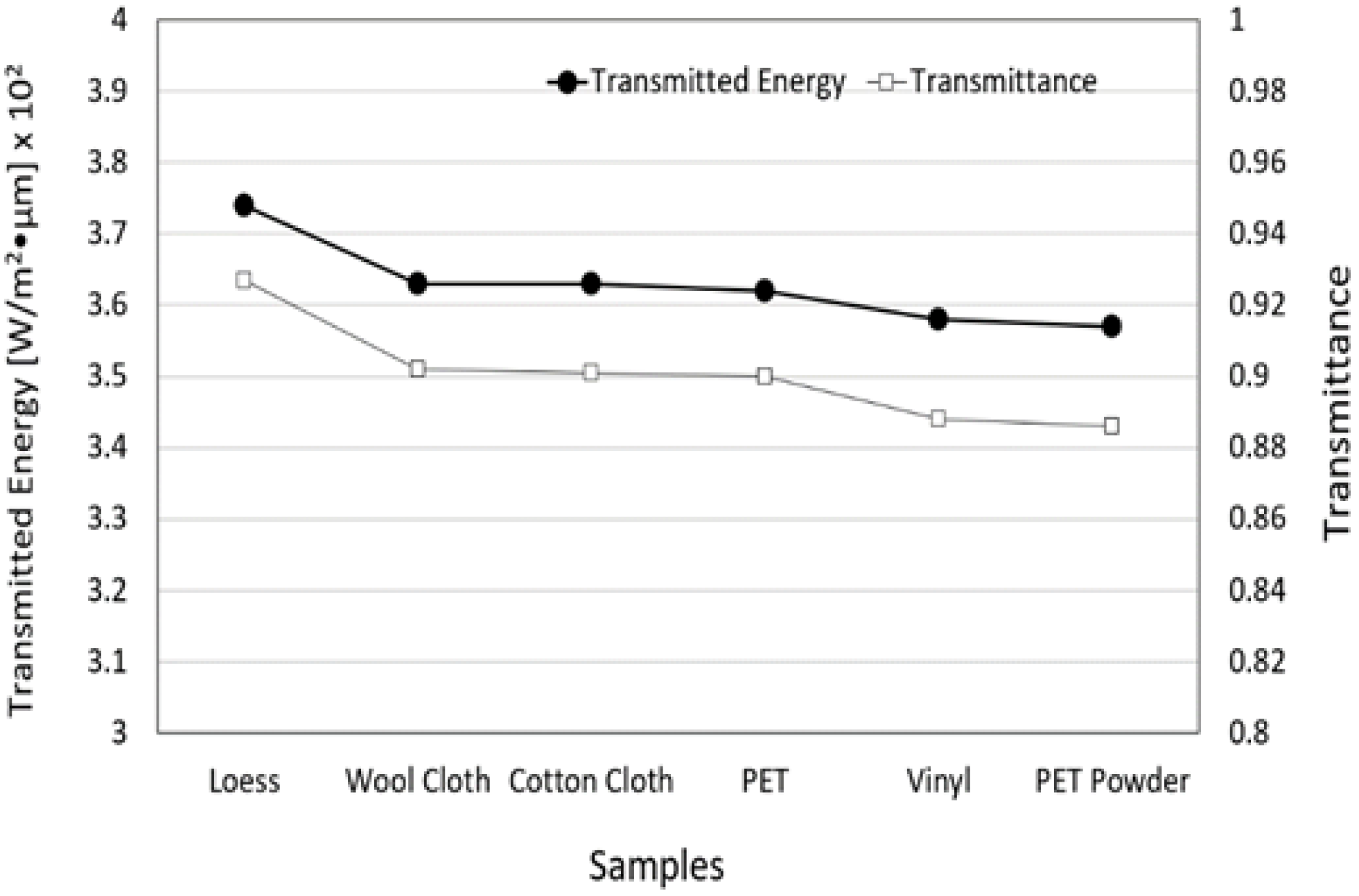
3.5. Transmitted Energy and Transmittance of FIR Emitted from Loess Bio-Ball for Five Materials
3.6. Energy Conversion between FIR Emitted from Loess Bio-Ball and Cellular Water Molecules, and Its Effect on Improving Blood Flow
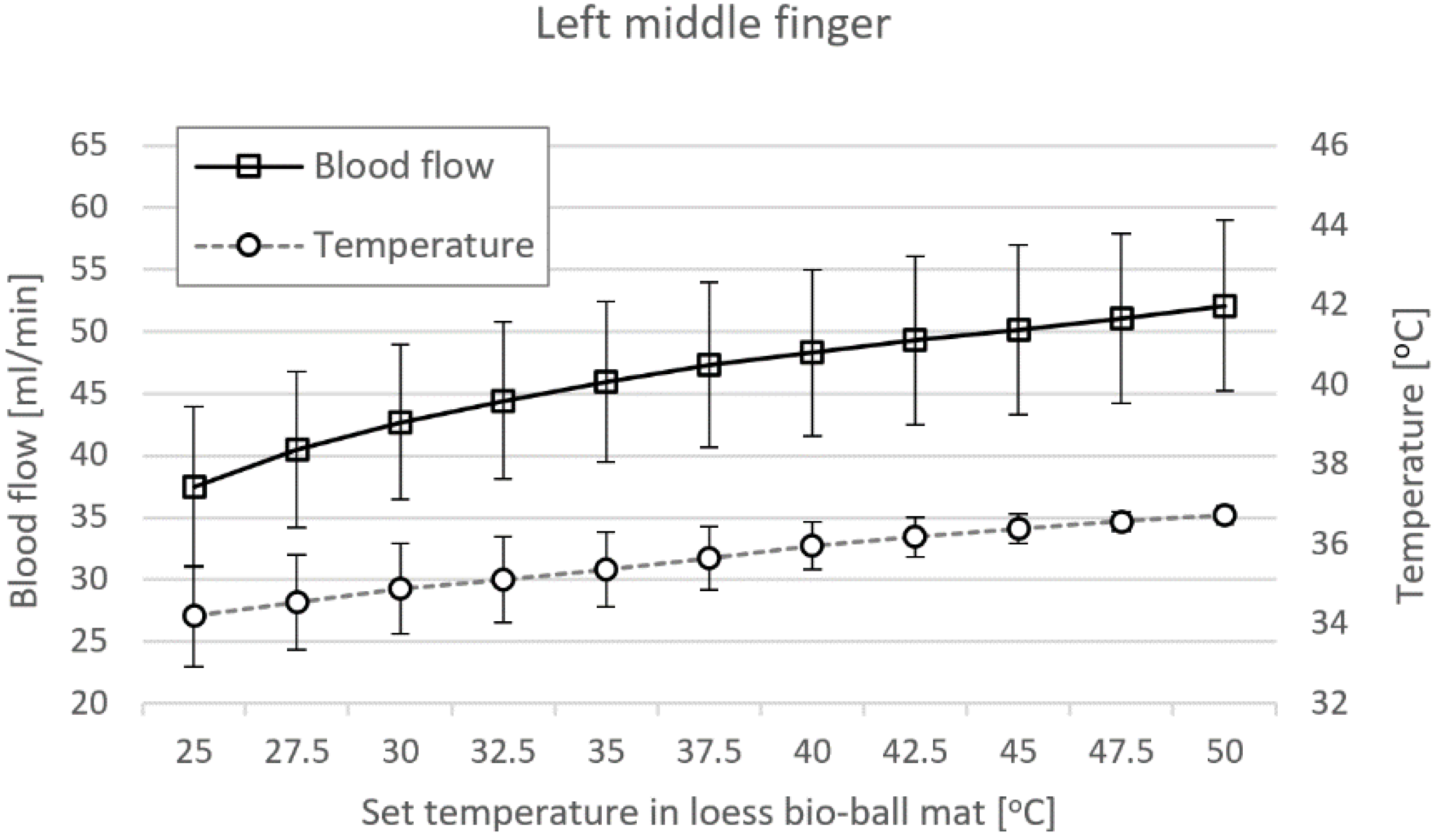
3.7. Blood Flow Measured at LMF and RMF When Using Loess Bio-Ball Mat
4. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, W.S.; Toyama, T.S.; Choi, Y.J.; Woo, B.S. Preclinical efficacy examination on healing practices and experiences of users for pillows and mattresses of loess ball bio-products. Procedia Eng. 2016, 102, 399–409. [Google Scholar] [CrossRef]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic activity of loess soil in organic and conventional farming systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, H.H.; Hahm, S.C. Autonomic nerve change after loess bedding radiating far-infrared ray and energy. J. Naturop. 2020, 9, 27–32. [Google Scholar] [CrossRef]
- Jang, H.; Xing, S.; Kim, J.; So, S. Loess classification by region using machine learning property values and reliability assessment methods. Sci. Adv. Mater. 2021, 13, 1136–1143. [Google Scholar] [CrossRef]
- Hao, Z.; Li, X.; Gao, R.; An, M.; Zhang, J.; Wen, F.; Zhou, B.; Xue, Q. A new method for evaluating the homogeneity and structure of remolded loess samples with the air permeability coefficient. Appl. Sci. 2022, 12, 9412. [Google Scholar] [CrossRef]
- Choi, W.S.; Toyama, T.S.; Choi, Y.J. Preclinical efficacy examination on healing practices and experiences of users for S4 bio-balls bed of loess bio-balls products. Acad. J. Sci. Res. 2020, 8, 104–113. [Google Scholar]
