Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Inoculum Preparation
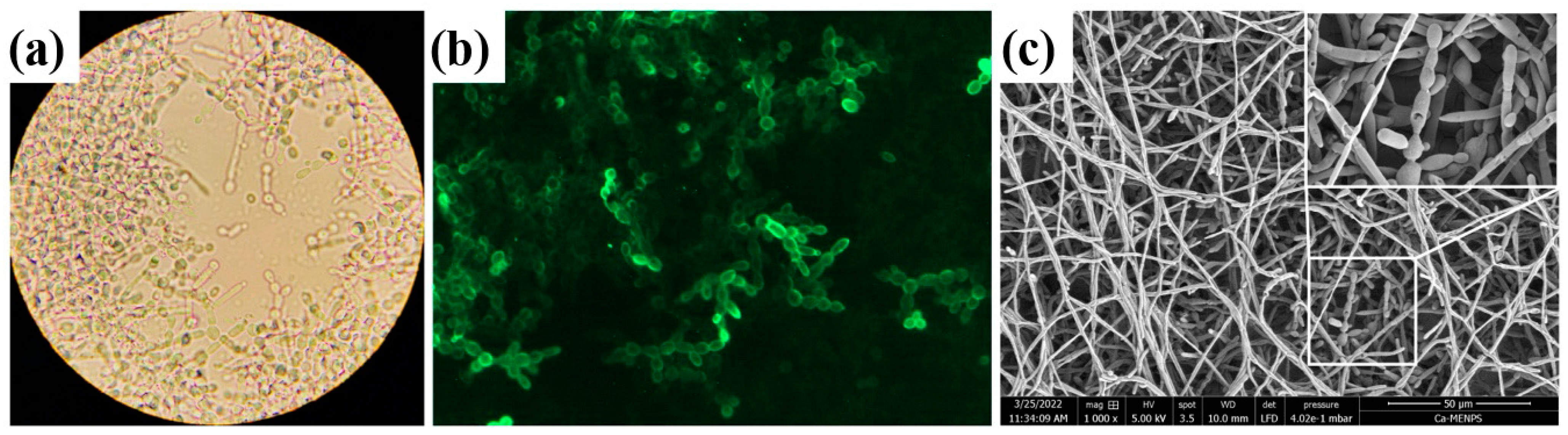
2.2. In Vitro Biofilm Formation by C. albicans ATCC 10231
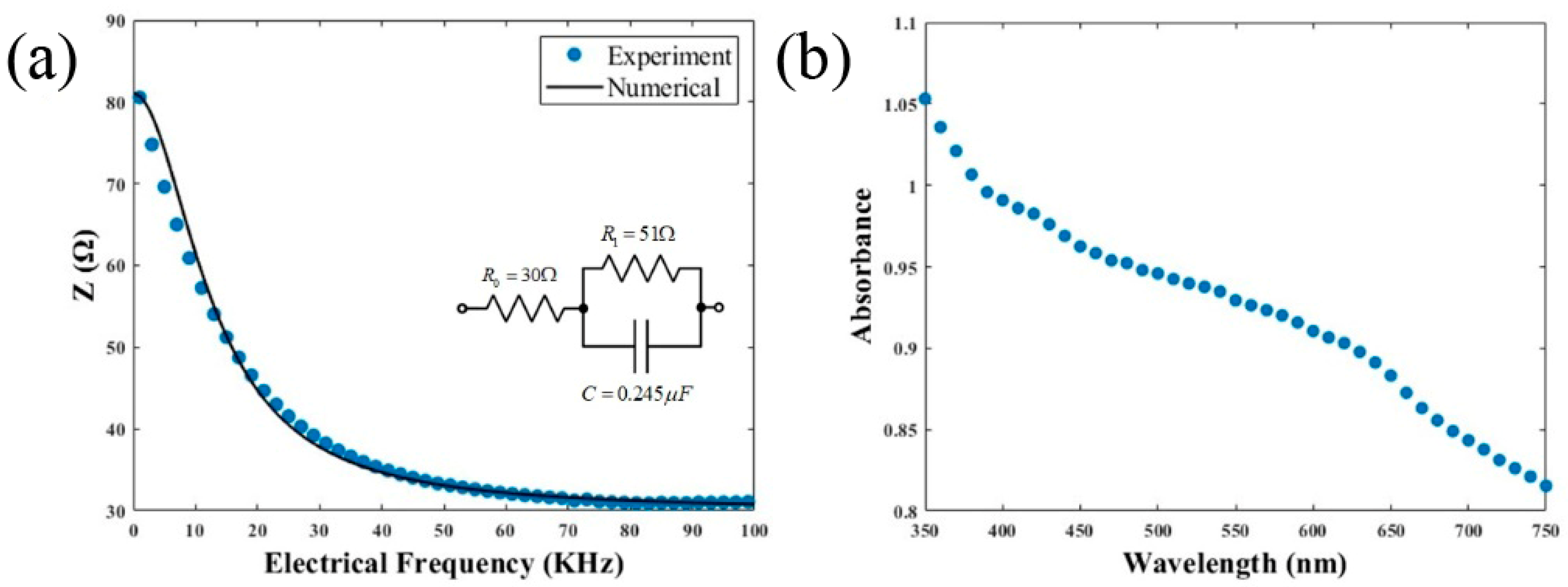
2.3. Electrochemical Impedance Explorations in C. albicans ATCC 10231
2.4. Spectral Absorbance in C. albicans ATCC 10231 Biofilm
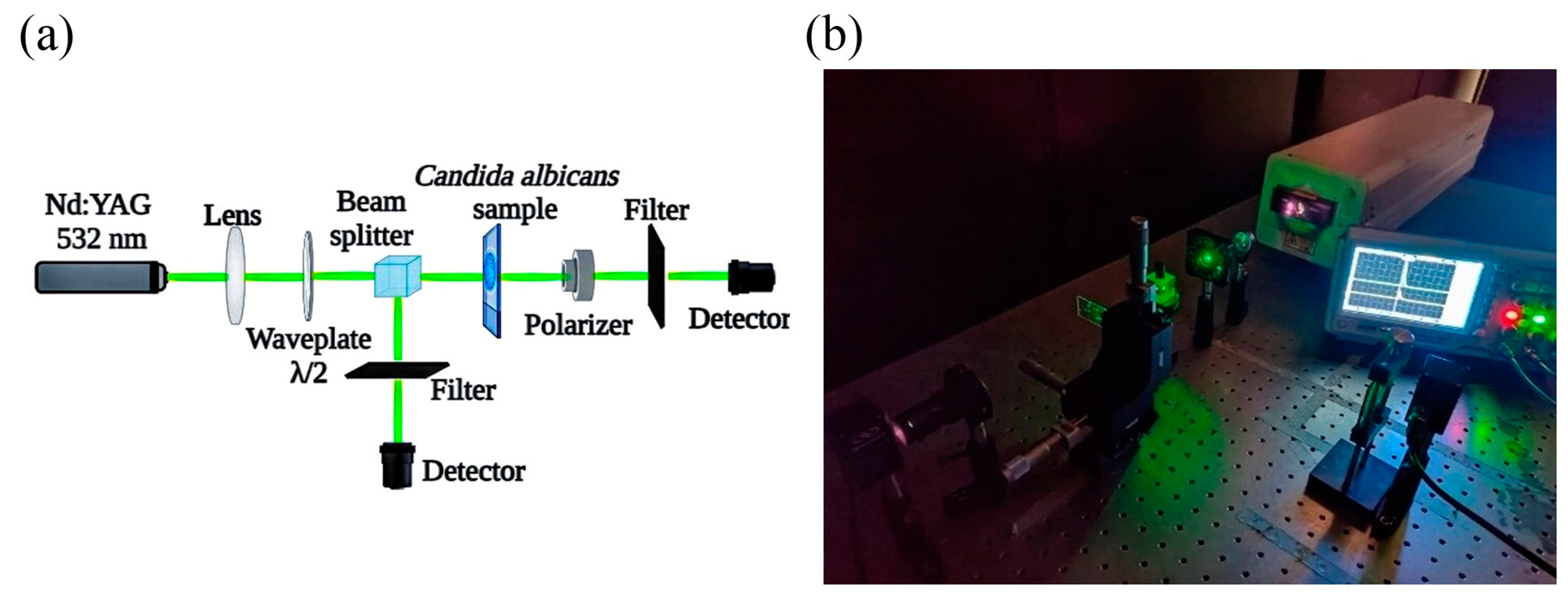
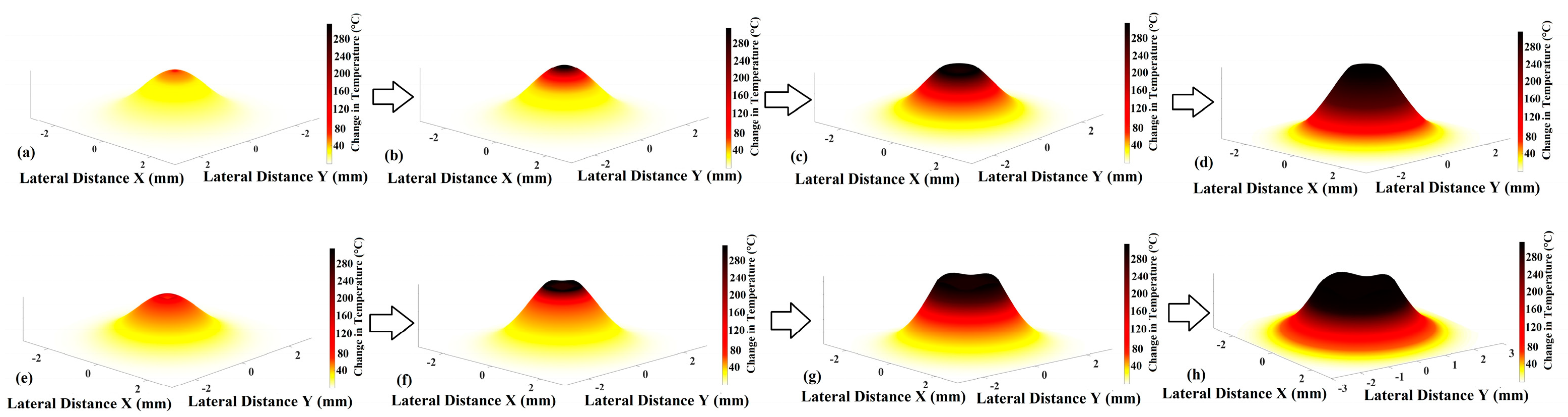
2.5. Studies of Ablation Effect over C. albicans ATCC 10231 Biofilms
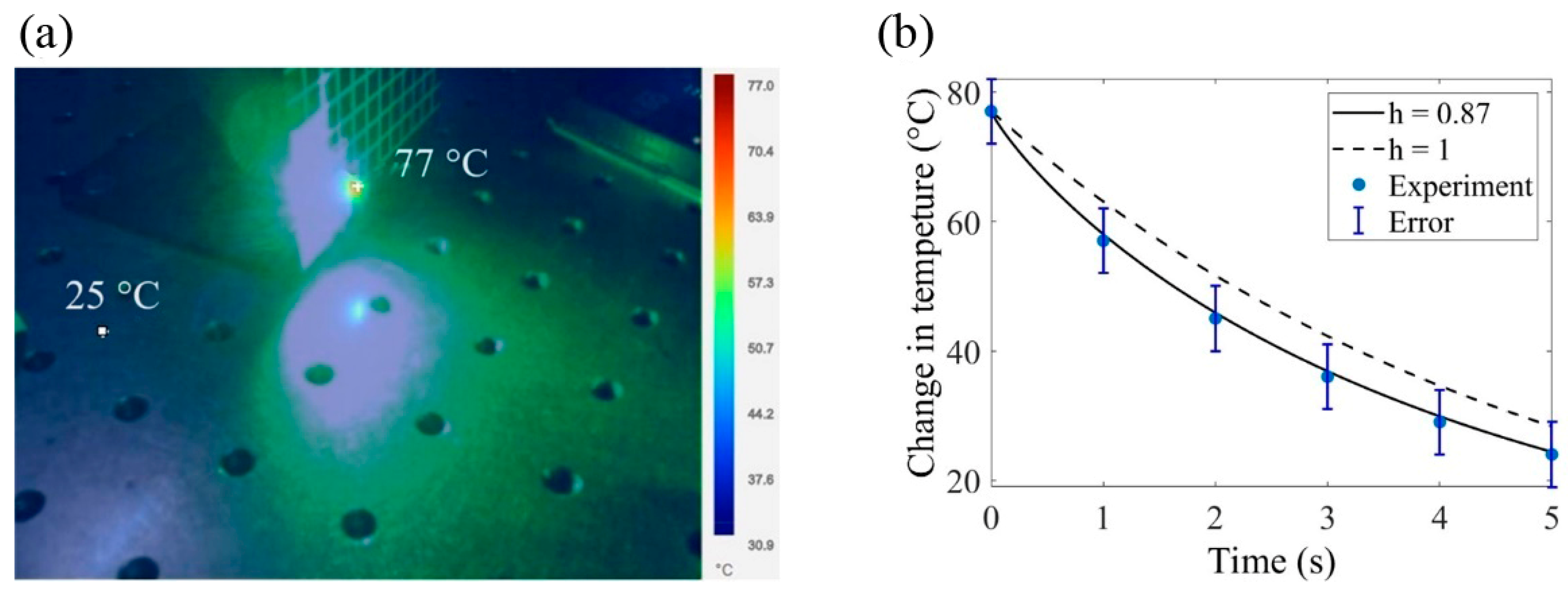
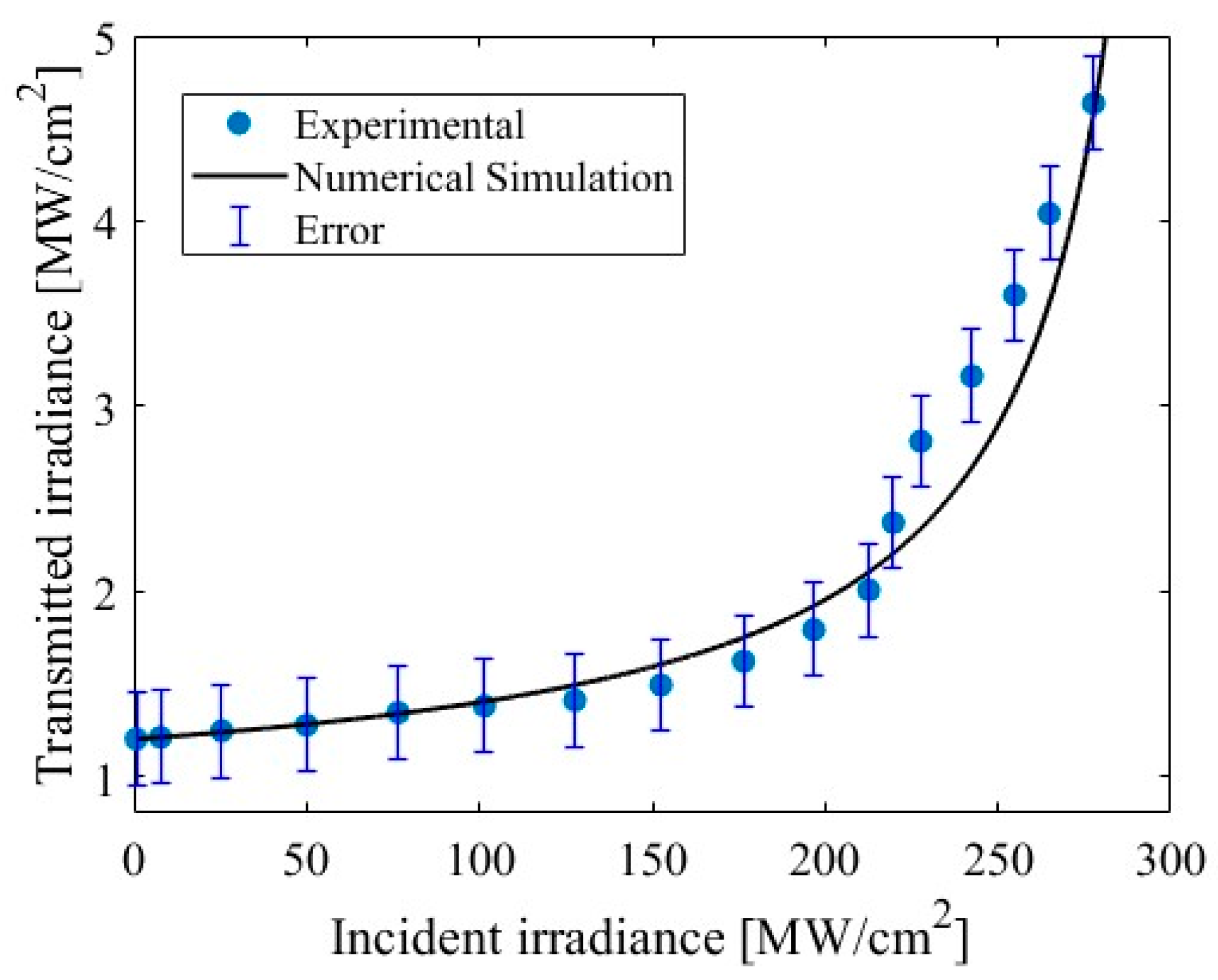
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, D.; Lv, X.; Xue, L.; Yang, N.; Hu, Y.; Weng, L.; Fu, N.; Wang, L.; Dong, X. A Lipase-Responsive Antifungal Nanoplatform for Synergistic Photodynamic/Photothermal/Pharmaco-Therapy of Azole-Resistant Candida albicans Infections. Chem. Commun. 2019, 55, 15145–15148. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, K.F.; Sousa, M.S.; Valverde, J.V.P.; Olivati, C.A.; Souto, P.C.S.; Silva, J.R.; de Souza, N.C. Fractal Analysis and Mathematical Models for the Investigation of Photothermal Inactivation of Candida albicans Using Carbon Nanotubes. Colloids Surf. B Biointerfaces 2019, 180, 393–400. [Google Scholar] [CrossRef]
- Cao, F.; Wei, C.; Ma, G.; Hou, L.; Zhang, R.; Mei, L.; Qin, Q. Synthesis of Photothermal Antimicrobial Cotton Gauze Using AuNPs as Photothermal Transduction Agents. RSC Adv. 2021, 11, 25976–25982. [Google Scholar] [CrossRef]
- Cuadrado, C.F.; Díaz-Barrios, A.; Campaña, K.O.; Romani, E.C.; Quiroz, F.; Nardecchia, S.; Debut, A.; Vizuete, K.; Niebieskikwiat, D.; Ávila, C.E.; et al. Broad-Spectrum Antimicrobial ZnMintPc Encapsulated in Magnetic-Nanocomposites with Graphene Oxide/MWCNTs Based on Bimodal Action of Photodynamic and Photothermal Effects. Pharmaceutics 2022, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Liu, L.; Feng, L. Photothermal-Responsive Conjugated Polymer Nanoparticles for the Rapid and Effective Killing of Bacteria. ACS Appl. Bio Mater. 2018, 1, 27–32. [Google Scholar] [CrossRef]
