Optimized Decellularization of a Porcine Fasciocutaneaous Flap
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
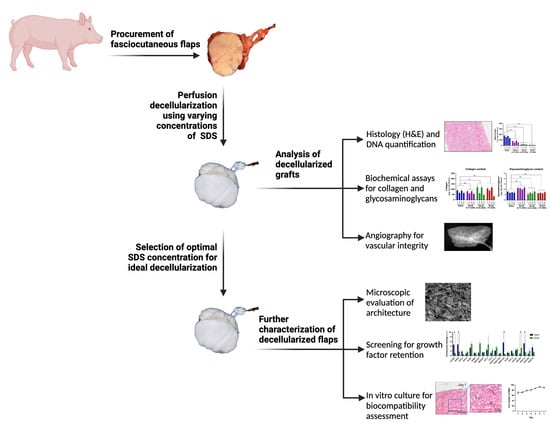
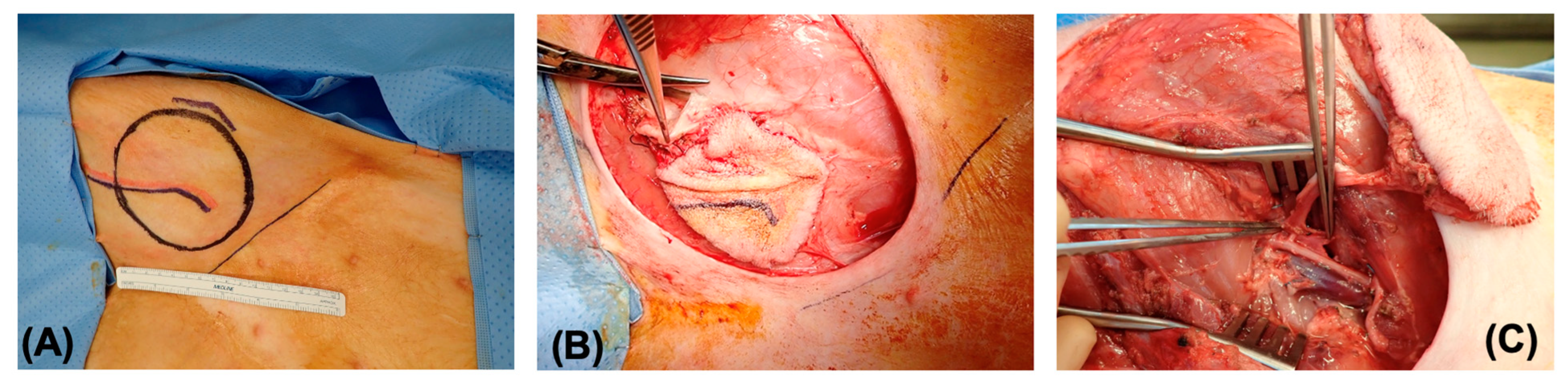
2.2. Procurement of Fasciocutaneous Flaps
2.3. Preparation of Decellularized Fasciocutaneous Flaps
2.4. Measurement of DNA, Collagen, Glycosaminoglycans and Growth Factors in Decellularized Flaps
2.5. Histological Analysis
2.6. Angiographic Imaging and Scanning Electron Microscopy
2.7. In Vitro Biocompatibility of Decellularized Flaps
2.8. Statistical Analysis
3. Results
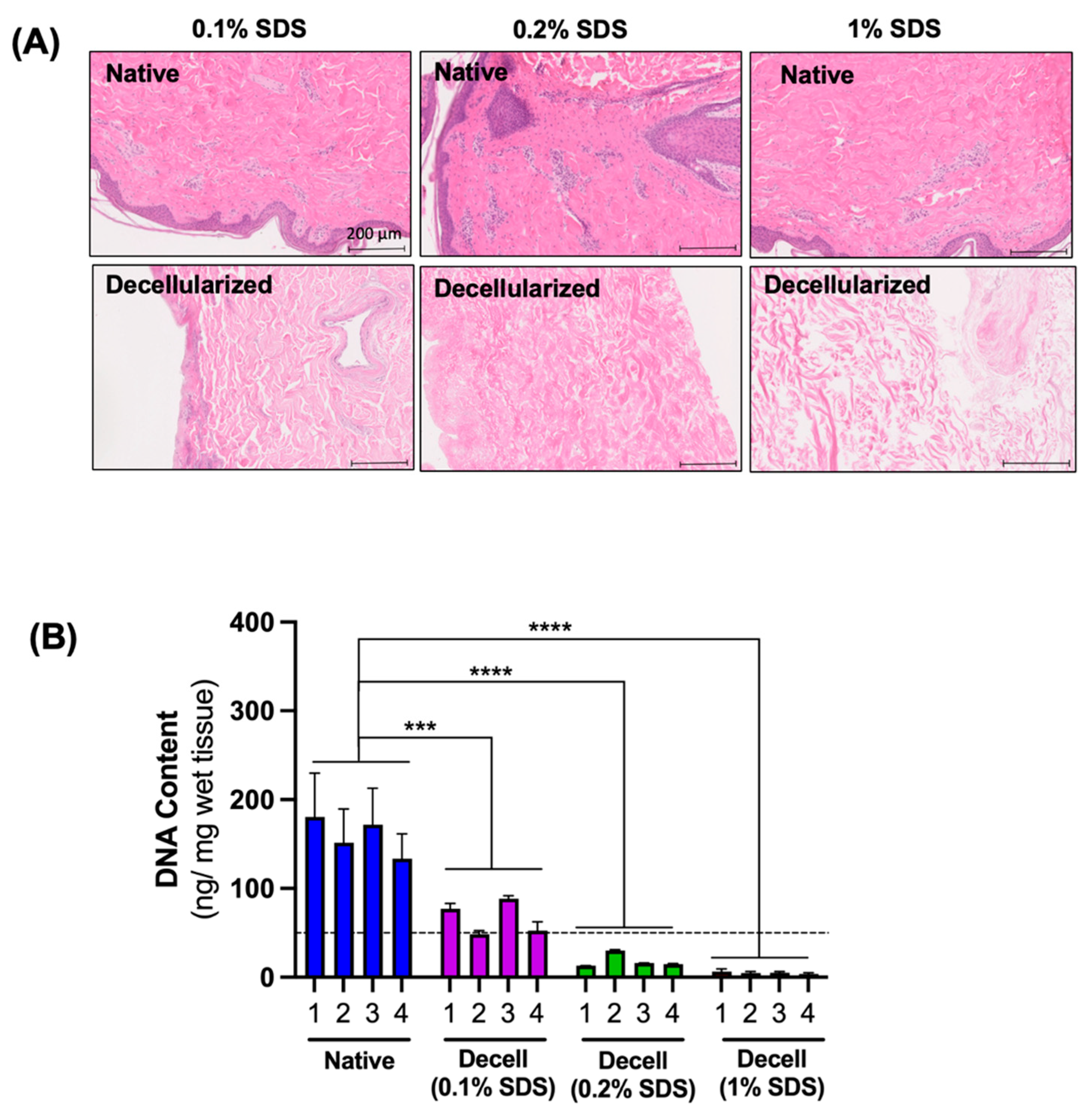
3.1. The Effect of SDS Concentration on the Efficiency of Cell Removal
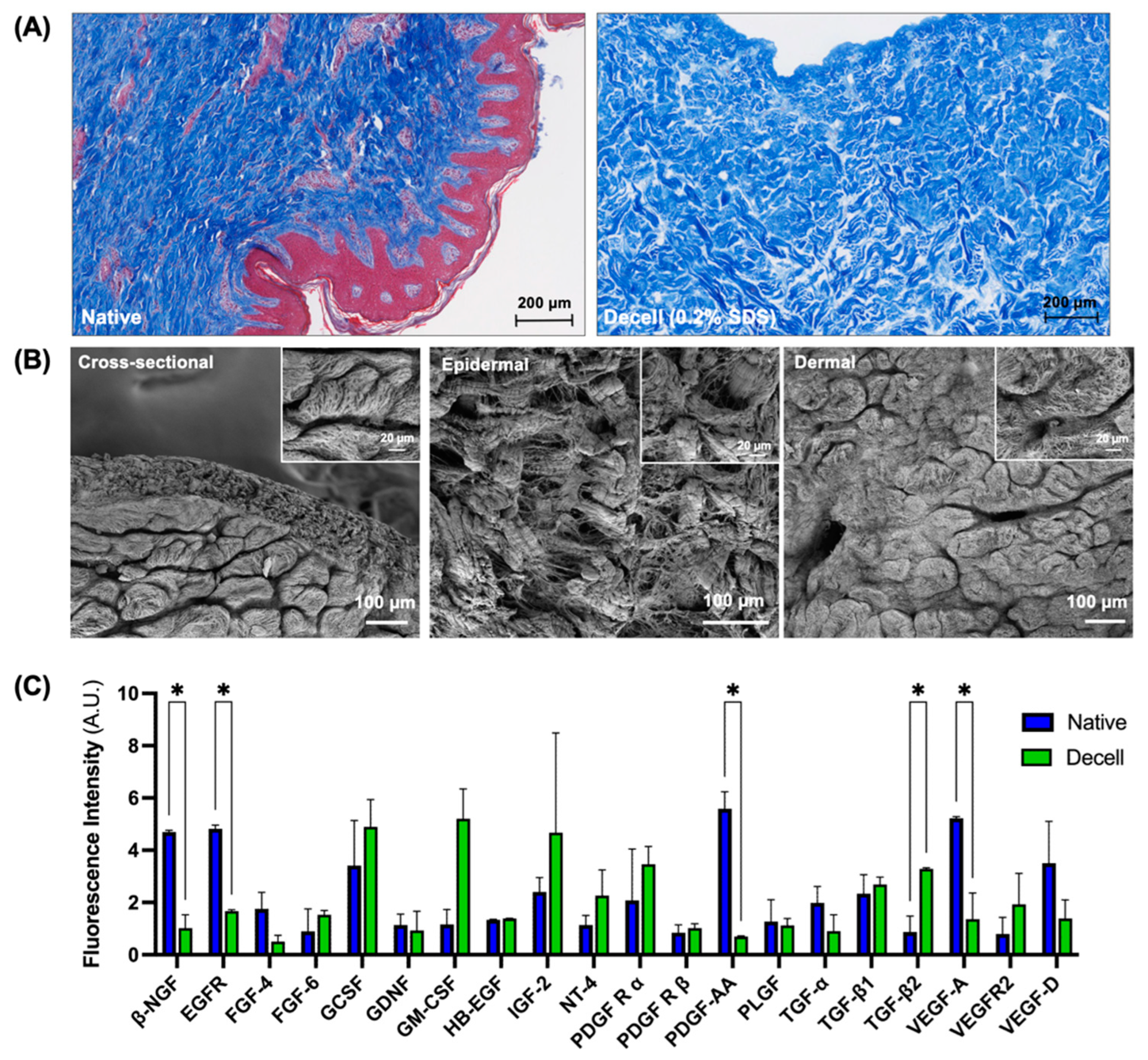
3.2. Preservation of Extracellular Matrix Components and Vascular Microarchitecture
3.3. Characterization of the Flaps Decellularized Using the Optimized Protocol
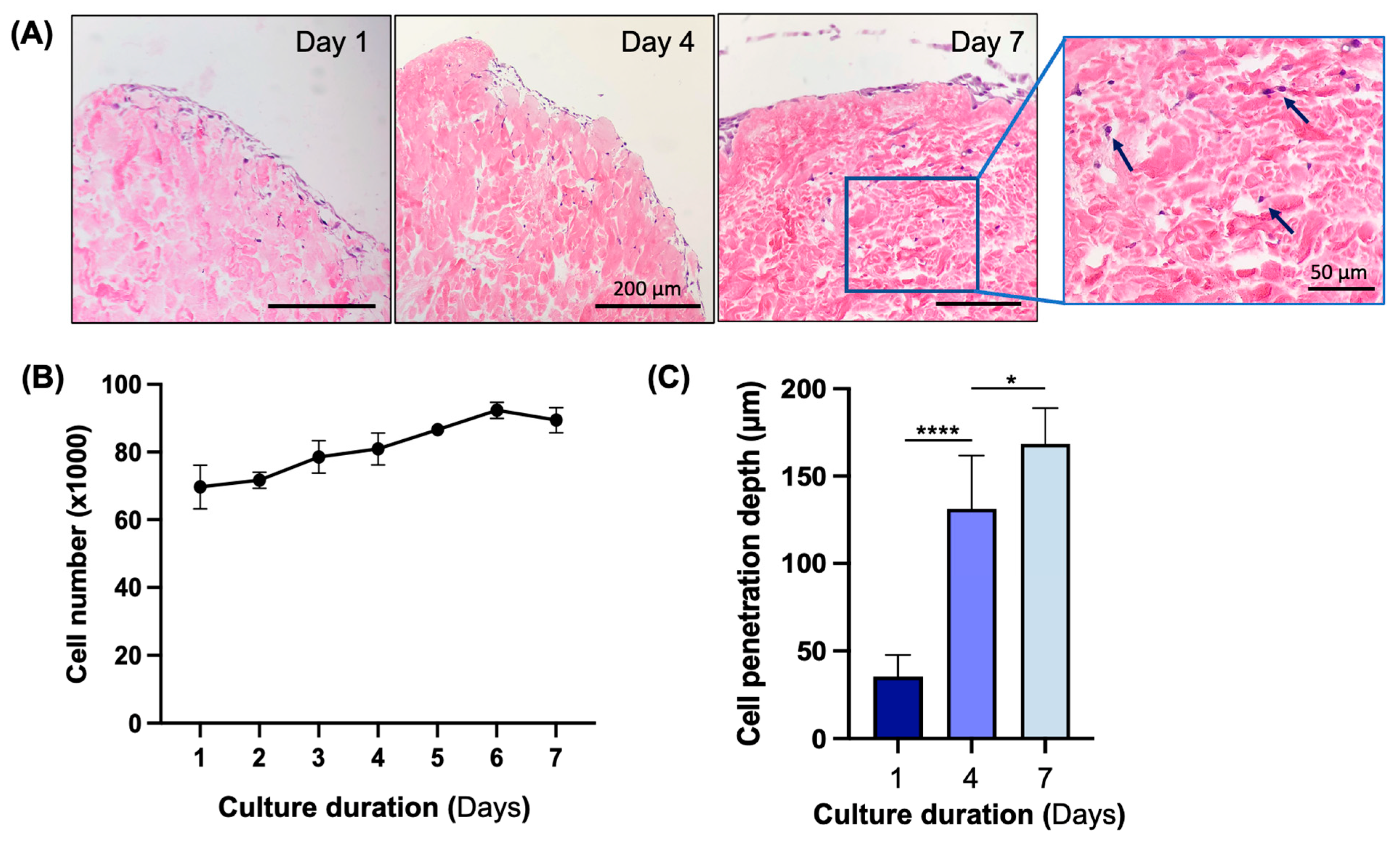
3.4. In Vitro Biocompatibility Assessment of Decellularized Flap
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semer, N.B.; Sullivan, S.R.; Meara, J.G. Plastic surgery and global health: How plastic surgery impacts the global burden of surgical disease. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1244–1248. [Google Scholar] [CrossRef]