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photon Lasers Med. 2012, 1, 255–266. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Z.; Liu, N.F.; Feng, S.Q.; Tong, Y.; Zhang, J.F.; Constantinides, J.; Lazzeri, D.; Grassetti, L.; Nicoli, F.; et al. Efficacy and safety of far infrared radiation in lymphedema treatment: Clinical evaluation and laboratory analysis. Lasers Med. Sci. 2017, 32, 485–494. [Google Scholar] [CrossRef]
- Leung, T.K.; Lin, S.L.; Yang, T.S.; Yang, J.C.; Lin, Y.S. The influence of ceramic far-infrared ray (cFIR) irradiation on water hydrogen bonding and its related chemo-physical properties. Hydrol. Current Res. 2014, 5, 1000174. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sardana, S.; Hamblin, M.R. Role of opsins and light or heat activated transient receptor potential ion channels in the mechanisms of photobiomodulation and infrared therapy. J. Photochem. Photobiol. 2023, 13, 100160. [Google Scholar] [CrossRef]
- Yamashita, K. The effects of the far-infrared ray (FIR) energy radiation on living body. In Blood Cell: An Overview of Studies in Hematology; Moschandreou, T.E., Ed.; Intechopen: London, UK, 2012; pp. 271–273. [Google Scholar] [CrossRef]
- Chen, T.C.; Huang, Y.C.; Chou, T.Y.; Hsu, S.T.; Chen, M.Y.; Nosaka, K. Effects of far-infrared radiation lamp therapy on recovery from muscle damage induced by eccentric exercise. Eur. J. Sport Sci. 2023, 23, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Chang, S.P.; Chi, L.M.; Lin, L.M. The effectiveness of far-infrared irradiation on foot skin surface temperature and heart rate variability in healthy adults over 50 years of age: A randomized study. Medicine 2020, 99, e23366. [Google Scholar] [CrossRef] [PubMed]
