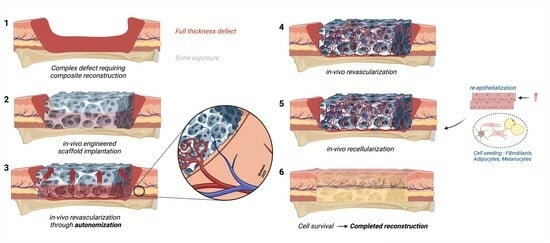
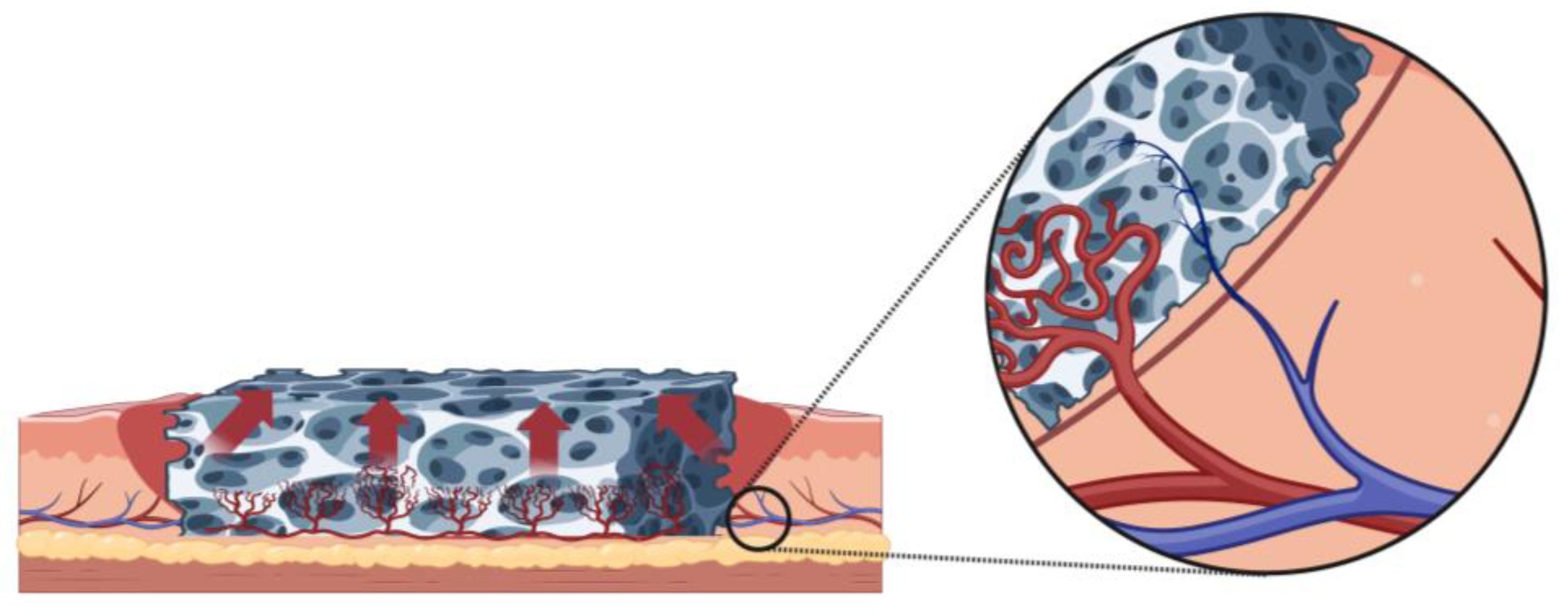
The Autonomization Principle in Vascularized Flaps: An Alternative Strategy for Composite Tissue Scaffold In Vivo Revascularization
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Search strategy
- (b)
- Exclusion criteria
- (i)
- Excluded during title/abstract screening: studies lacking original data; studies with non-human subjects; studies in any language other than English or French; unavailable full manuscripts.
- (ii)
- Excluded during full-text analysis: articles describing flap failure without describing the vascular compromise; articles describing flap survival after surgical revision of the anastomosis; external intervention prior to pedicle division, or flap survival following a delayed vascular compromise later than 2 weeks for head, neck and hand] and 3 weeks for all other sites.
- (c)
- Data extraction
- (d)
- Statistical analysis
- (e)
- Bias assessment
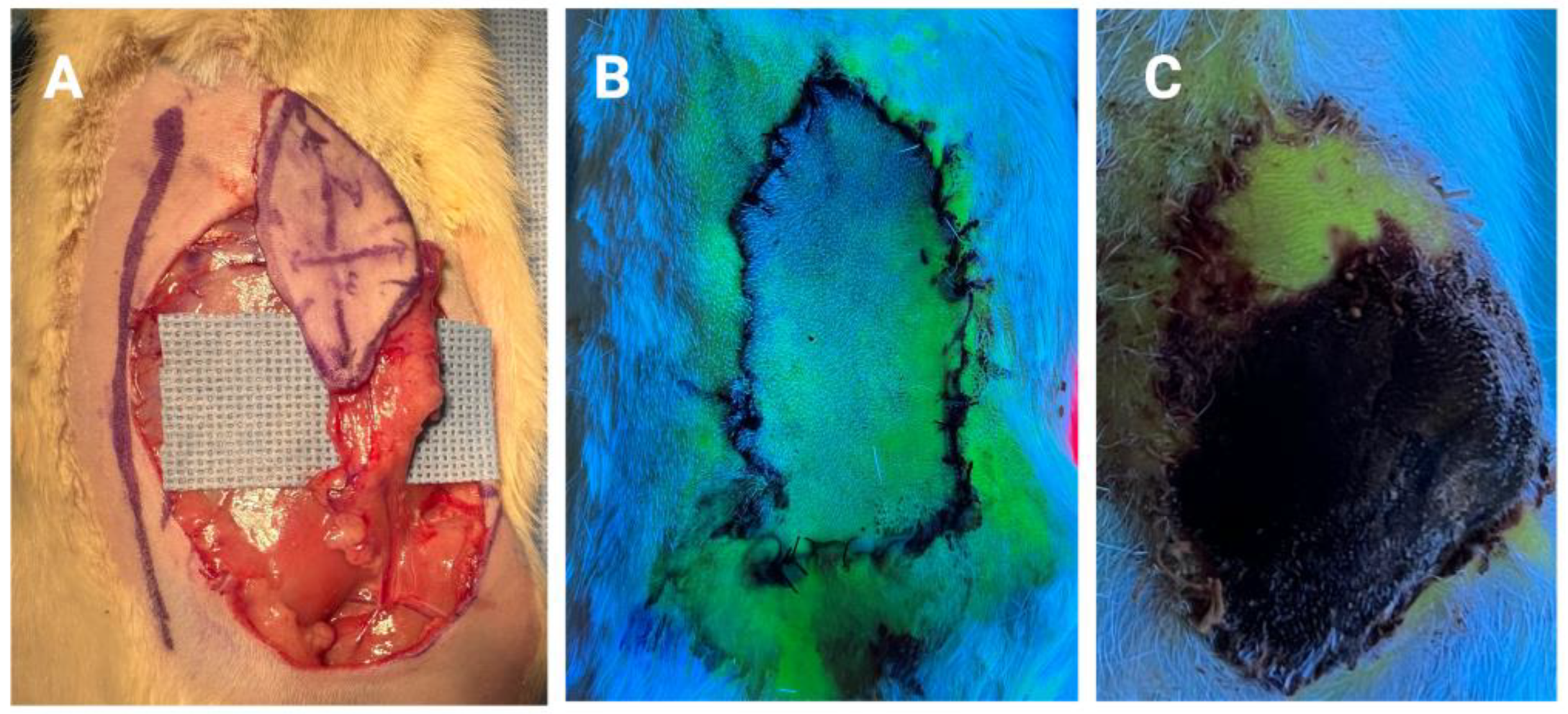
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Concern | Rationale for Concern | |
|---|---|---|
| 1. Concerns regarding specification of study eligibility criteria | Study size was not restriced. | Studies with small sample sizes were included due to limited data availability. |
| 2. Concerns regarding methods used to identify and/or select studies | Eligibility criteria may have excluded pertinent papers in other languages. | Papers not in English or French were excluded. |
| 3. Concerns regarding methods used to collect data and appraise studies | Not all study characteristics were available for review. | Some papers did not include potentially relevant information. |
| 4. Concerns regarding the synthesis and findings | Synthesis may not have included all the studies it should have. | Some relevant studies may not have been found during the search process. |
| RISK OF BIAS IN THE REVIEW | ||
| Describe whether conclusions were supported by the evidence: the conclusions were supported by the evidence gathered. | ||
| A. Did the interpretation of findings address all of the concerns identified in Domains 1 to 4? (Y)/PY/PN/N/NI B. Was the relevance of identified studies to the review’s research question appropriately considered? (Y)/PY/PN/N/NI C. Did the reviewers avoid emphasizing results on the basis of their statistical significance? (Y)/PY/PN/N/NI | ||
| Risk of bias in the review RISK: (LOW)/HIGH/UNCLEAR Rationale for risk: Bias is unavoidable, but foreseeable risks of bias were mitigated. | ||
References
- Chan, J.K.; Harry, L.; Williams, G.; Nanchahal, J. Soft-tissue reconstruction of open fractures of the lower limb: Muscle versus fasciocutaneous flaps. Plast. Reconstr. Surg. 2012, 130, 284e–295e. [Google Scholar] [CrossRef]
- Fox, C.M.; Beem, H.M.; Wiper, J.; Rozen, W.M.; Wagels, M.; Leong, J.C. Muscle versus fasciocutaneous free flaps in heel reconstruction: Systematic review and meta-analysis. J. Reconstr. Microsurg. 2015, 31, 59–66. [Google Scholar] [CrossRef]
- Kovar, A.; Colakoglu, S.; Iorio, M.L. A Systematic Review of Muscle and Fasciocutaneous Flaps in the Treatment of Extremity Osteomyelitis: Evidence for Fasciocutaneous Flap Use. Plast. Reconstr. Surg. Glob. Open 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Ludolph, I.; Cai, A.; Arkudas, A.; Lang, W.; Rother, U.; Horch, R.E. Indocyanine green angiography and the old question of vascular autonomy—Long term changes of microcirculation in microsurgically transplanted free flaps. Clin. Hemorheol. Microcirc. 2019, 72, 421–430. [Google Scholar] [CrossRef]
- Shaye, D.A. The history of nasal reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 259–264. [Google Scholar] [CrossRef]
- Abdurehim, Y.; Yasin, Y.; JX, K.T.; Wu, P.A.; Liang, X.N.; Xukurhan, A.; Yong, J.; Alim, N.; Kuyax, P.; Mirzak, M.; et al. Application of three-staged paramedian forehead flap in reconstruction and repair of full-thickness nasal defect. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021, 56, 374–380. [Google Scholar] [CrossRef]
- Millard, D.R., Jr. Forehead Flap in Immediate Repair of Head, Face and Jaw. Am. J. Surg. 1964, 108, 508–513. [Google Scholar] [CrossRef]
- Piers, J.H. Reconstruction of nose by means of forehead flap. Ned. Tijdschr. Geneeskd. 1953, 97, 2518–2523. [Google Scholar]
- Benkler, M.; Pressler, M.P.; Hallac, R.R.; Patel, S.; Seaward, J.R.; Kane, A.A. Early Pediatric Nasal Reconstruction Utilizing the Tagliacozzi Flap. J. Craniofac. Surg. 2019, 30, 2073–2075. [Google Scholar] [CrossRef]
- Wilson, D.E.; Maves, M.D. Tagliacotian cross-arm flap reconstruction of facial defects. Otolaryngol. Head Neck Surg. 1986, 94, 219–223. [Google Scholar] [CrossRef]
- Greig, A.; Gohritz, A.; Geishauser, M.; Muhlbauer, W. Heinrich von Pfalzpaint, Pioneer of Arm Flap Nasal Reconstruction in 1460, More Than a Century Before Tagliacozzi. J. Craniofac. Surg. 2015, 26, 1165–1168. [Google Scholar] [CrossRef]
