Simultaneous High-Speed Video Laryngoscopy and Acoustic Aerodynamic Recordings during Vocal Onset of Variable Sound Pressure Level: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
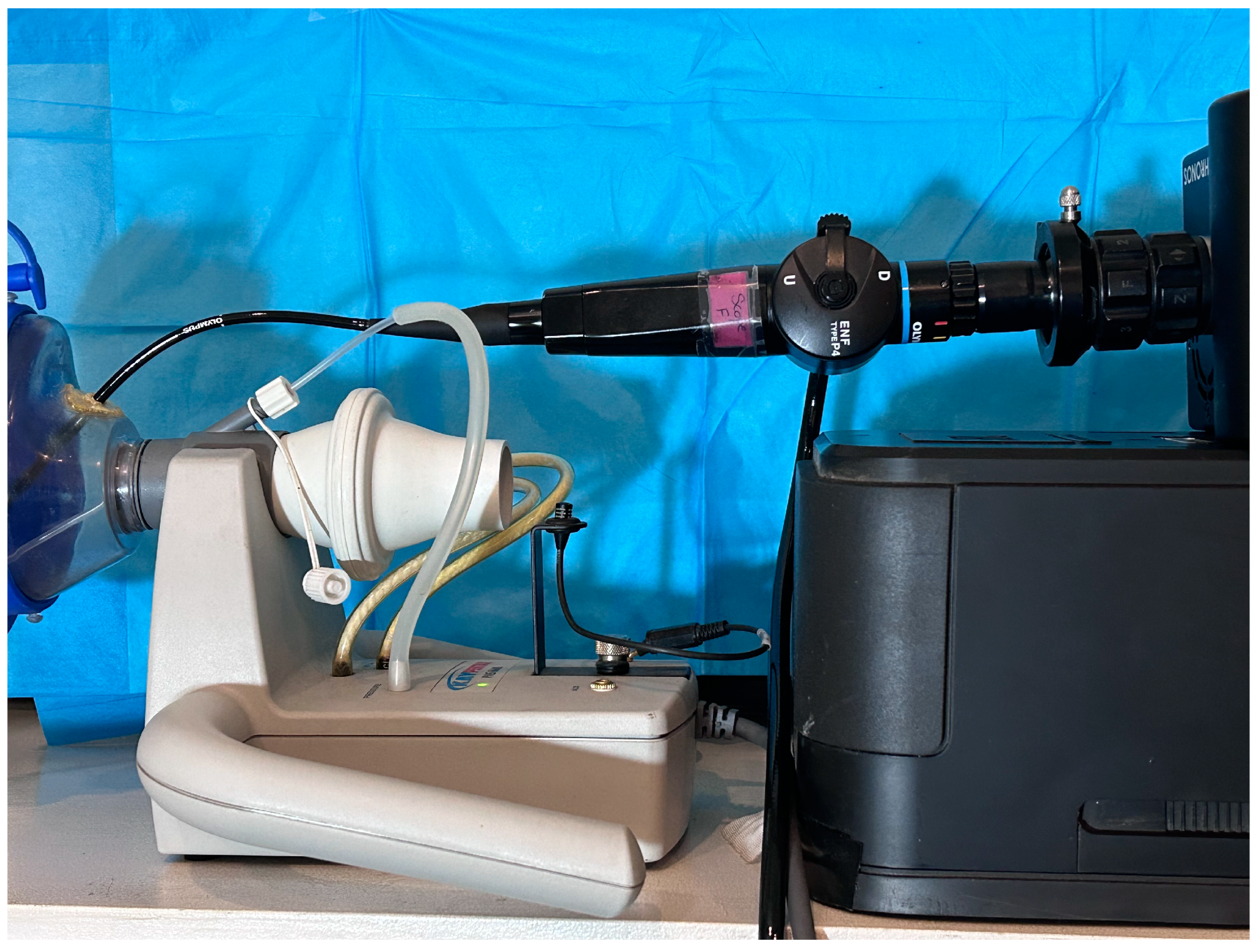
2.1. Equipment Setup
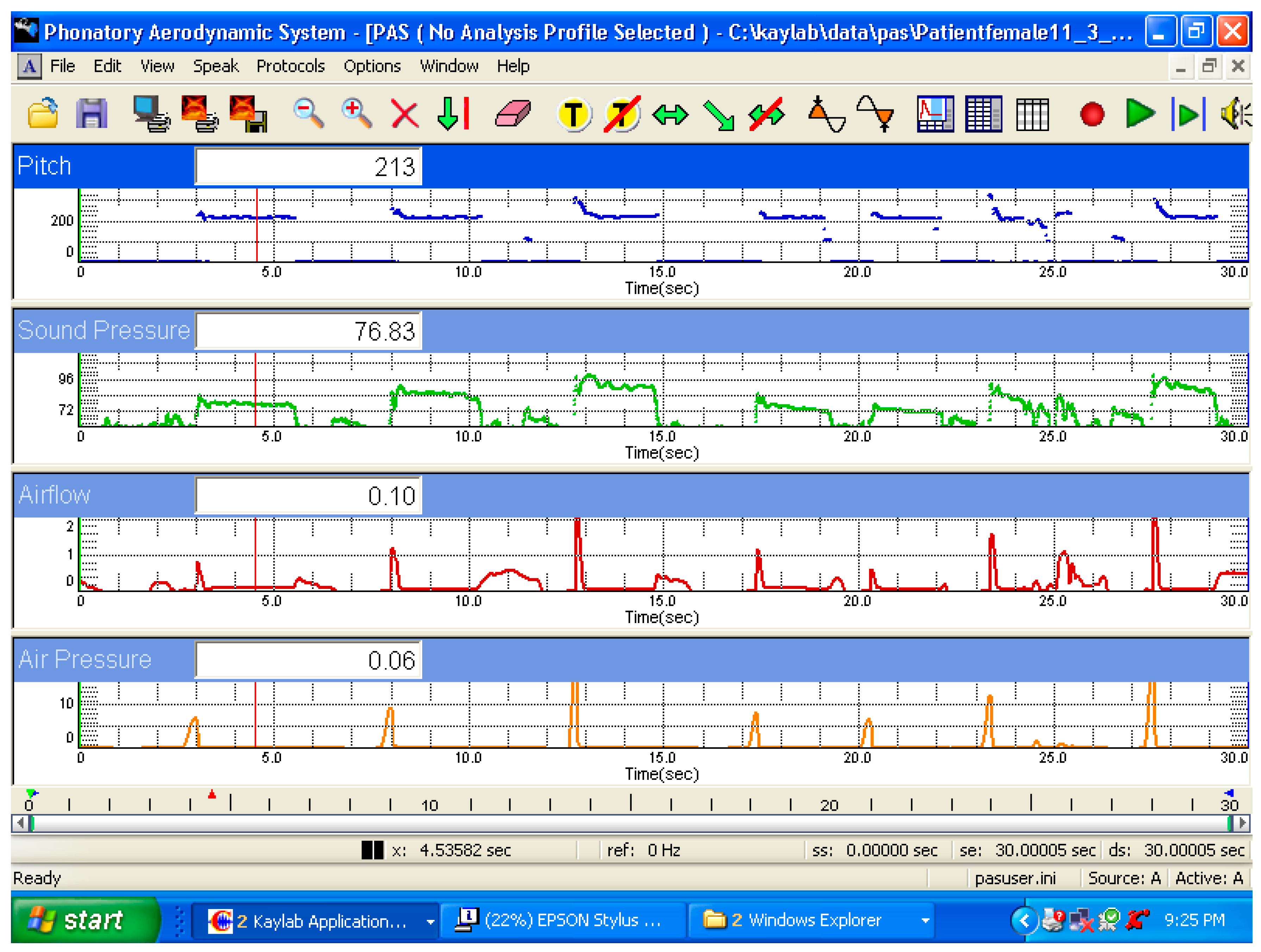
2.1.1. PAS
2.1.2. High-Speed Video Camera Setting
2.1.3. Flexible Laryngoscopy
2.2. Subject Recording
2.2.1. Subject Instructions
2.2.2. Recording of Aerodynamic Data
2.2.3. Recording of HSV
3. Analysis
3.1. Aerodynamic and Acoustic Analysis
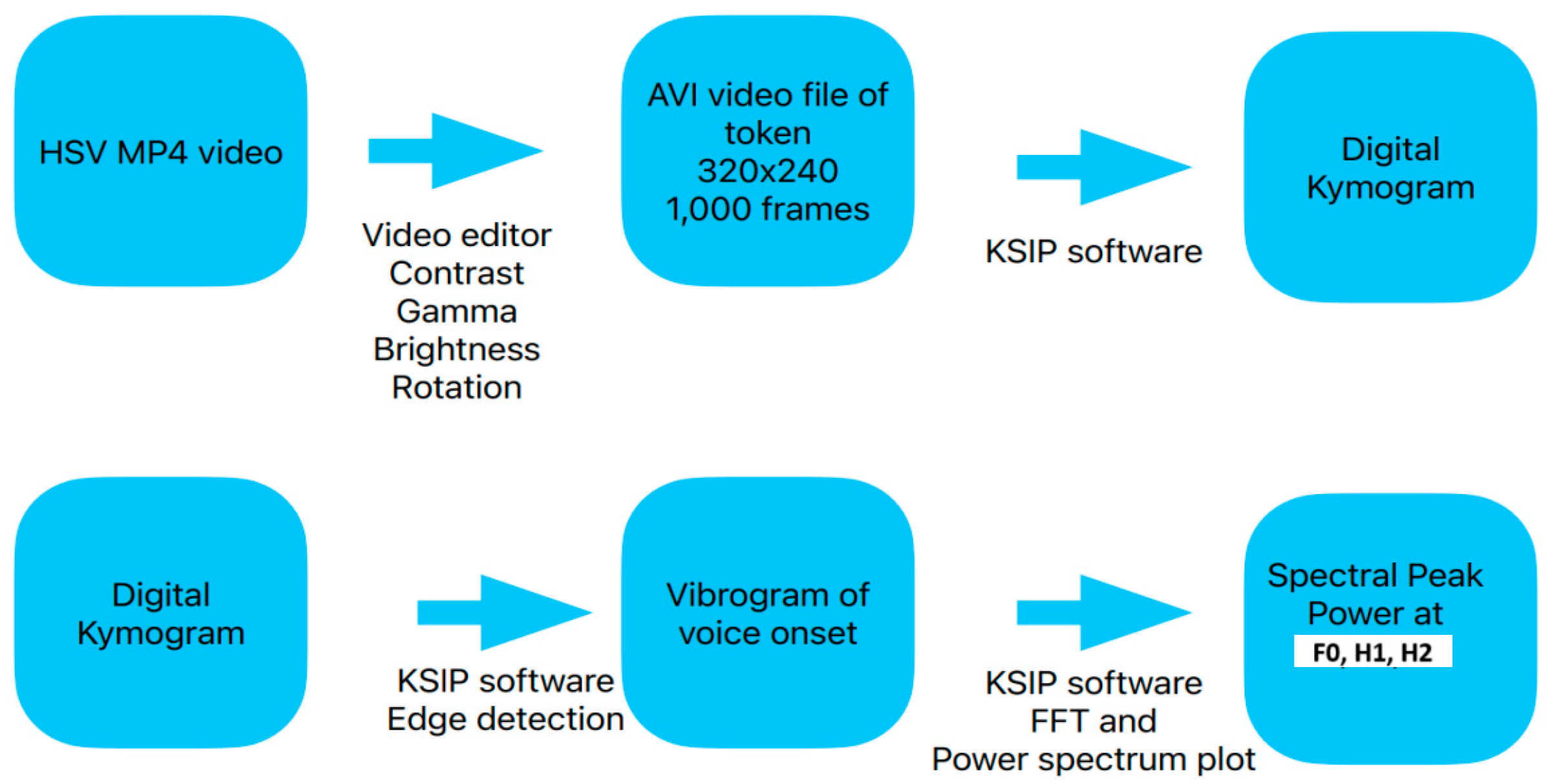
3.2. Video Editing and Conversion
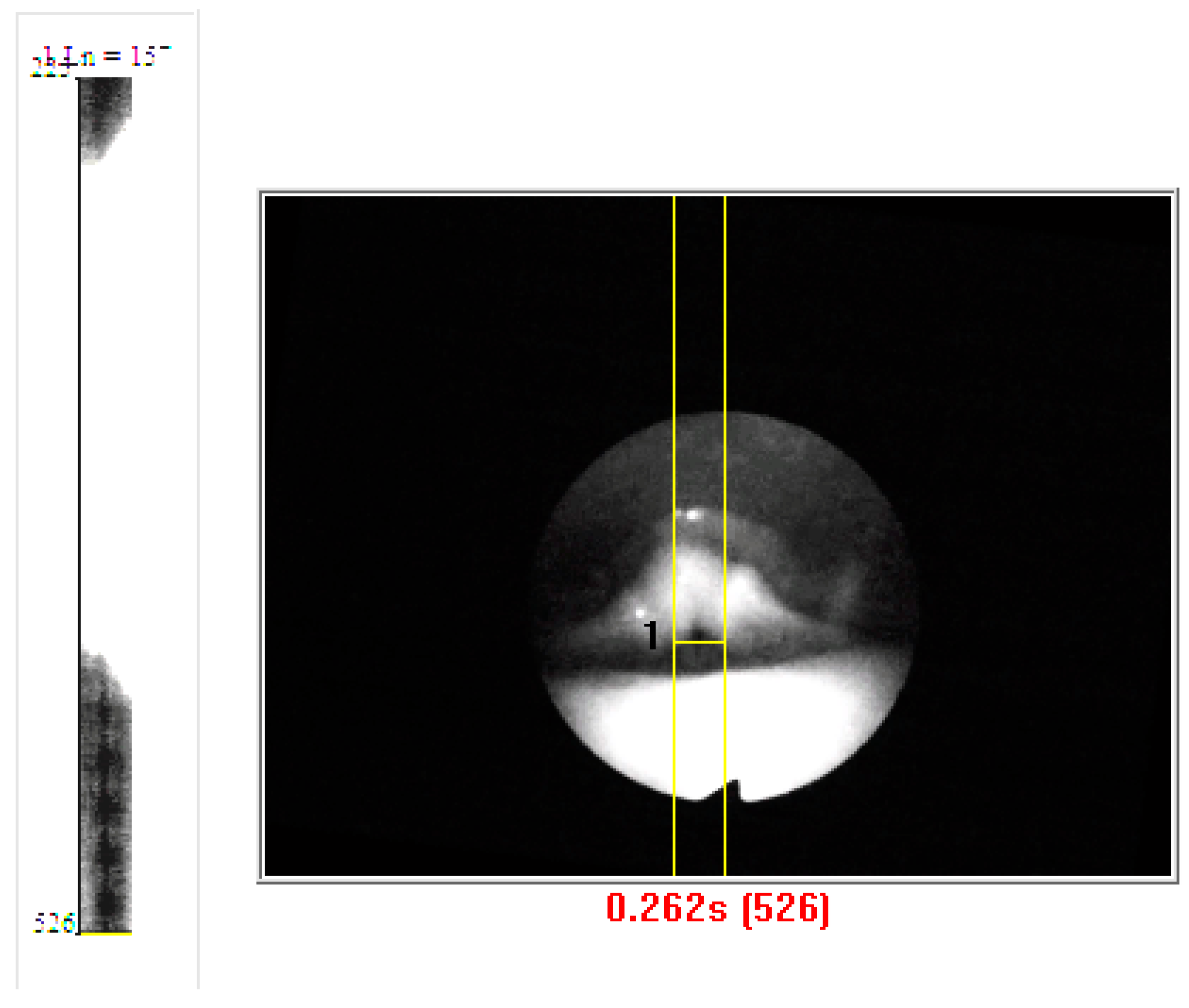
3.3. Vibrogram Analysis
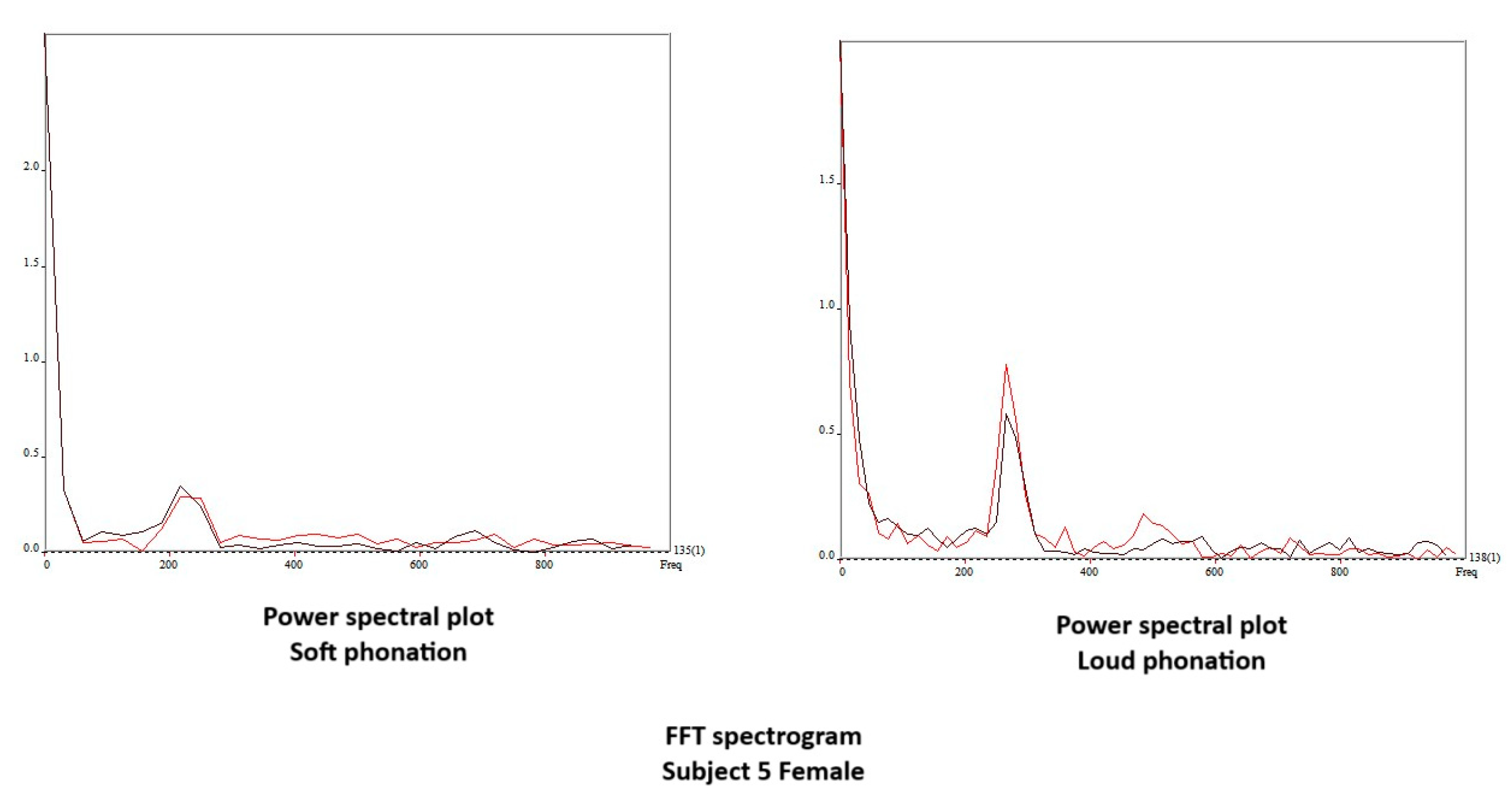
4. Results
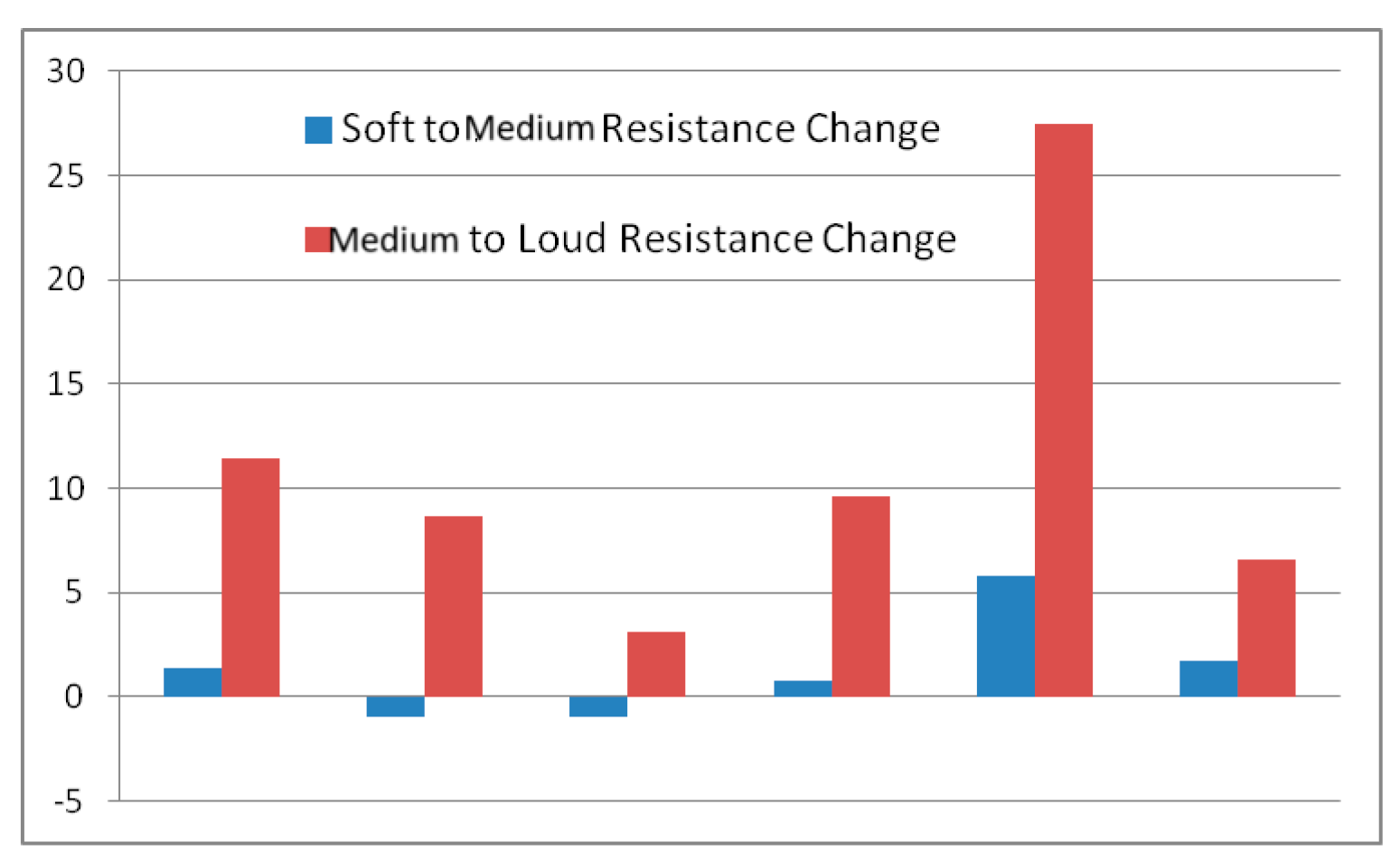
4.1. Aerodynamic Changes during the Production of Different SPL
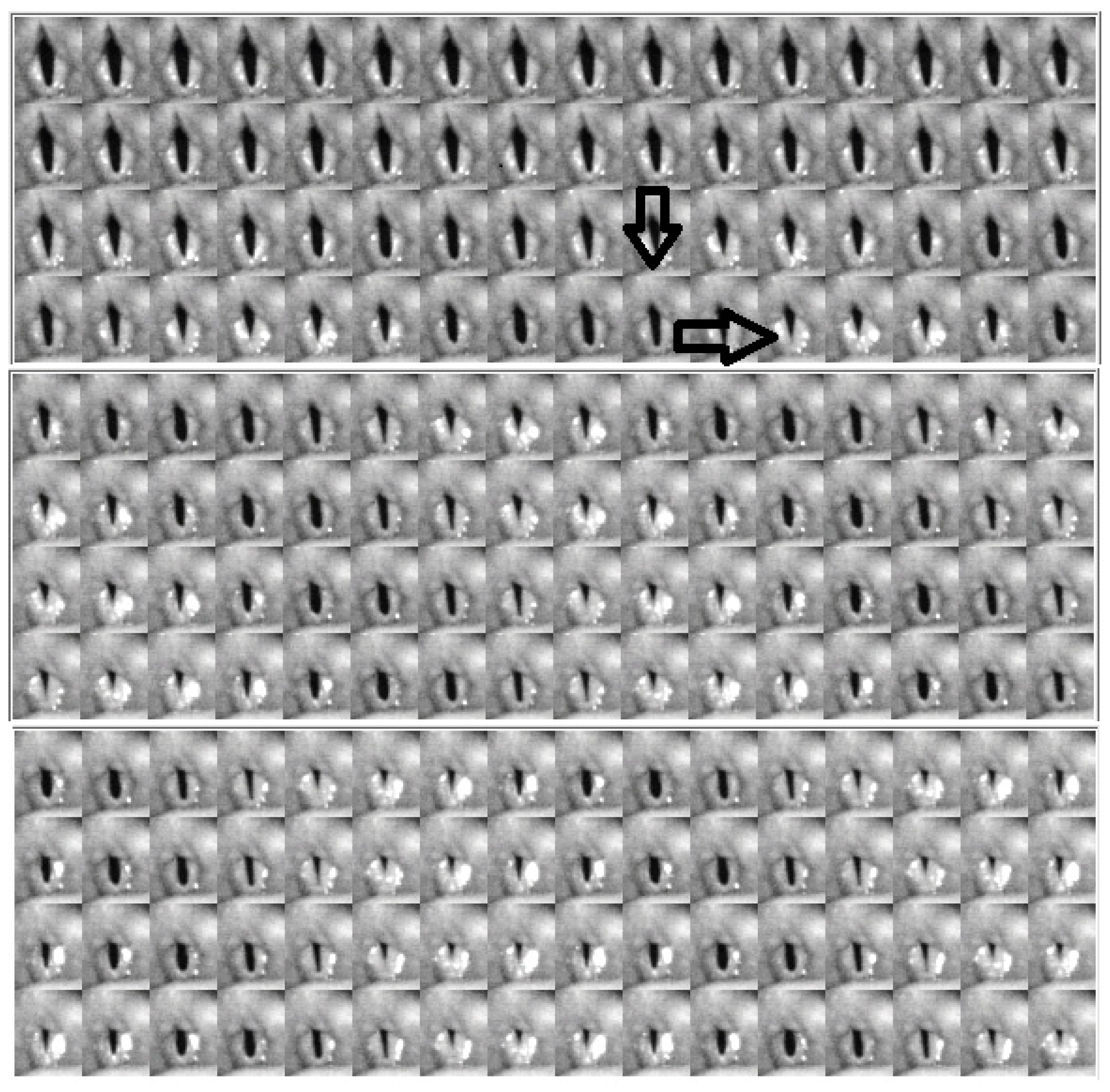
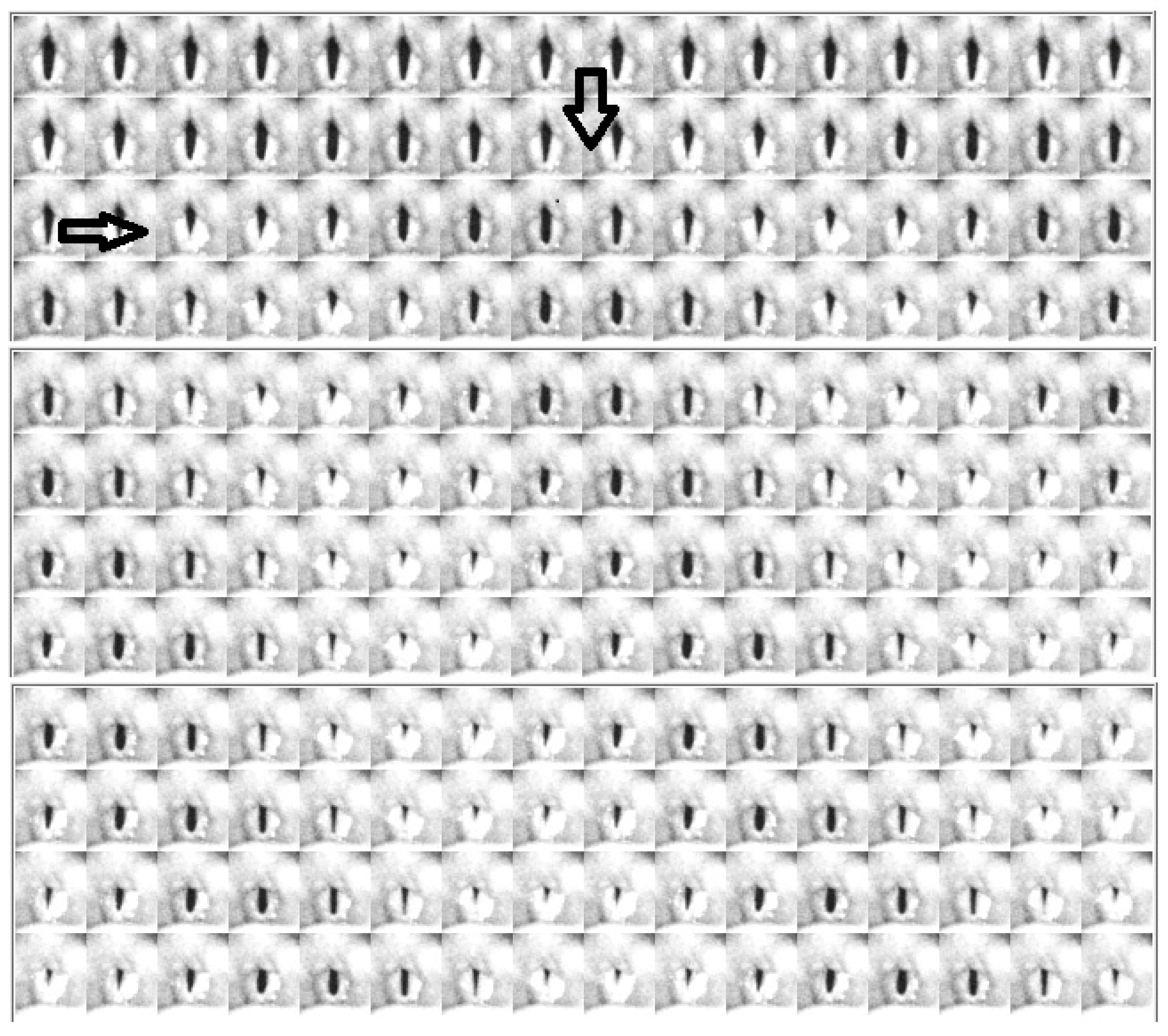
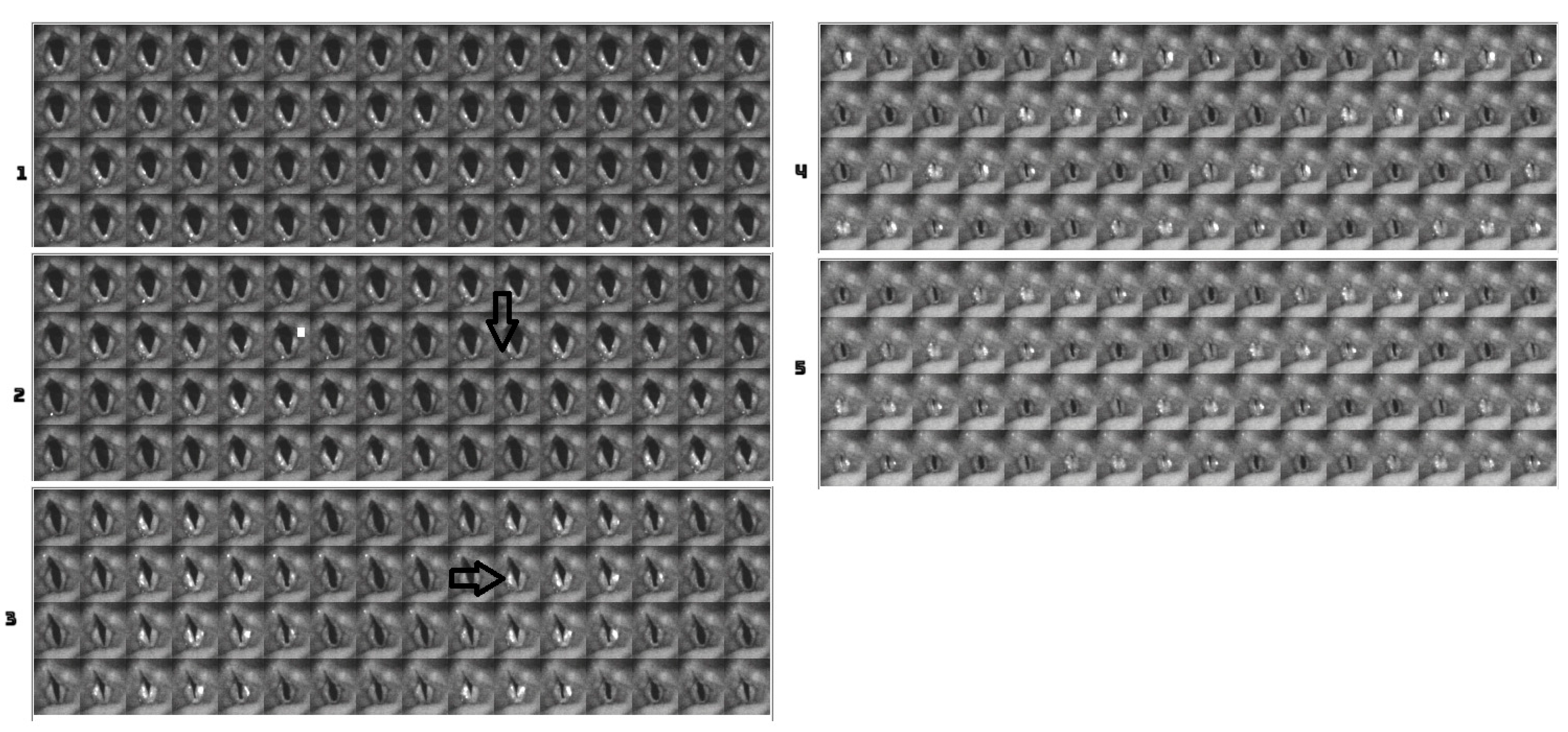
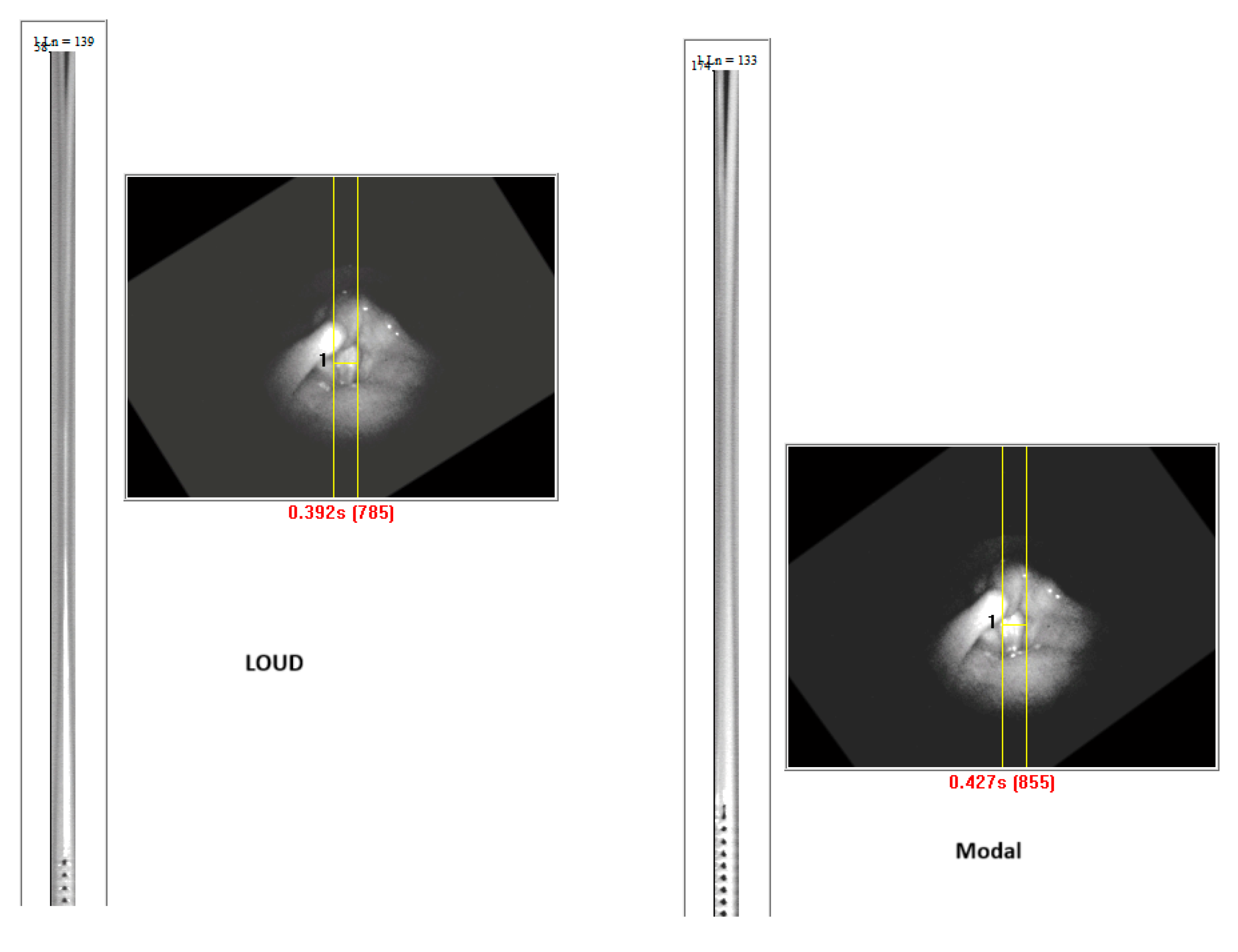
4.2. Descriptive Findings of High-Speed Video of Vocal Onset
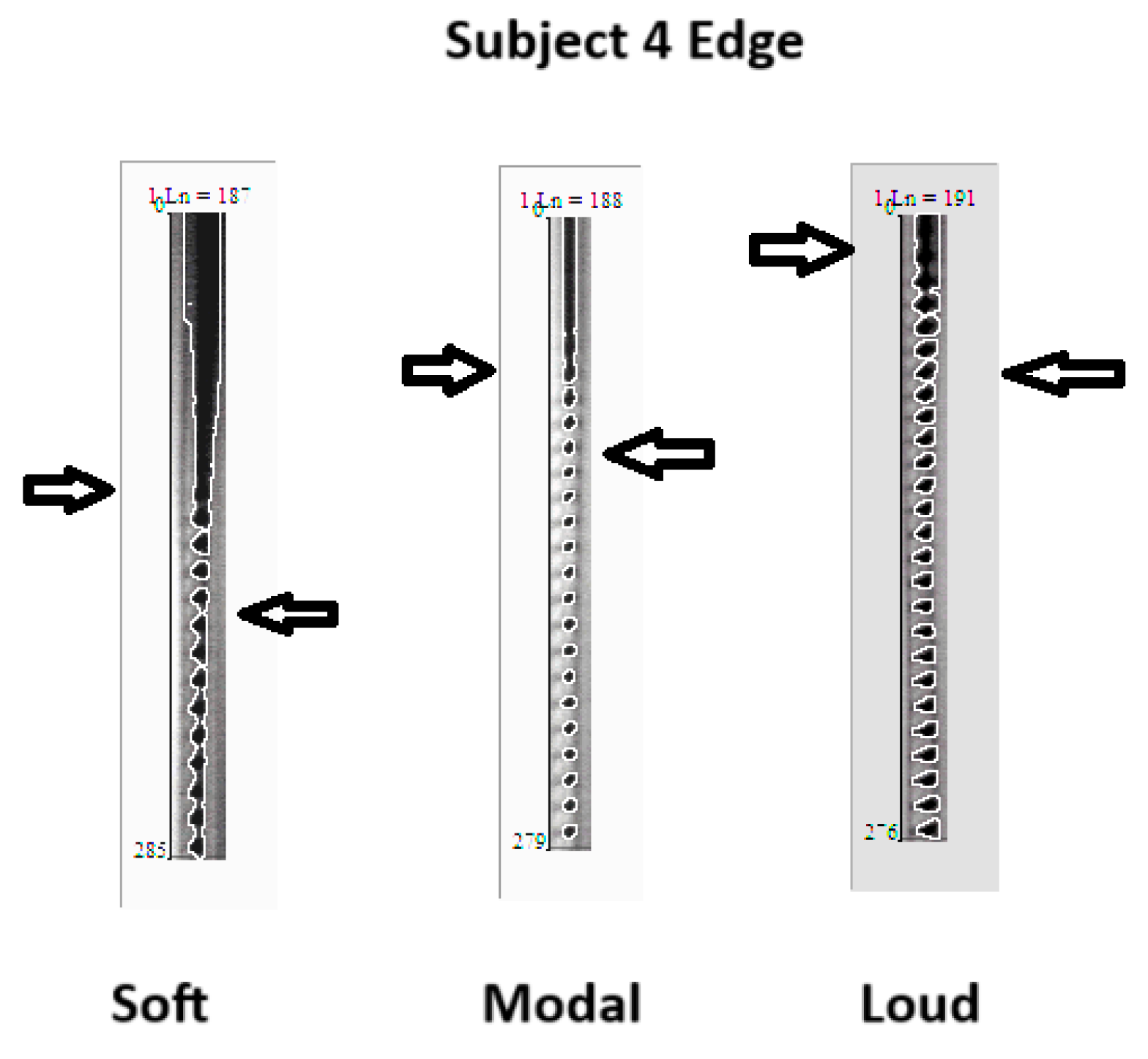
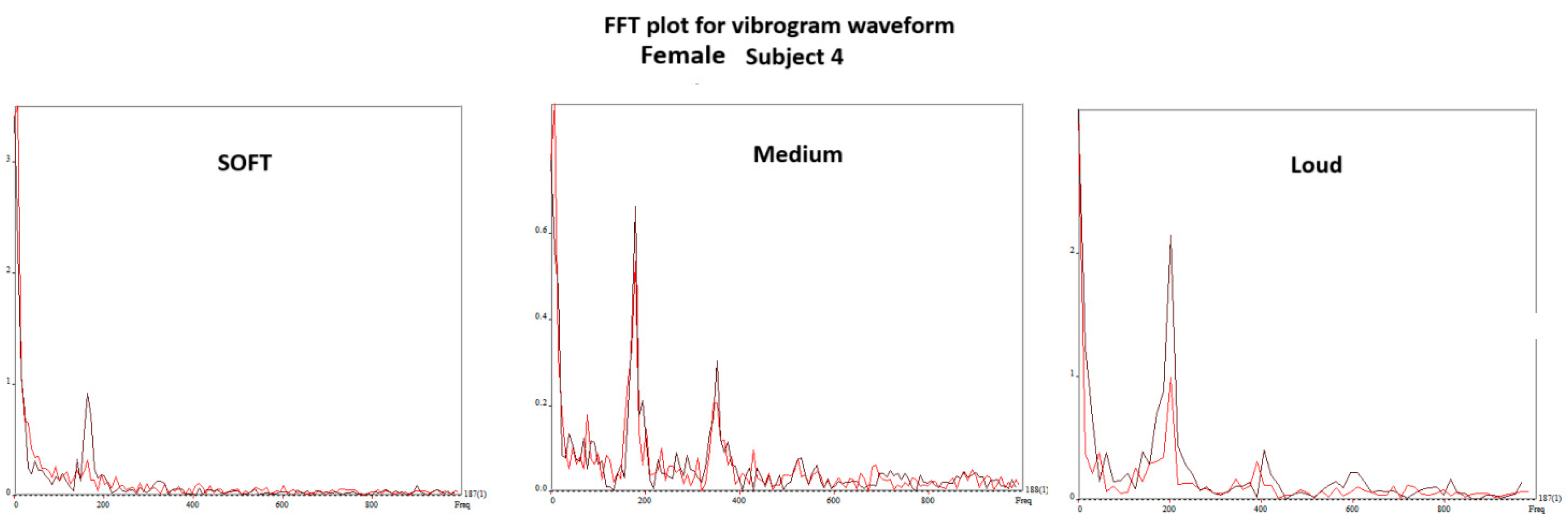
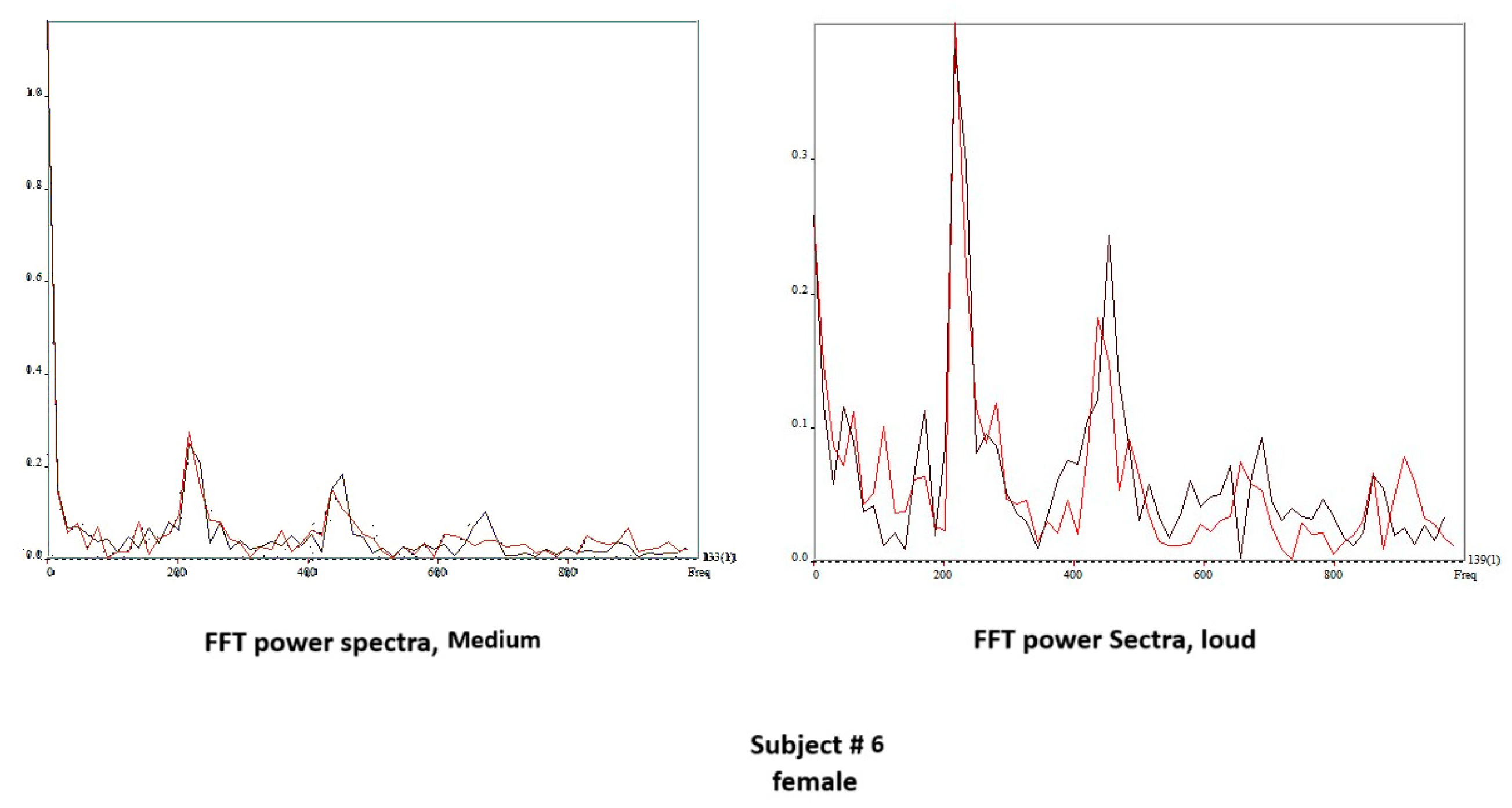