- Chaseling, G.K.; Crandall, C.G.; Gagnon, D. Skin blood flow measurements during heat stress: Technical and analytical considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, 57–69. [Google Scholar] [CrossRef]
- Wang, J.; Casati, G.; Benenti, G. Classical physics and blackbody radiation. Phys. Rev. Lett. 2022, 128, 134101. [Google Scholar] [CrossRef]
- Dervieux, E.; Guerrero, F.; Uhring, W.; Giroux-Metgès, M.A.; Théron, M. Skin temperature influence on transcutaneous carbon dioxide (Co2) conductivity and skin blood flow in healthy subjects at arm and wrist. Front. Physiol. 2023, 14, 1293752. [Google Scholar] [CrossRef]
- Välisuo, P. Optical methods for assessing skin flap survival. Biophotonics Med. Appl. 2015, 331–346. [Google Scholar] [CrossRef]
- Kiyokura, T.; Mino, S.; Shimada, J. Wearable Laser Blood Flowmeter. NTT Tech. Rev. 2006, 4, 38–43. Available online: https://ntt-review.jp/archive/ntttechnical.php?contents=ntr200601038.pdf (accessed on 19 February 2024).
- Saha, M.; Dremin, V.; Rafailov, I.; Dunaev, A.; Sokolovski, S.; Rafailov, E. Wearable Laser Doppler Flowmetry Sensor: A Feasibility Study with Smoker and Non-Smoker Volunteers. Biosensors 2020, 10, 201. Available online: https://www.mdpi.com/2079-6374/10/12/201 (accessed on 12 December 2023). [CrossRef]
- Choi, W.S.; Toyama, T.S.; Choi, Y.J. Healing formulation of South Korea loess powder as a bio material and its application to environmental-friendly bio products. In Proceedings of the 8th World Congress on Particle Technology, Orlando, FL, USA, 22–26 April 2018; Available online: https://aiche.confex.com/aiche/wcpt18/meetingapp.cgi/Paper/508433 (accessed on 14 January 2024).