- Desai, M.H.; Herndon, D.N.; Rutan, R.L.; Parker, J. An unusual donor site, a lifesaver in extensive burns. J. Burn. Care Rehabil. 1988, 9, 637–639. [Google Scholar] [CrossRef]
- Harris, B.N.; Bewley, A.F. Minimizing free flap donor-site morbidity. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 447–452. [Google Scholar] [CrossRef]
- Chang, E.I.; Chang, E.I.; Soto-Miranda, M.A.; Zhang, H.; Nosrati, N.; Robb, G.L.; Chang, D.W. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast. Reconstr. Surg. 2013, 132, 1383–1391. [Google Scholar] [CrossRef]
- Knott, P.D.; Seth, R.; Waters, H.H.; Revenaugh, P.C.; Alam, D.; Scharpf, J.; Meltzer, N.E.; Fritz, M.A. Short-term donor site morbidity: A comparison of the anterolateral thigh and radial forearm fasciocutaneous free flaps. Head Neck 2016, 38 (Suppl. S1), E945–E948. [Google Scholar] [CrossRef]
- Tessier, P.; Kawamoto, H.; Matthews, D.; Posnick, J.; Raulo, Y.; Tulasne, J.F.; Wolfe, S.A. Autogenous bone grafts and bone substitutes--tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast. Reconstr. Surg. 2005, 116, 6S–24S; discussion 92S–94S. [Google Scholar] [CrossRef]
- Ferri, J.; Piot, B.; Ruhin, B.; Mercier, J. Advantages and limitations of the fibula free flap in mandibular reconstruction. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1997, 55, 440–448; discussion 448–449. [Google Scholar] [CrossRef]