4.3. DKG Tracing and Analysis of the Vibrogram Waveform
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timcke, R. Die Synchron-stroboskopie von menschlichen Stimmlippen bzw. ähnlichen Schallquellen und Messung der öffungszeit. [Synchronous stroboscopy of the vocal cords in man and analogous sources of sound and the duration of opening]. Z. Laryngol. Rhinol. 1956, 35, 331–335. [Google Scholar]
- Schönharl, E. Die Stroboskopie in der Praktischen Laryngologie; Georg Thieme: Stuttgart, Germany, 1960. [Google Scholar]
- Woo, P. Stroboscopy; Plural Publishing: San Diego, CA, USA, 2010. [Google Scholar]
- Moore, G.P.; White, F.D.; von Leden, H. Ultra high speed photography in laryngeal physiology. J. Speech Hear. Disord. 1962, 27, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Kiritani, S.; Imagawa, H. High speed digital image analysis of laryngeal behavior in runnins speech. Annu. Bull. RILP 1987, 21, 25–40. [Google Scholar]
- Svec, J.G.; Sram, F.; Schutte, H.K. Videokymography in voice disorders: What to look for? Ann. Otol. Rhinol. Laryngol. 2007, 116, 172–180. [Google Scholar] [CrossRef]
- Werner-Kukuk, E.; von Leden, H. Vocal initiation: High speed cinematographic studies on normal subjects. Folia Phoniatr. Logop. 1970, 22, 107–116. [Google Scholar] [CrossRef]