- Gong, N.; Maharjan, N.; Liu, H.; Li, H.; Feng, W.; Meng, T.L.; Cao, J.; Tan, C.K.I.; Wei, Y.; Xie, H.; et al. Laser-Induced in-Plane Curving of Ripples on Biomedical Stainless Steel and Their Relationship to Biological Functions. Mater. Technol. 2022, 37, 3089–3099. [Google Scholar] [CrossRef]
- Kim, H.M.; Cho, B.R. Small-Molecule Two-Photon Probes for Bioimaging Applications. Chem. Rev. 2015, 115, 5014–5055. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, Q.; Tian, D.; Yang, X.; Liu, S.; Sun, X.; Chen, Q.; Fang, B.; Liang, H.; Li, L.; et al. Vaginal Epithelial Cell Membrane-Based Phototherapeutic Decoy Confers a “Three-in-One” Strategy to Treat against Intravaginal Infection of Candida albicans. ACS Nano 2023, 17, 12160–12175. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Kamrani, O. Effectiveness of Antimicrobial Photodynamic Therapy with Indocyanine Green against the Standard and Fluconazole-Resistant Candida albicans. Lasers Med. Sci. 2021, 36, 1971–1977. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Hamblin, M.R.; Mohamed, T. The Effect of Femtosecond Laser Irradiation on the Growth Kinetics of Staphylococcus Aureus: An in Vitro Study. J. Photochem. Photobiol. B Biol. 2021, 221, 112240. [Google Scholar] [CrossRef]
- Nisah, K.; Ramli, M.; Iqhrammullah, M.; Mitaphonna, R.; Hartadi, B.S.; Abdulmadjid, S.N.; Md Sani, N.D.; Idroes, R.; Safitri, E. Controlling the Diffusion of Micro-Volume Pb Solution on Hydrophobic Polyurethane Membrane for Quantitative Analysis Using Laser-Induced Breakdown Spectroscopy (LIBS). Arab. J. Chem. 2022, 15, 103812. [Google Scholar] [CrossRef]
- Joshi, K.M.; Shelar, A.; Kasabe, U.; Nikam, L.K.; Pawar, R.A.; Sangshetti, J.; Kale, B.B.; Singh, A.V.; Patil, R.; Chaskar, M.G. Biofilm Inhibition in Candida albicans with Biogenic Hierarchical Zinc-Oxide Nanoparticles. Biomater. Adv. 2022, 134, 112592. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.Y.; Tomblinson, C.M.; Ocal, I.T.; Vikram, H.R.; Lott, D.G. Laryngeal Cryptococcosis: Literature Review and Guidelines for Laser Ablation of Fungal Lesions. Laryngoscope 2016, 126, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, R.; ter Haar, G.; Nightingale, K.; Marks, L.; Natarajan, S. Methods of Monitoring Thermal Ablation of Soft Tissue Tumors—A Comprehensive Review. Med. Phys. 2022, 49, 769–791. [Google Scholar] [CrossRef]