- Diaz-Siso, J.R.; Borab, Z.M.; Plana, N.M.; Parent, B.; Stranix, J.T.; Rodriguez, E.D. Vascularized Composite Allotransplantation: Alternatives and Catch-22s. Plast. Reconstr. Surg. 2018, 142, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. [Google Scholar] [CrossRef]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Livesey, S.A.; Herndon, D.N.; Hollyoak, M.A.; Atkinson, Y.H.; Nag, A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995, 60, 1–9. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Guyette, J.P.; Gilpin, S.E.; Charest, J.M.; Tapias, L.F.; Ren, X.; Ott, H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014, 9, 1451–1468. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Lupon, E.; Lellouch, A.G.; Acun, A.; Andrews, A.R.; Oganesyan, R.; Goutard, M.; Taveau, C.B.; Lantieri, L.A.; Cetrulo, C.L.; Uygun, B.E. Engineering Vascularized Composite Allografts Using Natural Scaffolds: A Systematic Review. Tissue Eng. Part B Rev. 2021, 28, 677–693. [Google Scholar] [CrossRef]
- Adil, A.; Xu, M.; Haykal, S. Recellularization of Bioengineered Scaffolds for Vascular Composite Allotransplantation. Front. Surg. 2022, 9, 843677. [Google Scholar] [CrossRef]