- Woo, P. High-speed Imaging of Vocal Fold Vibration Onset Delay: Normal Versus Abnormal. J. Voice 2017, 31, 307–312. [Google Scholar] [CrossRef]
- Unger, J.; Meyer, T.; Herbst, C.T.; Fitch, W.T.; Dollinger, M.; Lohscheller, J. Phonovibrographic wavegrams: Visualizing vocal fold kinematics. J. Acoust. Soc. Am. 2013, 133, 1055–1064. [Google Scholar] [CrossRef]
- Gould, W.J.; Kojima, H.; Lambiase, A. A technique for stroboscopic examination of the vocal folds using fiberoptics. Arch. Otolaryngol. 1979, 105, 285. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.; Baxter, P. Flexible Fiber-Optic High-Speed Imaging of Vocal Fold Vibration: A Preliminary Report. J. Voice 2017, 31, 175–181. [Google Scholar] [CrossRef]
- Woo, P. Vibratory Characteristics of Diplophonia Studied by High Speed Video and Vibrogram Analysis. J. Voice 2019, 33, 7–15. [Google Scholar] [CrossRef]
- Chen, W.; Woo, P.; Murry, T. Spectral analysis of digital kymography in normal adult vocal fold vibration. J. Voice 2014, 28, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Rzepakowska, A.; Sielska-Badurek, E.; Zurek, M.; Osuch-Wojcikiewicz, E.; Niemczyk, K. Narrow