- Maliszewska, I.; Wanarska, E.; Tylus, W. Sulfonated Hydroxyaluminum Phthalocyanine-Biogenic Au/Ag Alloy Nanoparticles Mixtures for Effective Photo-Eradication of Candida albicans. Photodiagnosis Photodyn. Ther. 2020, 32, 102016. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, X.; Liu, S.; Hu, Y.; Ma, D. Near-Infrared Light-Triggered Nitric Oxide Release Combined with Low-Temperature Photothermal Therapy for Synergetic Antibacterial and Antifungal. Smart Mater. Med. 2021, 2, 302–313. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. The Effect of Laser Fluence for Enhancing the Antibacterial Activity of NiO Nanoparticles by Pulsed Laser Ablation in Liquid Media. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100382. [Google Scholar] [CrossRef]
- El-Gendy, A.O.; Nawaf, K.T.; Ahmed, E.; Samir, A.; Hamblin, M.R.; Hassan, M.; Mohamed, T. Preparation of Zinc Oxide Nanoparticles Using Laser-Ablation Technique: Retinal Epithelial Cell (ARPE-19) Biocompatibility and Antimicrobial Activity When Activated with Femtosecond Laser. J. Photochem. Photobiol. B 2022, 234, 112540. [Google Scholar] [CrossRef]
- Mescia, L.; Bia, P.; Caratelli, D. Fractional-Calculus-Based Electromagnetic Tool to Study Pulse Propagation in Arbitrary Dispersive Dielectrics. Phys. Status Solidi A 2019, 216, 1800557. [Google Scholar] [CrossRef]
- Vieira, L.C.; Costa, R.S.; Valério, D. An Overview of Mathematical Modelling in Cancer Research: Fractional Calculus as Modelling Tool. Fractal Fract. 2023, 7, 595. [Google Scholar] [CrossRef]
- Khan, M.; Rasheed, A.; Anwar, M.S.; Hussain, Z.; Shahzad, T. Modelling Charge Carrier Transport with Anomalous Diffusion and Heat Conduction in Amorphous Semiconductors Using Fractional Calculus. Phys. Scr. 2021, 96, 045204. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Nowicka, J.; Pajączkowska, M.; Matys, J.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Dobrzyński, M.; Dominiak, M. Effects of Nd:YAG Laser Irradiation on the Growth of Candida albicans and Streptococcus Mutans: In Vitro Study. Lasers Med. Sci. 2019, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Acosta, L.D.; Pérez-Camacho, O.; Acosta, R.; Escobar, D.M.; Gallardo, C.A.; Sánchez-Vargas, L.O. Reduction of Candida albicans Biofilm Formation by Coating Polymethyl Methacrylate Denture Bases with a Photopolymerized Film. J. Prosthet. Dent. 2020, 124, 605–613. [Google Scholar] [CrossRef]