- Tallent, A. Closure of a pharyngostoma with a bipedicled flap of scalp in the Leon Dufourmentel chin-strap. Ann. Chir. Plast. 1963, 8, 123–127. [Google Scholar]
- Burget, G.C.; Menick, F.J. Aesthetic restoration of one-half the upper lip. Plast. Reconstr. Surg. 1986, 78, 583–593. [Google Scholar] [CrossRef]
- McGregor, I.A.; Jackson, I.T. The groin flap. Br. J. Plast. Surg. 1972, 25, 3–16. [Google Scholar] [CrossRef]
- Jayes, P.H. Cross-leg flaps; a review of 60 cases. Br. J. Plast. Surg. 1950, 3, 1–5. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Srinivasan, K.; Ghildiyal, H.; Singh, M.; Jain, A.; Kapadia, T.; Tambotra, A. Review of Cross-Leg Flaps in Reconstruction of Posttraumatic Lower Extremity Wounds in a Microsurgical Unit. Indian J. Plast. Surg. 2019, 52, 117–124. [Google Scholar] [CrossRef]
- Chen, H.; El-Gammal, T.A.; Wei, F.; Chen, H.; Noordhoff, M.S.; Tang, Y. Cross-leg free flaps for difficult cases of leg defects: Indications, pitfalls, and long-term results. J. Trauma 1997, 43, 486–491. [Google Scholar] [CrossRef]
- Manrique, O.J.; Bishop, S.N.; Ciudad, P.; Adabi, K.; Martinez-Jorge, J.; Moran, S.L.; Huang, T.; Vijayasekaran, A.; Chen, S.H.; Chen, H.C. Lower Extremity Limb Salvage with Cross Leg Pedicle Flap, Cross Leg Free Flap, and Cross Leg Vascular Cable Bridge Flap. J. Reconstr. Microsurg. 2018, 34, 522–529. [Google Scholar] [CrossRef]
- Wolff, K.D.; Mucke, T.; Lehmbrock, J.; Loeffelbein, D.J.; Kesting, M.R.; Holzle, F. Rapid autonomisation of a combined fibular- and anterolateral thigh flap transferred by a wrist carrier to an irradiated and vessel depleted neck. J. Surg. Oncol. 2009, 99, 123–126. [Google Scholar] [CrossRef]
- Menick, F.J. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast. Reconstr. Surg. 2002, 109, 1839–1855; discussion 1856–1861. [Google Scholar] [CrossRef]
- Burget, G.C.; Walton, R.L. Optimal use of microvascular free flaps, cartilage grafts, and a paramedian forehead flap for aesthetic reconstruction of the nose and adjacent facial units. Plast. Reconstr. Surg. 2007, 120, 1171–1207. [Google Scholar] [CrossRef]
- Lam, H.Y.; Sulaiman, W.A.W.; Ismail, W.F.W.; Halim, A.S. Cross-Leg Free Flap: Crossing the Border Zone of Ischemic Limb-A Case Report of Limb Salvage Procedure following a Delayed Diagnosis of Popliteal Artery Injury. Arch. Plast. Surg. 2023, 50, 188–193. [Google Scholar] [CrossRef]
- Jacobson, A.S.; Eloy, J.A.; Park, E.; Roman, B.; Genden, E.M. Vessel-depleted neck: Techniques for achieving microvascular reconstruction. Head Neck 2008, 30, 201–207. [Google Scholar] [CrossRef]
- Duisit, J.; Maistriaux, L.; Taddeo, A.; Orlando, G.; Joris, V.; Coche, E.; Behets, C.; Lerut, J.; Dessy, C.; Cossu, G.; et al. Bioengineering a Human Face Graft: The Matrix of Identity. Ann. Surg. 2017, 266, 754–764. [Google Scholar] [CrossRef]
- Duisit, J.; Orlando, G.; Debluts, D.; Maistriaux, L.; Xhema, D.; de Bisthoven, Y.J.; Galli, C.; Peloso, A.; Behets, C.; Lengelé, B.; et al. Decellularization of the Porcine Ear Generates a Biocompatible, Nonimmunogenic Extracellular Matrix Platform for Face Subunit Bioengineering. Ann. Surg. 2018, 267, 1191–1201. [Google Scholar] [CrossRef]
- Oganesyan, R.V.; Lellouch, A.G.; Acun, A.; Lupon, E.; Taveau, C.B.; Burlage, L.C.; Lantieri, L.A.; Randolph, M.A.; Cetrulo, C.L.; Uygun, B.E. Acellular Nipple Scaffold Development, Characterization, and Preliminary Biocompatibility Assessment in a Swine Model. Plast. Reconstr. Surg. 2022, 151, 618e–629e. [Google Scholar] [CrossRef]
- Lupon, E.; Lellouch, A.G.; Acun, A.; Andrews, A.R.; Oganesyan, R.; Goutard, M.; Taveau, C.B.; Lantieri, L.A.; Cetrulo, C.L.; Uygun, B.E. Engineering Vascularized Composite Allografts Using Natural Scaffolds: A Systematic Review. Tissue Eng. Part B Rev. 2022, 28, 677–693. [Google Scholar] [CrossRef]
- Pozzo, V.; Romano, G.; Goutard, M.; Lupon, E.; Tawa, P.; Acun, A.; Andrews, A.R.; Taveau, C.B.; Uygun, B.E.; Randolph, M.A.; et al. A Reliable Porcine Fascio-Cutaneous Flap Model for Vascularized Composite Allografts Bioengineering Studies. JoVE J. Vis. Exp. 2022, 181, e63557. [Google Scholar] [CrossRef]
- Scarano, A.; Barros, R.R.; Iezzi, G.; Piattelli, A.; Novaes, A.B., Jr. Acellular dermal matrix graft for gingival augmentation: A preliminary clinical, histologic, and ultrastructural evaluation. J. Periodontol. 2009, 80, 253–259. [Google Scholar] [CrossRef]
- Taufique, Z.M.; Bhatt, N.; Zagzag, D.; Lebowitz, R.A.; Lieberman, S.M. Revascularization of AlloDerm Used during Endoscopic Skull Base Surgery. J. Neurol. Surg. B Skull Base. 2019, 80, 46–50. [Google Scholar] [CrossRef]
- Capito, A.E.; Tholpady, S.S.; Agrawal, H.; Drake, D.B.; Katz, A.J. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann. Plast. Surg. 2012, 68, 495–500. [Google Scholar] [CrossRef]