band imaging for risk stratification of glottic cancer within leukoplakia. Head Neck 2018, 40, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Kiritani, S.; Imagawa, H. High speed digital image analysis of laryngeal behavior in running speech. In Vocal physiology: Voice Production. Mechanisms, and Functions; Fujimura, O., Ed.; Raven Press: New York, NY, USA, 1988; pp. 335–345. [Google Scholar]
- Plaat, B.E.; van der Laan, B.F.; Wedman, J.; Halmos, G.B.; Dikkers, F.G. Distal chip versus fiberoptic laryngoscopy using endoscopic sheaths: Diagnostic accuracy and image quality. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Schutte, H.K. A new generation videokymography for routine clinical vocal fold examination. Laryngoscope 2006, 116, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Dejonckere, P.H.; Bradley, P.; Clemente, P.; Cornut, G.; Crevier-Buchman, L.; Friedrich, G.; Van De Heyning, P.; Remacle, M.; Woisard, V. A basic protocol for functional assessment o f voice pathology, especially for investigating the efficacy of (phonosurgical) treatments and evaluating new assessment techniques. Guideline elaborated by the Committee on Phoniatrics of the European Laryngological Society (ELS). Eur. Arch. Oto-Rhino-Laryngol. 2001, 258, 77–82. [Google Scholar]