- Yasui, H.; Takahashi, K.; Taki, S.; Shimizu, M.; Koike, C.; Umeda, K.; Rahman, S.; Akashi, T.; Nguyen, V.S.; Nakagawa, Y.; et al. Near Infrared Photo-Antimicrobial Targeting Therapy for Candida albicans. Adv. Ther. 2021, 4, 2000221. [Google Scholar] [CrossRef]
- Yook, K.-D.; kim, j.-w.; Song, Y.-K. In Vitro Study on the Effects of Photodynamic Inactivation Using Methyl Pheophorbide a, PhotoMed, PhotoCure, and 660 Nm Diode Laser on Candida albicans. Photodiagnosis Photodyn. Ther. 2022, 38, 102871. [Google Scholar] [CrossRef]
- Godoy, G.G.S.M.; de Andrade, V.M.; Dondeo, F.; Conceição, K.; Capella, A. Effect of Laser Thermochemical Treatment of Ti–6Al–4V Alloy on Candida albicans Biofilm Growth. Mater. Chem. Phys. 2023, 294, 127055. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.-H.; Sun, D.-W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Kang, K.; Platten, F. Electric-Field Induced Modulation of Amorphous Protein Aggregates: Polarization, Deformation, and Reorientation. Sci. Rep. 2022, 12, 3061. [Google Scholar] [CrossRef]
- Mahler, H.-C.; Friess, W.; Grauschopf, U.; Kiese, S. Protein Aggregation: Pathways, Induction Factors and Analysis. J. Pharm. Sci. 2009, 98, 2909–2934. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric Field Effects on Proteins—Novel Perspectives on Food and Potential Health Implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef]
- Das, S.; Jacob, R.S.; Patel, K.; Singh, N.; Maji, S.K. Amyloid Fibrils: Versatile Biomaterials for Cell Adhesion and Tissue Engineering Applications. Biomacromolecules 2018, 19, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Pulsed Electric Field Improved Protein Digestion of Beef during In-Vitro Gastrointestinal Simulation. LWT 2019, 102, 45–51. [Google Scholar] [CrossRef]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies Applied to Microalgal Biotechnology—Applications, Techniques and Future Trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef]