- Menon, N.G.; Rodriguez, E.D.; Byrnes, C.K.; Girotto, J.A.; Goldberg, N.H.; Silverman, R.P. Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in the rabbit model. Ann. Plast. Surg. 2003, 50, 523–527. [Google Scholar] [CrossRef]
- Gerli, M.F.M.; Guyette, J.P.; Evangelista-Leite, D.; Ghoshhajra, B.B.; Ott, H.C. Perfusion decellularization of a human limb: A novel platform for composite tissue engineering and reconstructive surgery. PLoS ONE 2018, 13, e0191497. [Google Scholar] [CrossRef]
- Adil, A.; Xu, M.; Haykal, S. Recellularization of Bioengineered Scaffolds for Vascular Composite Allotransplantation. Front. Surg. 2022, 9, 843677. [Google Scholar] [CrossRef]
- Ahmed, E.; Saleh, T.; Xu, M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells 2021, 10, 1787. [Google Scholar] [CrossRef]
- Acun, A.; Oganesyan, R.; Jaramillo, M.; Yarmush, M.L.; Uygun, B.E. Human-Origin iPSC-Based Recellularization of Decellularized Whole Rat Livers. Bioengineering 2022, 9, 219. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Calloway, H.E.; Moubayed, S.P.; Most, S.P. Cost-effectiveness of Early Division of the Forehead Flap Pedicle. JAMA Facial Plast. Surg. 2017, 19, 418–420. [Google Scholar] [CrossRef]
- Abdelmegeed, A.G.; Abulezz, T.A.; Abo-Saeda, M.A.; Allam, K.A. Perforator-Based Pedicled Cross-Leg Flaps in Pediatric Patients: A New Idea to Increase Flap Reach. Ann. Plast. Surg. 2021, 86, 568–572. [Google Scholar] [CrossRef]
- Rubio-Gallegos, F.; Núñez-González, S.; Gault, C.; Simancas-Racines, D.; Basantes-García, E. McGregor inguinal flap for coverage of large soft tissue losses due to high-voltage electrical burns in the upper limb: A retrospective study. Int. J. Burns Trauma 2019, 9, 52–58. [Google Scholar]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Berkane, Y.; Alana Shamlou, A.; Reyes, J.; Lancia, H.H.; Filz von Reiterdank, I.; Bertheuil, N.; Uygun, B.E.; Uygun, K.; Austen, W.G., Jr.; Cetrulo, C.L., Jr.; et al. The Superficial Inferior Epigastric Artery Axial Flap to Study Ischemic Preconditioning Effects in a Rat Model. J. Vis. Exp. 2023, e64980. [Google Scholar] [CrossRef]
- Semashko, D.; Song, Y.; Silverman, D.G.; Weinberg, H. Ischemic induction of neovascularization: A study by fluorometric analysis. Microsurgery 1985, 6, 244–248. [Google Scholar] [CrossRef]
- Akcal, A.; Sirvan, S.S.; Karsidag, S.; Görgülü, T.; Akcal, M.A.; Ozagari, A.; Tatlidede, S. Combination of ischemic preconditioning and postconditioning can minimise skin flap loss: Experimental study. J. Plast. Surg. Hand Surg. 2016, 50, 233–238. [Google Scholar] [CrossRef]
- Ulker, P.; Ozkan, O.; Amoroso, M.; Aslan, M.; Bassorgun, I.; Ubur, M.C.; Ünal, K.; Ozcan, F.; Ozkan, O. Does ischemic preconditioning increase flap survival by ADORA2B receptor activation? Clin. Hemorheol. Microcirc. 2020, 75, 151–162. [Google Scholar] [CrossRef]
- Mucke, T.; Borgmann, A.; Wagenpfeil, S.; Gunzinger, R.; Nobauer, C.; Lange, R.; Slotta-Huspenina, J.; Holzle, F.; Wolff, K.D. Autonomization of epigastric flaps in rats. Microsurgery 2011, 31, 472–478. [Google Scholar] [CrossRef]
- McKnight, C.D.; Winn, S.R.; Gong, X.; Hansen, J.E.; Wax, M.K. Revascularization of rat fasciocutaneous flap using CROSSEAL with VEGF protein or plasmid DNA expressing VEGF. Otolaryngol. Head Neck Surg. 2008, 139, 245–249. [Google Scholar] [CrossRef]
- Angelos, P.C.; Winn, S.R.; Kaurin, D.S.; Holland, J.; Wax, M.K. Evaluating revascularization and flap survival using vascular endothelial growth factor in an irradiated rat model. Arch. Facial Plast. Surg. 2011, 13, 185–189. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.; Riviere, J. The pig as a model for human skin research. In Swine in Biomedical Research: Update on Animal Models; Sage: Washington, DC, USA, 2005; pp. 17–22. [Google Scholar]
- Tsur, H.; Daniller, A.; Strauch, B. Neovascularization of skin flaps: Route and timing. Plast. Reconstr. Surg. 1980, 66, 85–90. [Google Scholar] [CrossRef]
- Vergote, T.; Arnaud, E. Forum on tissue expansion. Neovascularization of skin flap from expanded anatomical arteriovenous pedicle. An initial experimental study. Ann. Chir. Plast. Esthet. 1993, 38, 69–74. [Google Scholar]
- Young, C.M. The revascularization of pedicle skin flaps in pigs: A functional and morphologic study. Plast. Reconstr. Surg. 1982, 70, 455–464. [Google Scholar] [CrossRef]
- Park, S.S.; Rodeheaver, G.T.; Levine, P.A. Role of ischemic gradient in neovascularization of interpolated skin flaps. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 886–889. [Google Scholar] [CrossRef]
- Klöppel, M.; Nguyen, T.H.; Graf, P.; Laubenbacher, C.; Höhnke, C.; Schwaiger, M.; Biemer, E. Neovascularization of pre-formed tissue flaps in relation to arteriovenous blood flow of the implanted vascular pedicle. Experimental study in the rabbit. Langenbecks Arch. Chir. Suppl. Kongressbd 1997, 114, 1379–1380. [Google Scholar]