- Henderson, P.W.; Nagineni, V.V.; Harper, A.; Bavinck, N.; Sohn, A.M.; Krijgh, D.D.; Jimenez, N.; Weinstein, A.L.; Spector, J.A. Development of an acellular bioengineered matrix with a dominant vascular pedicle. J. Surg. Res. 2010, 164, 1–5. [Google Scholar] [CrossRef]
- Qu, J.; Van Hogezand, R.M.; Zhao, C.; Kuo, B.J.; Carlsen, B.T. Decellularization of a Fasciocutaneous Flap for Use as a Perfusable Scaffold. Ann. Plast. Surg. 2015, 75, 112–116. [Google Scholar] [CrossRef]
- Bengur, F.B.; Chen, L.; Schilling, B.K.; Komatsu, C.; Figlioli, G.M.; Marra, K.G.; Kokai, L.E.; Solari, M.G. Automated Decellularization of the Rodent Epigastric Free Flap: A Comparison of Sodium Dodecyl Sulfate-Based Protocols. J. Reconstr. Microsurg. 2023, 39, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Adil, A.; Karoubi, G.; Haykal, S. Procurement and Decellularization of Rat Hindlimbs using an Ex Vivo Perfusion-based Bioreactor for Vascularized Composite Allotransplantation. J. Vis. Exp. 2022, 9, 184. [Google Scholar] [CrossRef]
- Jank, B.J.; Goverman, J.; Guyette, J.P.; Charest, J.M.; Randolph, M.; Gaudette, G.R.; Gershlak, J.R.; Purschke, M.; Javorsky, E.; Nazarian, R.M.; et al. Creation of a Bioengineered Skin Flap Scaffold with a Perfusable Vascular Pedicle. Tissue Eng. Part A 2017, 23, 696–707. [Google Scholar] [CrossRef]
