Bio-Hybrid Magnetic Robots: From Bioengineering to Targeted Therapy
Abstract
1. Introduction
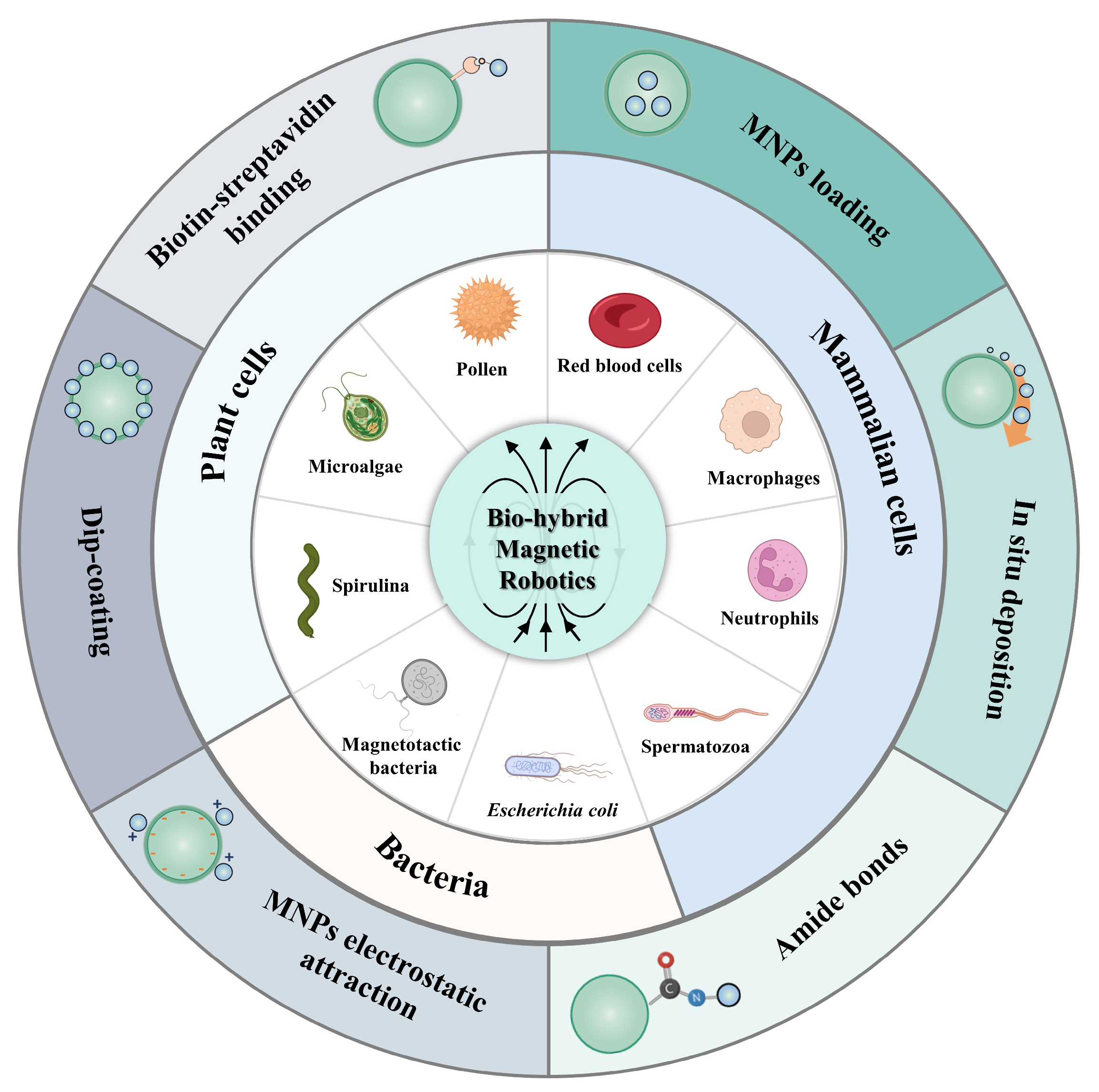
2. Mammalian Cells
2.1. Red Blood Cells
2.2. Neutrophils
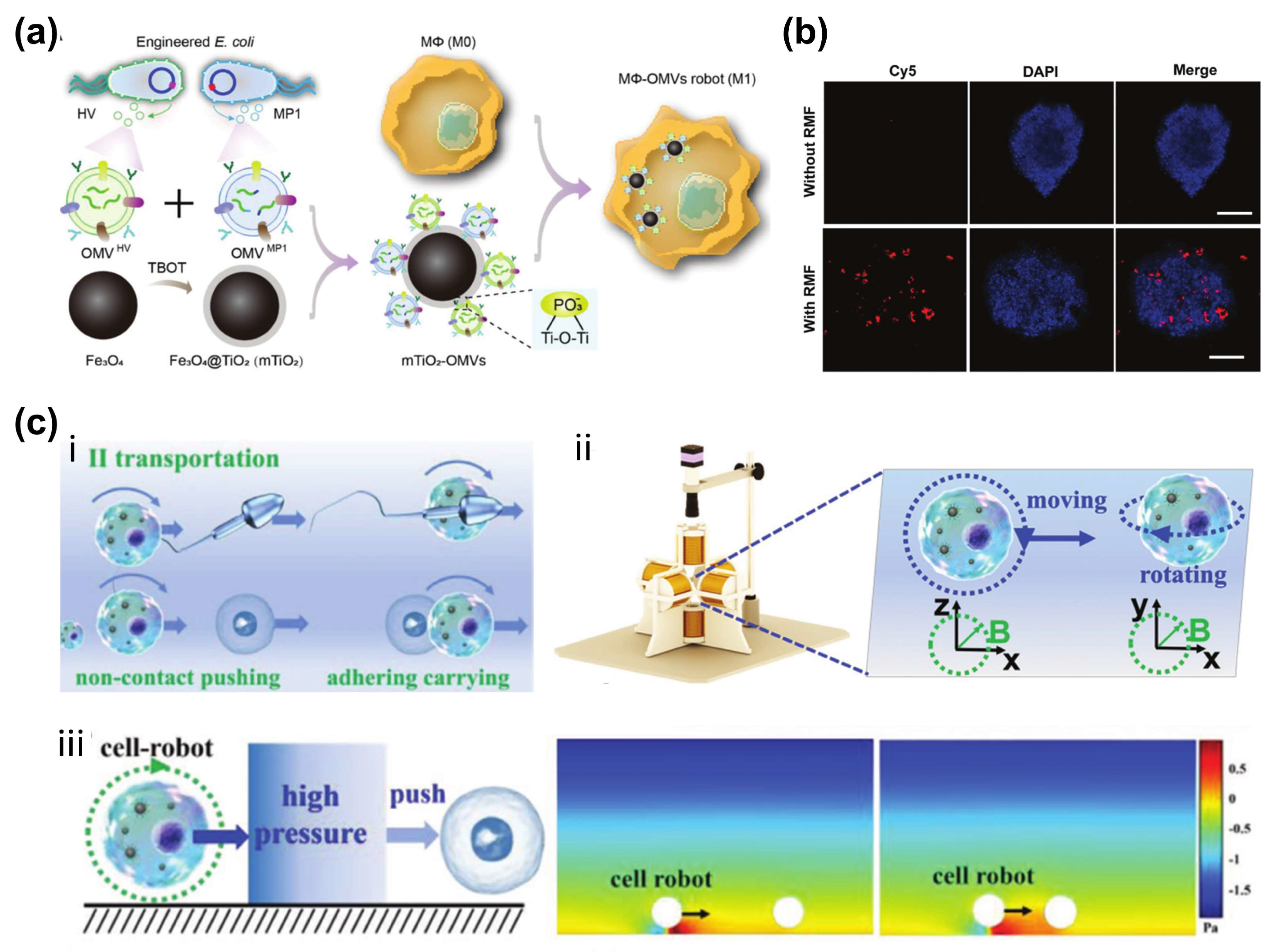
2.3. Macrophages
2.4. Sperm
3. Plant Cells
3.1. Microalgae
3.2. Pollen
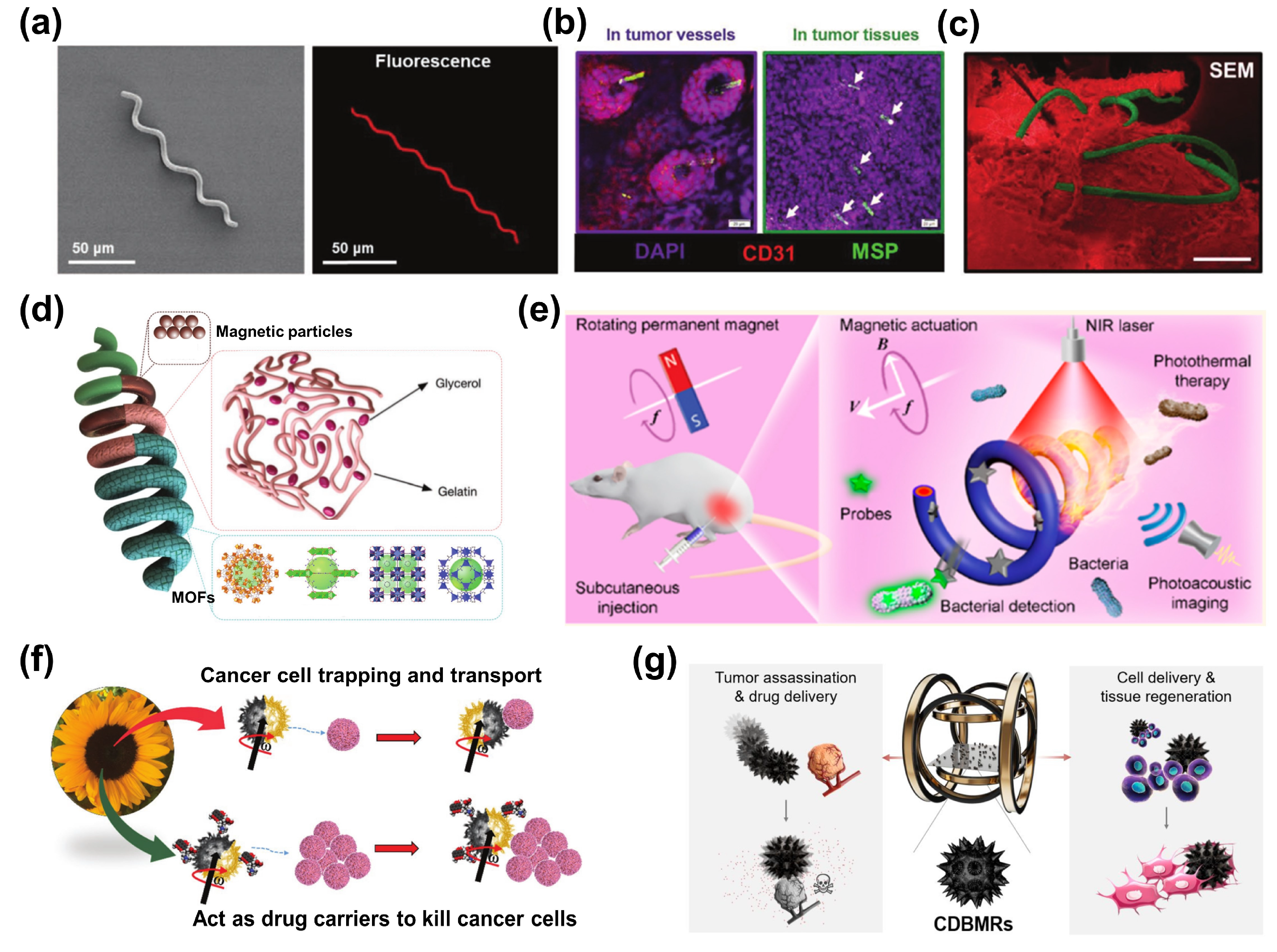
4. Bacteria
4.1. Magnetotactic Bacteria
4.2. Escherichia coli

5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Soto, F.; Wang, J.; Ahmed, R.; Demirci, U. Medical Micro/Nanorobots in Precision Medicine. Adv. Sci. 2020, 7, 2002203. [Google Scholar] [CrossRef] [PubMed]
- Sitti, M. Miniature soft robots—road to the clinic. Nat. Rev. Mater. 2018, 3, 74–75. [Google Scholar] [CrossRef]
- Ghosh, A.; Xu, W.; Gupta, N.; Gracias, D.H. Active matter therapeutics. Nano Today 2020, 31, 100836. [Google Scholar] [CrossRef] [PubMed]
- Mundaca-Uribe, R.; Askarinam, N.; Fang, R.H.; Zhang, L.; Wang, J. Towards multifunctional robotic pills. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Mou, F.; Cao, C.; Yu, L.; Li, Z.; Guan, J. Swarming magnetic nanorobots bio-interfaced by heparinoid-polymer brushes for in vivo safe synergistic thrombolysis. Sci. Adv. 2023, 9, eadk7251. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Jiang, L.; Xu, N.; Li, W.; Wang, L.; Wu, Y.; Wang, J.; He, Z.; Sun, F.; et al. Ultrasound-driven BaTiO3 nanorobots patching immunologic barrier to cure chronic rheumatoid arthritis. J. Adv. Ceram. 2023, 12, 1105–1117. [Google Scholar]
- Dai, B.; Wang, J.; Xiong, Z.; Zhan, X.; Dai, W.; Li, C.-C.; Feng, S.-P.; Tang, J. Programmable artificial phototactic microswimmer. Nat. Nanotechnol. 2016, 11, 1087–1092. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Yang, L.; Vong, C.I.; Chan, K.F.; Wu, W.K.K.; Kwong, T.N.Y.; Lo, N.W.S.; Ip, M.; Wong, S.H.; et al. Real-time tracking of fluorescent magnetic spore-based microrobots for remote detection of C. diff toxins. Sci. Adv. 2019, 5, eaau9650. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, T.; Wang, J.; Xu, J.; Shen, T.; Zhang, T.; Zhang, B.; Gao, S.; Zhao, C.; Yang, M.; et al. Self-Propelled Janus Nanocatalytic Robots Guided by Magnetic Resonance Imaging for Enhanced Tumor Penetration and Therapy. J. Am. Chem. Soc. 2023, 145, 11019–11032. [Google Scholar] [CrossRef]
- Choi, J.; Hwang, J.; Kim, J.Y.; Choi, H. Recent Progress in Magnetically Actuated Microrobots for Targeted Delivery of Therapeutic Agents. Adv. Healthc. Mater. 2021, 10, e2001596. [Google Scholar] [CrossRef]