- Hoang, N.T.; Kloeppel, M.; Werner, J.; Staudenmaier, R.; Biemer, E. Proposed new method for angiographically quantifying neovascularization in prefabricated flaps. Microsurgery 2005, 25, 220–226. [Google Scholar] [CrossRef]
- Huang, S.R.; Li, X.Y.; Liu, L.F.; Su, J.R.; He, J.K. Experimental study and clinical application of early pedicle division of skin flap by ligation. Zhonghua Wai Ke Za Zhi 2006, 44, 762–764. [Google Scholar]
- Hom, D.B.; Baker, S.R.; Graham, L.M.; McClatchey, K.D. Utilizing angiogenic agents to expedite the neovascularization process in skin flaps. Laryngoscope 1988, 98, 521–526. [Google Scholar] [CrossRef]
- Stepnick, D.W.; Peterson, M.K.; Bodgan, C.; Davis, J.; Wasman, J.; Mailer, K. Effects of tumor necrosis factor alpha and vascular permeability factor on neovascularization of the rabbit ear flap. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 667–672. [Google Scholar] [CrossRef]
- Xu, N.; Guo, S.; Wang, Y.; Sun, Q.; Wang, C. Transplantation of adipose tissue-derived stromal cells promotes the survival of venous-congested skin flaps in rabbit ear. Cell Biochem. Biophys. 2015, 71, 557–563. [Google Scholar] [CrossRef]
- Xie, K.; Huang, M.; Zheng, Y.; Chen, D.; Hu, J.; Zheng, J. Effect of Antilogous Platelet-Rich Plasma on the Revascularization of Rabbit Prefabricated Flap. Med. Sci. Monit. 2022, 28, e937718. [Google Scholar] [CrossRef]
- Hallock, G.G. The complete classification of flaps. Microsurgery 2004, 24, 157–161. [Google Scholar] [CrossRef]
- Hallock, G.G. Preliminary assessment of laser Doppler flowmetry for determining timing of division of the cross-finger flap. J. Hand Surg. Am. 1990, 15, 898–901. [Google Scholar] [CrossRef]
- McGrath, M.H.; Adelberg, D.; Finseth, F. The intravenous fluorescein test: Use in timing of groin flap division. J. Hand Surg. Am. 1979, 4, 19–22. [Google Scholar] [CrossRef]
- Gatti, J.E.; LaRossa, D.; Brousseau, D.A.; Silverman, D.G. Assessment of neovascularization and timing of flap division. Plast. Reconstr. Surg. 1984, 73, 396–402. [Google Scholar] [CrossRef]
- George, A.; Cunha-Gomes, D.; Thatte, R.L. Early division of pedicled flaps using a simple device: A new technique. Br. J. Plast. Surg. 1996, 49, 119–122. [Google Scholar] [CrossRef]
- Sabapathy, S.R.; Bajantri, B. Indications, selection, and use of distant pedicled flap for upper limb reconstruction. Hand Clin. 2014, 30, 185–199. [Google Scholar] [CrossRef]
- Al-Qattan, M.M.; Al-Qattan, A.M. Defining the Indications of Pedicled Groin and Abdominal Flaps in Hand Reconstruction in the Current Microsurgery Era. J. Hand Surg. Am. 2016, 41, 917–927. [Google Scholar] [CrossRef]
- Jokuszies, A.; Niederbichler, A.D.; Hirsch, N.; Kahlmann, D.; Herold, C.; Vogt, P.M. The pedicled groin flap for defect closure of the hand. Oper. Orthop. Traumatol. 2010, 22, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Long, C.D.; Granick, M.S.; Solomon, M.P. The cross-leg flap revisited. Ann. Plast. Surg. 1993, 30, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; He, L.; Yin, D.; Niu, Y.; Jin, Z.; Li, J.J.; Wang, Q.K.; Zhou, T. Modified donor blood flow-preserved cross-leg anterolateral thigh flap procedure for complex lower extremity reconstruction. J. Orthop. Surg. Res. 2022, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- Serel, S.; Kaya, B.; Demiralp, O.; Can, Z. Cross-leg free anterolateral thigh perforator flap: A case report. Microsurgery 2006, 26, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Chang, S.; Zhang, Z.; Wu, X.; Wu, B.; Qi, J.; Wei, Z. Parallel Cross-Leg Free Flap with Posterior Tibial Artery Perforator Pedicle Propeller Cable Bridge Flap for the Treatment of Lower Extremity Wounds: A Case Series Report. J. Investig. Surg. 2022, 35, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, E.K.; Demir, C.; Tekfiliz, İ.; Alagoz, M.S. An idea for bringing the recipient pedicle of cross leg free flap closer: Fasciocutaneous flap above pedicle. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Mucke, T.; Wolff, K.D.; Rau, A.; Kehl, V.; Mitchell, D.A.; Steiner, T. Autonomization of free flaps in the oral cavity: A prospective clinical study. Microsurgery 2012, 32, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Beckler, A.D.; Ezzat, W.H.; Seth, R.; Nabili, V.; Blackwell, K.E. Assessment of Fibula Flap Skin Perfusion in Patients Undergoing Oromandibular Reconstruction: Comparison of Clinical Findings, Fluorescein, and Indocyanine Green Angiography. JAMA Facial Plast. Surg. 2015, 17, 422–426. [Google Scholar] [CrossRef]
- Mücke, T.; Fichter, A.M.; Schmidt, L.H.; Mitchell, D.A.; Wolff, K.D.; Ritschl, L.M. Indocyanine green videoangiography-assisted prediction of flap necrosis in the rat epigastric flap using the flow(®) 800 tool. Microsurgery 2017, 37, 235–242. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Spataro, E.A.; Kandathil, C.K.; Most, S.P. Neovascularization Perfusion of Melolabial Flaps Using Intraoperative Indocyanine Green Angiography. JAMA Facial Plast. Surg. 2019, 21, 230–236. [Google Scholar] [CrossRef]