- Schneider, B.; Denk, D.M.; Bigenzahn, W. Functional results after external vocal fold medialization thyroplasty with the titanium vocal fold medialization implant. Laryngoscope 2003, 113, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, E.B.; Oates, J.; Dacakis, G.; Grant, C. Phonetograms, aerodynamic measurements, self-evaluations, and auditory perceptual ratings of male-to-female transsexual voice. J. Voice 2010, 24, 511–522. [Google Scholar] [CrossRef]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and validation of the voice handicap index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- Dejonckere, P.H. Perceptual and laboratory assessment of dysphonia. Otolaryngol. Clin. N. Am. 2000, 33, 731–750. [Google Scholar] [CrossRef]
- Woo, P. Objective measures of laryngeal imaging: What have we learned since Dr. Paul Moore. J. Voice 2014, 28, 69–81. [Google Scholar] [CrossRef]
- Dejonckere, P.H.; Crevier, L.; Elbaz, E.; Marraco, M.; Millet, B.; Remacle, M.; Woisard, V. Quantitative rating of video-laryngostroboscopy: A reliability study. Rev. Laryngol.-Otol.-Rhinol. 1998, 119, 259–260. [Google Scholar]
- Chhetri, D.K.; Neubauer, J.; Bergeron, J.L.; Sofer, E.; Peng, K.A.; Jamal, N. Effects of Asymmetric Superior Laryngeal Nerve Stimulation on Glottic Posture, Acoustics, Vibration. Laryngoscope 2013, 123, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.; Woo, P.; Saxman, J.H.; Murry, T. A comparison of sung and spoken phonation onset gestures using high-speed digital imaging. J. Voice 2012, 26, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Blalock, P.D. Vocal fatigue and dysphonia in the professional voice user: Bogart-Bacall syndrome. Laryngoscope 1988, 98, 493–498. [Google Scholar] [CrossRef]
| Subject | Sex | Age | Token | SPL dB | Frequency Hz | Flow cc/sec | Pressure cm | Resistance Pressure/Flow | dB Change | Resistance Change | Resistance Change per dB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 33 | soft | 74 | 110 | 210 | 3 | 14.28571 | |||
| 1 | male | 33 | modal | 85 | 109 | 170 | 5 | 29.41176 | 11 | 15.12605 | 1.375095 |
| 1 | male | 33 | loud | 88 | 110 | 110 | 7 | 63.63636 | 3 | 34.2246 | 11.4082 |
| 2 | male | 29 | soft | 70 | 102 | 79 | 4 | 50.63291 | |||
| 2 | male | 29 | modal | 83 | 121 | 132 | 5 | 37.87879 | 13 | −12.7541 | −0.98109 |