- Diller, E.; Giltinan, J.; Lum, G.Z.; Ye, Z.; Sitti, M. Six-degree-of-freedom magnetic actuation for wireless microrobotics. Int. J. Rob. Res. 2016, 35, 114–128. [Google Scholar] [CrossRef]
- Norton, J.C.; Slawinski, P.R.; Lay, H.S.; Martin, J.W.; Cox, B.F.; Cummins, G.; Desmulliez, M.P.Y.; Clutton, R.E.; Obstein, K.L.; Cochran, S.; et al. Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci. Robot. 2019, 4, eaav7725. [Google Scholar] [CrossRef] [PubMed]
- Chesnitskiy, A.V.; Gayduk, A.E.; Seleznev, V.A.; Prinz, V.Y. Bio-Inspired Micro- and Nanorobotics Driven by Magnetic Field. Materials 2022, 15, 7781. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Fischer, P. Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, R.W.; Sitti, M. Bio-Hybrid Cell-Based Actuators for Microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef] [PubMed]
- Loghin, D.; Tremblay, C.; Mohammadi, M.; Martel, S. Exploiting the responses of magnetotactic bacteria robotic agents to enhance displacement control and swarm formation for drug delivery platforms. Int. J. Rob. Res. 2017, 36, 1195–1210. [Google Scholar] [CrossRef]
- Alapan, Y.; Yasa, O.; Yigit, B.; Yasa, I.C.; Erkoc, P.; Sitti, M. Microrobotics and Microorganisms: Biohybrid Autonomous Cellular Robots. Annu. Rev. Control Robot. Auton. Syst. 2019, 2, 205–230. [Google Scholar] [CrossRef]