- Tang, J.; Du, N.; Doyle, P.S. Compression and Self-Entanglement of Single DNA Molecules under Uniform Electric Field. Proc. Natl. Acad. Sci. 2011, 108, 16153–16158. [Google Scholar] [CrossRef]
- Novianti, V.; Damayanti, L.; Adenan, A.; Malinda, Y. Effectiveness of Red Fruit (Pandanus conoideus Lam.) on Candida albicans (ATCC 10231) in the Field of Prosthodontics: An Experimental Study. J. Int. Oral Health 2020, 12, 260. [Google Scholar] [CrossRef]
- Ermenlieva, n. synergistic interaction between lamiaceae essential oils and antifungal drugs against Candida albicans ATCC 10231. Farmacia 2022, 70, 720–725. [Google Scholar] [CrossRef]
- Su, X.; Ren, R.; Wu, Y.; Li, S.; Ge, C.; Liu, L.; Xu, Y. Study of Biochip Integrated with Microelectrodes Modified by Poly-Dopamine-Co-Chitosan Composite Gel for Separation, Enrichment and Detection of Microbes in the Aerosol. Biosens. Bioelectron. 2021, 176, 112931. [Google Scholar] [CrossRef]
- Camarillo-Márquez, O.; Córdova-Alcántara, I.M.; Hernández-Rodríguez, C.H.; García-Pérez, B.E.; Martínez-Rivera, M.A.; Rodríguez-Tovar, A.V. Antagonistic Interaction of Staphylococcus Aureus Toward Candida Glabrata During in Vitro Biofilm Formation Is Caused by an Apoptotic Mechanism. Front. Microbiol. 2018, 9, 2031. [Google Scholar] [CrossRef]
- Hernandez-Jaimes, C.; Vazquez-Arenas, J.; Vernon-Carter, J.; Alvarez-Ramirez, J. A Nonlinear Cole–Cole Model for Large-Amplitude Electrochemical Impedance Spectroscopy. Chem. Eng. Sci. 2015, 137, 1–8. [Google Scholar] [CrossRef]
- Koch, C.; Bürgers, R.; Hahnel, S. Candida albicans Adherence and Proliferation on the Surface of Denture Base Materials. Gerodontology 2013, 30, 309–313. [Google Scholar] [CrossRef]
- Takada, T.; Fukuchi, M.; Nezu, T.; Nagano-Takebe, F.; Endo, K. Evaluation of Thermal Conductivity and Esthetic Quality of Denture Base Resin Composites with Acrylic Polymer and Nanodiamonds. J. Appl. Polym. Sci. 2021, 138, 51436. [Google Scholar] [CrossRef]
- Hernández-Acosta, M.A.; Soto-Ruvalcaba, L.; Martínez-González, C.L.; Trejo-Valdez, M.; Torres-Torres, C. Optical Phase-Change in Plasmonic Nanoparticles by a Two-Wave Mixing. Phys. Scr. 2019, 94, 125802. [Google Scholar] [CrossRef]