- Christensen, J.M.; Baumann, D.P.; Myers, J.N.; Buretta, K.; Sacks, J.M. Indocyanine green near-infrared laser angiography predicts timing for the division of a forehead flap. Eplasty 2012, 12, e41. [Google Scholar]
- Schlosshauer, T.; Rothenberger, J.; Heiss, C.; Rieger, U.M. The role of near-infrared fluorescence imaging with indocyanine green dye in pedicle division with the paramedian forehead flap. Int. Wound J. 2021, 18, 881–888. [Google Scholar] [CrossRef]
- Converse, J.M.; Wood-Smith, D. Experiences with the forehead island flap with a subcutaneous pedicle. Plast. Reconstr. Surg. 1963, 31, 521–527. [Google Scholar] [CrossRef]
- Surowitz, J.B.; Most, S.P. Use of laser-assisted indocyanine green angiography for early division of the forehead flap pedicle. JAMA Facial Plast. Surg. 2015, 17, 209–214. [Google Scholar] [CrossRef]
- Rudy, S.F.; Abdelwahab, M.; Kandathil, C.K.; Most, S.P. Paramedian forehead flap pedicle division after 7 days using laser-assisted indocyanine green angiography. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, A.; Ameerally, P.; Wildan, T. Free flap survival after arterial ligation. Fac. Dent. J. 2013, 4, 192–193. [Google Scholar] [CrossRef]
- Skrbic, S.; Stanec, Z. Early rupture of the arterial anastomoses with free flap survival. Injury 1995, 26, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, P.; Van Landuyt, K.; Hamdi, M.; Blondeel, P.; Matton, G.; Monstrey, S. Complete survival of a free flap after early pseudoaneurysm formation and pedicle thrombosis. Ann. Plast. Surg. 2001, 47, 332–335. [Google Scholar] [CrossRef]
- Branford, O.A.; Davis, M.; Schreuder, F. Free flap survival after traumatic pedicle avulsion in an obese diabetic patient. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 999–1000. [Google Scholar] [CrossRef]
- Amato, M.M.; Rodriguez, L.R.; Lineaweaver, W.C. Survival of free tissue transfer following internal jugular venous thrombosis. Plast. Reconstr. Surg. 1999, 104, 1406–1408. [Google Scholar] [CrossRef]
- Rothaus, K.O.; Acland, R.D. Free flap neo-vascularisation: Case report. Br. J. Plast. Surg. 1983, 36, 348–349. [Google Scholar] [CrossRef]
- Godden, D.R.; Thomas, S.J. Survival of a free flap after vascular disconnection at 9 days. Br. J. Oral Maxillofac. Surg. 2002, 40, 446–447. [Google Scholar] [CrossRef]
- Salgado, C.J.; Smith, A.; Kim, S.; Higgins, J.; Behnam, A.; Herrera, H.R.; Serletti, J.M. Effects of late loss of arterial inflow on free flap survival. J. Reconstr. Microsurg. 2002, 18, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Castling, B.; Avery, C. Re: Godden, D. R. P., Thomas S J. Survival of a free flap after vascular disconnection at 9 days. Br J Oral Maxillofac Surg 2002; 40: 446-447. Br. J. Oral Maxillofac. Surg. 2003, 41, 281. [Google Scholar] [CrossRef] [PubMed]
- Kissun, D.; Shaw, R.J.; Vaughan, E.D. Survival of a free flap after arterial disconnection at six days. Br. J. Oral Maxillofac. Surg. 2004, 42, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Chiummariello, S.; Cigna, E.; Scuderi, N. Salvage of a free flap after late total thrombosis of the flap and revascularisation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2004, 38, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Avery, B.S.; Edge, C.J. Survival of microvascular free flaps in head and neck surgery after early interruption of the vascular pedicle. Br. J. Oral Maxillofac. Surg. 2005, 43, 426–427. [Google Scholar] [CrossRef]
- Enajat, M.; Rozen, W.M.; Whitaker, I.S.; Audolfsson, T.; Acosta, R. How long are fasciocutaneous flaps dependant on their vascular pedicle: A unique case of SIEA flap survival. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, e347–e350. [Google Scholar] [CrossRef] [PubMed]
- Chubb, D.; Rozen, W.M.; Ashton, M.W. Early survival of a compromised fasciocutaneous flap without pedicle revision: Monitoring with photoplethysmography. Microsurgery 2010, 30, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.R.; Harsha, W.J.; Kim, N.; Hayden, R.E. Free flap survival despite early loss of the vascular pedicle. Head Neck 2011, 33, 1068–1071. [Google Scholar] [CrossRef]
- Nelson, J.A.; Kim, E.M.; Eftakhari, K.; Low, D.W.; Kovach, S.J.; Wu, L.C.; Serletti, J.M. Late venous thrombosis in free flap breast reconstruction: Strategies for salvage after this real entity. Plast. Reconstr. Surg. 2012, 129, 8e–15e. [Google Scholar] [CrossRef]
- Granzow, J.; Li, A.I.; Caton, A.; Boyd, J.B. Free Flap Survival Following Failure of the Vascular Pedicle. Ann. Plast. Surg. 2015, 75, 44–48. [Google Scholar] [CrossRef]