| 2 | male | 29 | loud | 89 | 139 | 89 | 8 | 89.88764 | 6 | 52.00885 | 8.668142 |
| 3 | male | 69 | soft | 85 | 125 | 100 | 2 | 20 | |||
| 3 | male | 69 | modal | 91 | 123 | 220 | 3 | 13.63636 | 6 | −6.36364 | −1.06061 |
| 3 | male | 69 | loud | 100 | 136 | 170 | 7 | 41.17647 | 9 | 27.54011 | 3.060012 |
| 4 | Female | 64 | soft | 74 | 161 | 100 | 4 | 40 | |||
| 4 | Female | 64 | modal | 84 | 157 | 110 | 5.3 | 48.18182 | 10 | 8.181818 | 0.818182 |
| 4 | Female | 64 | loud | 87 | 186 | 130 | 10 | 76.92308 | 3 | 28.74126 | 9.58042 |
| 5 | Female | 24 | soft | 76 | 212 | 100 | 6 | 60 | |||
| 5 | Female | 24 | modal | 85 | 215 | 80 | 9 | 112.5 | 9 | 52.5 | 5.833333 |
| 5 | Female | 24 | loud | 90 | 217 | 60 | 15 | 250 | 5 | 137.5 | 27.5 |
| 6 | Female | 30 | soft | 75 | 168 | 130 | 4.6 | 35.38462 | |||
| 6 | Female | 30 | modal | 86 | 182 | 130 | 7 | 53.84615 | 11 | 18.46154 | 1.678322 |
| 6 | Female | 30 | loud | 92 | 207 | 120 | 11.2 | 93.33333 | 6 | 39.48718 | 6.581197 |
| Frequency Hz | Flow cc/sec | Pressure cm H2O | ||
|---|---|---|---|---|
| Mean | Male | 119 | 142 | 4 |
| SD | 12 | 52 | 2 | |
| Mean | Female | 189 | 106 | 8 |
| SD | 24 | 24 | 3 |