- Javed, A.; Khan, N.; Bashir, S.; Ahmad, M.; Bashir, M. Thickness Dependent Structural, Electrical and Optical Properties of Cubic SnS Thin Films. Mater. Chem. Phys. 2020, 246, 122831. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.-H.; Hagan, D.J.; Van Stryland, E.W. Sensitive Measurement of Optical Nonlinearities Using a Single Beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Ge, S.; Dupuy, L.X.; MacDonald, M.P. In Situ Laser Manipulation of Root Tissues in Transparent Soil. Plant Soil 2021, 468, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhuang, J.; Kolb, J.F. Discrimination of Different Cell Monolayers before and after Exposure to Nanosecond Pulsed Electric Fields Based on Cole–Cole and Multivariate Analysis. J. Phys. D Appl. Phys. 2019, 52, 495401. [Google Scholar] [CrossRef]
- Huang, F.; Song, Y.; Chen, W.; Liu, Q.; Wang, Q.; Liu, W.; Wang, X.; Wang, W. Effects of Candida albicans Infection on Defense Effector Secretion by Human Oral Mucosal Epithelial Cells. Arch. Oral Biol. 2019, 103, 55–61. [Google Scholar] [CrossRef]
- Kwasny, D.; Tehrani, S.; Almeida, C.; Schjødt, I.; Dimaki, M.; Svendsen, W. Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor. Sensors 2018, 18, 2214. [Google Scholar] [CrossRef]
- Olaifa, K.; Nikodinovic-Runic, J.; Glišić, B.; Boschetto, F.; Marin, E.; Segreto, F.; Marsili, E. Electroanalysis of Candida albicans Biofilms: A Suitable Real-Time Tool for Antifungal Testing. Electrochim. Acta 2021, 389, 138757. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y. Photodynamic Therapy for the Treatment of Fungal Infections. Infect. Drug Resist. 2022, 15, 3251–3266. [Google Scholar] [CrossRef]
- El-Gendy, A.O.; Obaid, Y.; Ahmed, E.; Enwemeka, C.S.; Hassan, M.; Mohamed, T. The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study. Nanomaterials 2022, 12, 3757. [Google Scholar] [CrossRef]
- Failla, G.; Zingales, M. Advanced Materials Modelling via Fractional Calculus: Challenges and Perspectives. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20200050. [Google Scholar] [CrossRef]
- Matias, G.S.; Lermen, F.H.; Matos, C.; Nicolin, D.J.; Fischer, C.; Rossoni, D.F.; Jorge, L.M.M. A Model of Distributed Parameters for Non-Fickian Diffusion in Grain Drying Based on the Fractional Calculus Approach. Biosyst. Eng. 2023, 226, 16–26. [Google Scholar] [CrossRef]