- Wolff, K.D.; Mucke, T.; von Bomhard, A.; Ritschl, L.M.; Schneider, J.; Humbs, M.; Fichter, A.M. Free flap transplantation using an extracorporeal perfusion device: First three cases. J. Craniomaxillofac. Surg. 2016, 44, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.D. New aspects in free flap surgery: Mini-perforator flaps and extracorporeal flap perfusion. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.-D.; Ritschl, L.M.; von Bomhard, A.; Braun, C.; Wolff, C.; Fichter, A.M. In vivo perfusion of free skin flaps using extracorporeal membrane oxygenation. J. Cranio-Maxillofac. Surg. 2020, 48, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Jaffe, W.; London, N.J.; Varma, S.K. Free flap neovascularization: Myth or reality? J. Reconstr. Microsurg. 2004, 20, 31–34. [Google Scholar] [CrossRef]
- Pignatti, M.; Iwuagwu, F.C.; Browne, T.F. Late partial failure of a free ALT flap. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, e124–e127. [Google Scholar] [CrossRef]
- Giordano, L.; Galli, A.; Familiari, M.; Canta, D.; Irem, A.; Biafora, M.; Battista, R.A.; Bussi, M. Head and neck pedicled flap autonomization using a new high-resolution indocyanine green fluorescence video-angiography device. Head Neck 2022, 44, 1496–1499. [Google Scholar] [CrossRef]
- Mueller, S.; Wendl, C.M.; Ettl, T.; Klingelhöffer, C.; Geis, S.; Prantl, L.; Reichert, T.E.; Jung, E.M. Contrast-enhanced ultrasonography as a new method for assessing autonomization of pedicled and microvascular free flaps in head and neck reconstructive surgery. Clin. Hemorheol. Microcirc. 2017, 65, 317–325. [Google Scholar] [CrossRef]
- Tasch, C.; Pattiss, A.; Maier, S.; Lanthaler, M.; Pierer, G. Free Flap Outcome in Irradiated Recipient Sites: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4216. [Google Scholar] [CrossRef]
- Heitland, A.S.; Markowicz, M.P.; Koellensperger, E.; Schoth, F.; Pallua, N. Early and long-term evaluation of perfusion changes in free DIEP-flaps for breast reconstruction via IC-view and duplex ultrasound: Autonomous or peripheral perfusion? J. Reconstr. Microsurg. 2009, 25, 139–145. [Google Scholar] [CrossRef]
- Longo, B.; Laporta, R.; Sorotos, M.; Atzeni, M.; Santanelli di Pompeo, F. Complete DIEP flap survival following pedicle resection, 4 years after its transfer. Clinical evidence of autonomization. Case Reports Plast. Surg. Hand Surg. 2016, 3, 70–72. [Google Scholar] [CrossRef][Green Version]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34 (Suppl. S1), S82–S84. [Google Scholar] [CrossRef]
- Yoon, A.P.; Jones, N.F. Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: A review of the literature. Microsurgery 2016, 36, 604–612. [Google Scholar] [CrossRef]
- Payement, G.; Cariou, J.L.; Arcila, M.; Arrouvel, C.; Banzet, P. [Arterial phenomenon of autonomization of vascularized island flaps reviewed by expansion of the vascular pedicle. An experimental study in the rat]. Ann. Chir. Plast. Esthet. 1994, 39, 779–784. [Google Scholar]
- Vourtsis, S.A.; Spyriounis, P.K.; Agrogiannis, G.D.; Ionac, M.; Papalois, A.E. VEGF application on rat skin flap survival. J. Investig. Surg. 2012, 25, 14–19. [Google Scholar] [CrossRef]
- Efeoğlu, F.B.; Gökkaya, A.; Karabekmez, F.E.; Fırat, T.; Gorgu, M. Effects of omentin on flap viability: An experimental research on rats. J. Plast. Surg. Hand Surg. 2019, 53, 347–355. [Google Scholar] [CrossRef]
- Berkane, Y.; Lellouch, A.G.; Shamlou, A.A.; Goutard, M.; Tawa, P.; Uygun, B.E.; Randolph, M.A.; Cetrulo Jr, C.L.; Uygun, K. 121. Acellular Subnomothermic Machine Perfusion of Fasciocutaneous Flaps in Swine. Plast. Reconstr. Surg. Glob. Open 2023, 11, 76. [Google Scholar] [CrossRef]
- Berkane, Y.; Lellouch, A.G.; Goudot, G.; Shamlou, A.; Filz von Reiterdank, I.; Goutard, M.; Tawa, P.; Girard, P.; Bertheuil, N.; Uygun, B.E.; et al. Towards Optimizing Sub-Normothermic Machine Perfusion in Fasciocutaneous Flaps: A Large Animal Study. Bioengineering 2023, 10, 1415. [Google Scholar] [CrossRef]
- Li, K.; Tharwat, M.; Larson, E.L.; Felgendreff, P.; Hosseiniasl, S.M.; Rmilah, A.A.; Safwat, K.; Ross, J.J.; Nyberg, S.L. Re-Endothelialization of Decellularized Liver Scaffolds: A Step for Bioengineered Liver Transplantation. Front. Bioeng. Biotechnol. 2022, 10, 833163. [Google Scholar] [CrossRef]
- Uygun, B.E.; Yarmush, M.L. Engineered liver for transplantation. Curr. Opin. Biotechnol. 2013, 24, 893–899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acun, A.; Oganesyan, R.; Uygun, B.E. Liver Bioengineering: Promise, Pitfalls, and Hurdles to Overcome. Curr. Transplant. Rep. 2019, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Stabler, C.T.; Lecht, S.; Mondrinos, M.J.; Goulart, E.; Lazarovici, P.; Lelkes, P.I. Revascularization of decellularized lung scaffolds: Principles and progress. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1273–L1285. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Ni, J.; Mao, W.; Tian, C.; Xie, J.; Zhang, Y.; Wang, Y.; Wan, J.; Wang, K.; et al. Constructing a heparin-modified penile decellularized scaffold to improve re-endothelialization in organizational reconstruction. Transl. Androl. Urol. 2022, 11, 683–693. [Google Scholar] [CrossRef]