| Soft-Modal Resistance/dB | Modal-Loud Resistance/dB | |
|---|---|---|
| Mean | 1.27 | 11.15 |
| STD | 2.49 | 8.5 |
| Paired t-test | p-value < 0.01 | p-value > 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, P. Simultaneous High-Speed Video Laryngoscopy and Acoustic Aerodynamic Recordings during Vocal Onset of Variable Sound Pressure Level: A Preliminary Study. Bioengineering 2024, 11, 334. https://doi.org/10.3390/bioengineering11040334
Woo P. Simultaneous High-Speed Video Laryngoscopy and Acoustic Aerodynamic Recordings during Vocal Onset of Variable Sound Pressure Level: A Preliminary Study. Bioengineering. 2024; 11(4):334. https://doi.org/10.3390/bioengineering11040334
Chicago/Turabian StyleWoo, Peak. 2024. "Simultaneous High-Speed Video Laryngoscopy and Acoustic Aerodynamic Recordings during Vocal Onset of Variable Sound Pressure Level: A Preliminary Study" Bioengineering 11, no. 4: 334. https://doi.org/10.3390/bioengineering11040334
APA StyleWoo, P. (2024). Simultaneous High-Speed Video Laryngoscopy and Acoustic Aerodynamic Recordings during Vocal Onset of Variable Sound Pressure Level: A Preliminary Study. Bioengineering, 11(4), 334. https://doi.org/10.3390/bioengineering11040334