- Wang, X.; Qi, H.; Yang, X.; Xu, H. Analysis of the Time-Space Fractional Bioheat Transfer Equation for Biological Tissues during Laser Irradiation. Int. J. Heat Mass Transf. 2021, 177, 121555. [Google Scholar] [CrossRef]
- Martines-Arano, H.; Palacios-Barreto, S.; Castillo-Cruz, J.; Meda-Campaña, J.A.; García-Pérez, B.E.; Torres-Torres, C. Fractional Photodamage Triggered by Chaotic Attractors in Human Lung Epithelial Cancer Cells. Int. J. Therm. Sci. 2022, 181, 107734. [Google Scholar] [CrossRef]
- Ali, A.; Ovais, M.; Cui, X.; Rui, Y.; Chen, C. Safety Assessment of Nanomaterials for Antimicrobial Applications. Chem. Res. Toxicol. 2020, 33, 1082–1109. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans Biofilm by Pure Selenium Nanoparticles Synthesized by Pulsed Laser Ablation in Liquids. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Maione, A.; de Alteriis, E.; Carraturo, F.; Galdiero, S.; Falanga, A.; Guida, M.; Di Cosmo, A.; Maselli, V.; Galdiero, E. The Membranotropic Peptide GH625 to Combat Mixed Candida albicans/Klebsiella Pneumoniae Biofilm: Correlation between In Vitro Anti-Biofilm Activity and In Vivo Antimicrobial Protection. J. Fungi 2021, 7, 26. [Google Scholar] [CrossRef]
- Rahman, A.; Krause, B.; Hoang, T.B.; Guisbiers, G. Tailoring the Optical Properties of Selenium Nanoneedles by Pulsed Laser Ablation in Liquids: Implications for Solar Cells and Photocells. ACS Appl. Nano Mater. 2023, 6, 2258–2265. [Google Scholar] [CrossRef]
- Souza, S.O.; Raposo, B.L.; Sarmento-Neto, J.F.; Rebouças, J.S.; Macêdo, D.P.C.; Figueiredo, R.C.B.Q.; Santos, B.S.; Freitas, A.Z.; Cabral Filho, P.E.; Ribeiro, M.S.; et al. Photoinactivation of Yeast and Biofilm Communities of Candida albicans Mediated by ZnTnHex-2-PyP4+ Porphyrin. J. Fungi 2022, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Leonel, L.; Carvalho, M.L.; da Silva, B.M.; Zamuner, S.