- Nyirjesy, S.C.; Yu, J.; Dharmadhikari, S.; Liu, L.; Bergman, M.; Tan, Z.H.; VanKoevering, K.K.; Chiang, T. Successful Early Neovascularization in Composite Tracheal Grafts. Otolaryngol. Head Neck Surg. 2023, 169, 1035–1040. [Google Scholar] [CrossRef]

| PubMed ID (Reference #) | Year | Author | Article Type |
|---|---|---|---|
| 6190527 [90] | 1983 | Rothaus | Case report |
| 7493792 [86] | 1995 | Skbric | Case report |
| 10513925 [89] | 1999 | Amato | Case report |
| 11562041 [87] | 2001 | Ceulemans | Case report |
| 123797 [91] | 2002 | Godden | Case report |
| 12404130 [92] | 2002 | Salgado | Case series |
| 12946680 [93] | 2003 | Castling | Case report |
| 15013552 [94] | 2004 | Kissun | Case report |
| 15074725 [95] | 2004 | Ribuffo | Case report |
| 15908077 [96] | 2005 | Burns | Case series |
| 18495566 [88] | 2008 | Branford | Case report |
| 19446514 [97] | 2009 | Enajat | Case report |
| 20878730 [98] | 2010 | Chubb | Case report |
| 20175197 [99] | 2010 | Wise | Case report |
| 22186589 [100] | 2012 | Nelson | Case series |
| * [85] | 2013 | Hindocha | Case report |
| 25643188 [101] | 2015 | Granzow | Case series |
| 26752222 [102] | 2016 | Wolff | Case series |
| 28642191 [103] | 2017 | Wolff | Case report |
| 31874806 [104] | 2020 | Wolff | Case series |
| 32565139 [84] | 2021 | Rudy | Case series |
| 1st Author | Study Type | Age | Initial Pathology | Recipient Site | RxTh * | Smoking Status | Diabetes | Local Infection | Flap | Flap Classification | Flap Size | DBD * | Type of BD * | Partial Flap Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amato | Case report | 68 | SCC | Mandible | Yes | Previous | NA | No | Scapular | Perforator/Free | 4 | Venous | No | |
| Branford | Case report | 48 | Trauma | Heel | No | No | Yes | No | RFF | Septal/Free | 6 × 5 | 26 | Pedicle | Minor necrosis |
| Burns | Case series | 59 | SCC | Tongue | No | NA | NA | Yes | RFF | Septal/Free | 19 | Veinous | No | |
| Burns | Case series | 69 | SCC | Tongue | No | NA | NA | No | RFF | Septal/Free | 11 | Veinous | No | |
| Burns | Case series | 49 | Carcinoma | Tongue | NA | NA | NA | No | RFF | Septal/Free | 6 | Pedicle | No | |
| Castling | Case report | 52 | Adenoic cystic carninoma | Tongue | No | NA | NA | Yes | RFF | Septal/Free | 9 | Pedicle | No | |
| Ceulemans | Case report | 65 | Trauma | Ankle | No | No | No | Yes | TDAP | Perforator/Free | 18 | Pedicle | No | |
| Chubb | Case report | 50 | DCIS | Breast | Yes | No | NA | No | SGAP | Perforator/Free | 400 g | 7 | Pedicle | Epidermolysis, 10% Necrosis |
| Enajat | Case report | 64 | Carcinoma | Breast | Yes | No | NA | No | SIEA | Perforator/Free | 11 | Pedicle | 25% Necrosis | |
| Felcht | Case series | 75 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 6.3 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 70 | Carcinoma | Nasal dorsum | No | NA | NA | No | Forehead | Axial/Pedicled | 7.5 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 84 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 6.3 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 80 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 5.8 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 79 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 9 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 78 | Carcinoma | Nasal dorsum | No | NA | NA | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Felcht | Case series | 87 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 6.5 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 60 | Carcinoma | Nasal sidewalls | No | NA | NA | No | Forehead | Axial/Pedicled | 6.9 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 90 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 5.5 cm2 | 7 | Pedicle | No |
| Felcht | Case series | 87 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 3.4 cm2 | 8 | Pedicle | No |
| Felcht | Case series | 76 | Carcinoma | Nasal tip | No | NA | NA | No | Forehead | Axial/Pedicled | 6.3 cm2 | 11 | Pedicle | No |
| Felcht | Case series | 87 | Carcinoma | Nasal ala | No | NA | NA | No | Forehead | Axial/Pedicled | 7.3 cm2 | 7 | Pedicle | No |
| Godden | Case report | 40 | SCC | Tongue | No | NA | NA | Yes | RFF | Septal/Free | NA | 9 | Pedicle | No |
| Granzow | Case series | 76 | SCC | External Cheek | No | No | NA | No | Fibular | Septal/Free | 20 × 16 | 17 | Arterial | No |
| Granzow | Case series | 39 | Ameloblastoma | Intra-oral Cheek | No | No | NA | No | Fibular | Septal/Free | 27 × 10 | 11 | Pedicle | No |
| Hindocha | Case report | 55 | SCC | Buccas mucosa | No | Yes | No | No | RFF | Septal/Free | 12 | Arterial | No | |
| Kissun | Case report | 35 | SCC | Tongue | Yes | NA | NA | Yes | Radial Forearm flap | Septal/Free | 6 | Pedicle | No | |
| Nelson | Case series | 49 | Cancer | Breast | NA | NA | NA | No | DIEP | Perforator/Free | 5 | Venous | Yes | |
| Nelson | Case series | 52 | Cancer | Breast | NA | NA | NA | No | SGAP | Perforator/Free | 8 | Venous | No | |