- Kraehling, J.R.; Sessa, W.C. Contemporary approaches to modulating the nitric oxide–cGMP pathway in cardiovascular disease. Circ. Res. 2017, 120, 1174–1182. [Google Scholar] [CrossRef]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015, 263, 235–243. Available online: https://www.sciencedirect.com/science/article/pii/S0014488614003537 (accessed on 21 November 2023). [CrossRef] [PubMed]
- Witek, K.; Piotrowski, T.; Skwarek, A. Analysis of polymer foil heaters as infrared radiation sources. Mater. Sci. Eng. B 2012, 177, 1373–1377. [Google Scholar] [CrossRef]
- Cristiano, L. Use of infrared-based devices in aesthetic medicine and for beauty and wellness treatments. Infrared Phys. Technol. 2019, 102, 10299. [Google Scholar] [CrossRef]
- Steketee, J. Spectral emissivity of skin and pericardium. Phys. Med. Biol. 1973, 18, 686. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef]
- Geesink, H.J.H.; Jerman, I.; Meijer, D.K.F. The cradle of life via its coherent quantum frequencies. Water 2020, 11, 78–108. Available online: https://waterjournal.org/uploads/vol11/geesink/WATER.2020.1.Geesink.pdf (accessed on 13 January 2024).
- Stomp, M.; Huisman, J.; Stal, L.J.; Matthijs, H.C.P. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J. 2007, 1, 271–282. Available online: https://www.nature.com/articles/ismej200759 (accessed on 2 February 2024). [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef]
- Kuht, J.; Farmery, A.D. Body temperature and its regulation. Anaesth. Intensive Care Med. 2021, 22, 657–662. [Google Scholar] [CrossRef]
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Thermometry and interpretation of body temperature. Biomed. Eng. Lett. 2019, 9, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public Health 2020, 17, 7795. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Pan, J.; Ma, J.; Ge, X.; Xu, Z.; Wang, X.; Lv, Z. Advances in far-infrared research: Therapeutic mechanisms of disease and application in cancer detection. Lasers Med. Sci. 2024, 39, 41. [Google Scholar] [CrossRef] [PubMed]



| Analyte | Result | Line | Net Int. | BG Int. |
|---|---|---|---|---|
| SiO2 | 72.3262% | SiKa | 902.218 | 2.766 |
| Al2O3 | 15.9731% | AlKa | 280.657 | 12.753 |
| Fe2O3 | 5.9951% | FeKa | 349.073 | 1.330 |
| K2O | 2.1878% | K Ka | 102.760 | 0.742 |
| MgO | 1.0557% | MgKa | 6.590 | 0.480 |
| TiO2 | 0.9764% | TiKa | 13.524 | 0.169 |
| CaO | 0.4570% | CaKa | 16.625 | 0.434 |
| P2O5 | 0.3802% | P Ka | 7.140 | 0.619 |
| MnO | 0.1914% | MnKa | 8.758 | 0.787 |
| Na2O | 0.1912% | P Ka | 0.608 | 0.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Choi, W.C.; Jeon, G.R.; Kim, J.H.; Kim, M.S.; Kim, J.H. Characteristics of Far-Infrared Ray Emitted from Functional Loess Bio-Balls and Its Effect on Improving Blood Flow. Bioengineering 2024, 11, 380. https://doi.org/10.3390/bioengineering11040380
Choi YJ, Choi WC, Jeon GR, Kim JH, Kim MS, Kim JH. Characteristics of Far-Infrared Ray Emitted from Functional Loess Bio-Balls and Its Effect on Improving Blood Flow. Bioengineering. 2024; 11(4):380. https://doi.org/10.3390/bioengineering11040380
Chicago/Turabian StyleChoi, Yeon Jin, Woo Cheol Choi, Gye Rok Jeon, Jae Ho Kim, Min Seok Kim, and Jae Hyung Kim. 2024. "Characteristics of Far-Infrared Ray Emitted from Functional Loess Bio-Balls and Its Effect on Improving Blood Flow" Bioengineering 11, no. 4: 380. https://doi.org/10.3390/bioengineering11040380
APA StyleChoi, Y. J., Choi, W. C., Jeon, G. R., Kim, J. H., Kim, M. S., & Kim, J. H. (2024). Characteristics of Far-Infrared Ray Emitted from Functional Loess Bio-Balls and Its Effect on Improving Blood Flow. Bioengineering, 11(4), 380. https://doi.org/10.3390/bioengineering11040380