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT) Using Methylene Blue Inhibits the Viability of the Biofilm Produced by Candida albicans. Photodiagnosis Photodyn. Ther. 2019, 26, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Alves da Collina, G.; Freire, F.; da Silva Barbosa, V.; Bento Correa, C.; Reis Nascimento, H.; Ratto Tempestini Horliana, A.C.; Teixeira da Silva, D.d.F.; Araujo Prates, R.; Pavani, C. Photodynamic Antimicrobial Chemotherapy Action of Phenothiazinium Dyes in Planktonic Candida albicans Is Increased in Sodium Dodecyl Sulfate. Photodiagnosis Photodyn. Ther. 2020, 29, 101612. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Zhan, Y.; Jusuf, S.; Hui, J.; Dagher, Z.; Mansour, M.K.; Cheng, J. Photoinactivation of Catalase Sensitizes Candida albicans and Candida Auris to ROS-Producing Agents and Immune Cells. Adv. Sci. 2022, 9, 2104384. [Google Scholar] [CrossRef]
- Alkhtani, F. Efficacy of Chemical and Photoactivated Disinfectants against Candida albicans and Assessment of Hardness, Roughness, and Mass Loss of Acrylic Denture Base Resin. Photodiagnosis Photodyn. Ther. 2022, 39, 102911. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arano-Martinez, J.A.; Hernández-Benítez, J.A.; Martines-Arano, H.; Rodríguez-Tovar, A.V.; Trejo-Valdez, M.; García-Pérez, B.E.; Torres-Torres, C. Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms. Bioengineering 2024, 11, 333. https://doi.org/10.3390/bioengineering11040333
Arano-Martinez JA, Hernández-Benítez JA, Martines-Arano H, Rodríguez-Tovar AV, Trejo-Valdez M, García-Pérez BE, Torres-Torres C. Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms. Bioengineering. 2024; 11(4):333. https://doi.org/10.3390/bioengineering11040333
Chicago/Turabian StyleArano-Martinez, Jose Alberto, José Alejandro Hernández-Benítez, Hilario Martines-Arano, Aída Verónica Rodríguez-Tovar, Martin Trejo-Valdez, Blanca Estela García-Pérez, and Carlos Torres-Torres. 2024. "Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms" Bioengineering 11, no. 4: 333. https://doi.org/10.3390/bioengineering11040333
APA StyleArano-Martinez, J. A., Hernández-Benítez, J. A., Martines-Arano, H., Rodríguez-Tovar, A. V., Trejo-Valdez, M., García-Pérez, B. E., & Torres-Torres, C. (2024). Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms. Bioengineering, 11(4), 333. https://doi.org/10.3390/bioengineering11040333