| Ribuffo | Case report | 42 | Trauma | Ankle | No | NA | NA | No | RFF | Septal/Free | 8 × 4 | 11 | Venous | No |
| Rothaus | Case report | 17 | Trauma | Heel | No | No | No | No | Groin Flap | Axial/Free | 9 × 9 | 9 | Arterial | No |
| Rudy | Case series | 87 | Melanoma in situ | L Nasal dorsum | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 77 | BCC | L Nasal Ala | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 55 | BCC | L Nasal tip | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 74 | BCC | L Nasal tip | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 52 | BCC | R Nasal lateral wall | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 51 | BCC | R Nasal tip | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 58 | BCC | R Nasal Ala | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 65 | BCC | R Nasal Ala | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Rudy | Case series | 89 | BCC | R nasal tip | No | No | No | No | Forehead | Axial/Pedicled | 7 | Pedicle | No | |
| Salgado | Case series | 62 | SCC | Tongue | No | No | No | Yes | Fibular | Septal/Free | 8 | Pedicle | No | |
| Salgado | Case series | 38 | Trauma | Tongue | No | No | No | Yes | Fibular | Septal/Free | 10 | Pedicle | No | |
| Salgado | Case series | 61 | SCC | Mouth | No | No | Yes | Yes | Fibular | Septal/Free | 13 | Pedicle | No | |
| Salgado | Case series | 47 | SCC | Mouth | Yes | No | No | Yes | Fibular | Septal/Free | 20 | Pedicle | No | |
| Skbric | Case report | 37 | Trauma | Heel | No | NA | NA | No | RFF | Septal/Free | 12 | Arterial | No | |
| Wise | Case report | 69 | SCC | Tongue | Yes | Previous | No | Yes | ALT | Perforator/Free | 9 | Pedicle | No | |
| Wolff | Case report | 57 | Secondary defect | Shoulder | Yes | NA | NA | No | ALT | Perforator/Free | 13 × 8 | 6 | Pedicle | No |
| Wolff | Case series | 52 | SCC | Chin | Yes | NA | NA | No | ALT | Perforator/Free | 25 × 8 | 18 | Pedicle | No |
| Wolff | Case series | 77 | Carcinoma | Neck | Yes | NA | NA | No | ALT | Perforator/Free | 14 × 9 | 6 | Pedicle | Yes: epithelial + hilum |
| Wolff | Case series | 60 | Carcinoma | Cheek | No | NA | NA | No | ALT | Perforator/Free | 7 × 6 | 6 | Pedicle | Yes: epithelial |
| Wolff | Case series | 76 | CUP syndrom | Neck | Yes | NA | NA | No | RFF | Septal/Free | 8 × 6 | 5 | Pedicle | Yes: epidermis + dermis |
| Wolff | Case series | 70 | Glioblastoma | Occipital scalp | Yes | NA | NA | Yes | RFF | Septal/Free | 14 × 9 | 4 | Pedicle | Yes: 80% |
| Wolff | Case series | 66 | SCC | Cheek | Yes | No | NA | No | Fibular (septal) | Septal/Free | 6 × 4 | 13 | Pedicle | No |
| Flap Location | Number of Flaps | Day of Discontinuity (Mean ± SD) | Partial Loss n (Mean) | Earliest Full Autonomization (Days) |
|---|---|---|---|---|
| Head/Neck | 29 | 7.76 ± 3.17 | 4 (14%) | 4 |
| Intra-oral | 13 | 8.00 ± 4.06 | 0 (00%) | 6 |
| Limb | 6 | 13.67 ± 7.23 | 1 (17%) | 6 |
| Breast | 4 | 7.75 ± 2.50 | 3 (75%) | 8 |
| Total | 52 | 9.25 ± 4.46 | 8 (15%) | 4 |
| Vascularization Type | Number of Flaps | Day of Discontinuity | Partial Loss n (Mean) |
|---|---|---|---|
| (Mean ± SD) | |||
| Indirect (Musculocutaneous) | 12 | 8.67 ± 4.74 | 4 (33%) |
| Direct Septal | 18 | 12.00 ± 5.56 | 3 (17%) |
| Direct Axial | 22 | 7.31 ± 0.89 | 0 (00%) |
| Total | 52 | 9.25 ± 4.46 | 8 (15%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkane, Y.; Kostyra, D.M.; Chrelias, T.; Randolph, M.A.; Lellouch, A.G.; Cetrulo, C.L., Jr.; Uygun, K.; Uygun, B.E.; Bertheuil, N.; Duisit, J. The Autonomization Principle in Vascularized Flaps: An Alternative Strategy for Composite Tissue Scaffold In Vivo Revascularization. Bioengineering 2023, 10, 1440. https://doi.org/10.3390/bioengineering10121440
Berkane Y, Kostyra DM, Chrelias T, Randolph MA, Lellouch AG, Cetrulo CL Jr., Uygun K, Uygun BE, Bertheuil N, Duisit J. The Autonomization Principle in Vascularized Flaps: An Alternative Strategy for Composite Tissue Scaffold In Vivo Revascularization. Bioengineering. 2023; 10(12):1440. https://doi.org/10.3390/bioengineering10121440
Chicago/Turabian StyleBerkane, Yanis, David M. Kostyra, Theodoros Chrelias, Mark A. Randolph, Alexandre G. Lellouch, Curtis L. Cetrulo, Jr., Korkut Uygun, Basak E. Uygun, Nicolas Bertheuil, and Jérôme Duisit. 2023. "The Autonomization Principle in Vascularized Flaps: An Alternative Strategy for Composite Tissue Scaffold In Vivo Revascularization" Bioengineering 10, no. 12: 1440. https://doi.org/10.3390/bioengineering10121440
APA StyleBerkane, Y., Kostyra, D. M., Chrelias, T., Randolph, M. A., Lellouch, A. G., Cetrulo, C. L., Jr., Uygun, K., Uygun, B. E., Bertheuil, N., & Duisit, J. (2023). The Autonomization Principle in Vascularized Flaps: An Alternative Strategy for Composite Tissue Scaffold In Vivo Revascularization. Bioengineering, 10(12), 1440. https://doi.org/10.3390/bioengineering10121440