- Xu, M.S.; Karoubi, G.; Waddell, T.K.; Haykal, S. Procurement and Perfusion-Decellularization of Porcine Vascularized Flaps in a Customized Perfusion Bioreactor. J. Vis. Exp. 2022, 1, 186. [Google Scholar] [CrossRef]
- Pozzo, V.; Romano, G.; Goutard, M.; Lupon, E.; Tawa, P.; Acun, A.; Andrews, A.R.; Taveau, C.B.; Uygun, B.E.; Randolph, M.A.; et al. A Reliable Porcine Fascio-Cutaneous Flap Model for Vascularized Composite Allografts Bioengineering Studies. J. Vis. Exp. 2022, 31, 181. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Acun, A.; Oganesyan, R.; Uygun, K.; Yeh, H.; Yarmush, M.L.; Uygun, B.E. Liver donor age affects hepatocyte function through age-dependent changes in decellularized liver matrix. Biomaterials 2021, 270, 120689. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Gilbert, T.W. Strategies for tissue and organ decellularization. J. Cell. Biochem. 2012, 113, 2217–2222. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, V.; Acun, A.; Lupon, E.; Lellouch, A.G.; Oganesyan, R.; Andrews, A.R.; Lantieri, L.; Randolph, M.A.; Cetrulo, C.L.; Uygun, B.E. Reendothelialization of decellularized swine fasciocutaneous flap: A proof-of-concept study. In Proceedings of the IXA-CTRMS, Virtual, 23–25 September 2021. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupon, E.; Acun, A.; Taveau, C.B.; Oganesyan, R.; Lancia, H.H.; Andrews, A.R.; Randolph, M.A.; Cetrulo, C.L., Jr.; Lellouch, A.G.; Uygun, B.E. Optimized Decellularization of a Porcine Fasciocutaneaous Flap. Bioengineering 2024, 11, 321. https://doi.org/10.3390/bioengineering11040321
Lupon E, Acun A, Taveau CB, Oganesyan R, Lancia HH, Andrews AR, Randolph MA, Cetrulo CL Jr., Lellouch AG, Uygun BE. Optimized Decellularization of a Porcine Fasciocutaneaous Flap. Bioengineering. 2024; 11(4):321. https://doi.org/10.3390/bioengineering11040321
Chicago/Turabian StyleLupon, Elise, Aylin Acun, Corentin B. Taveau, Ruben Oganesyan, Hyshem H. Lancia, Alec R. Andrews, Mark A. Randolph, Curtis L. Cetrulo, Jr., Alexandre G. Lellouch, and Basak E. Uygun. 2024. "Optimized Decellularization of a Porcine Fasciocutaneaous Flap" Bioengineering 11, no. 4: 321. https://doi.org/10.3390/bioengineering11040321
APA StyleLupon, E., Acun, A., Taveau, C. B., Oganesyan, R., Lancia, H. H., Andrews, A. R., Randolph, M. A., Cetrulo, C. L., Jr., Lellouch, A. G., & Uygun, B. E. (2024). Optimized Decellularization of a Porcine Fasciocutaneaous Flap. Bioengineering, 11(4), 321. https://doi.org/10.3390/bioengineering11040321