- Dong, X.; Wu, W.; Pan, P.; Zhang, X.Z. Engineered Living Materials for Advanced Diseases Therapy. Adv. Mater. 2023, 33, e2304963. [Google Scholar] [CrossRef]
- Orozco, J.; García-Gradilla, V.; D’Agostino, M.; Gao, W.; Cortés, A.; Wang, J. Artificial Enzyme-Powered Microfish for Water-Quality Testing. ACS Nano 2013, 7, 818–824. [Google Scholar] [CrossRef]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-based DNA detection using catalytic nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef]
- Kagan, D.; Benchimol, M.J.; Claussen, J.C.; Chuluun-Erdene, E.; Esener, S.; Wang, J. Acoustic Droplet Vaporization and Propulsion of Perfluorocarbon-Loaded Microbullets for Targeted Tissue Penetration and Deformation. Angew. Chem. Int. Ed. 2012, 51, 7519–7522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, Y.; He, W.; Lin, X.; Sun, J.; He, Q. Self-Propelled Polymer-Based Multilayer Nanorockets for Transportation and Drug Release. Angew. Chem. Int. Ed. 2013, 52, 7000–7003. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zheng, M.; Ma, A.; Liu, L.; Cai, L. Cell/Bacteria-Based Bioactive Materials for Cancer Immune Modulation and Precision Therapy. Adv. Mater. 2021, 33, e2100241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Su, G.; Mei, J.; Li, J. A Review of Single-Cell Microrobots: Classification, Driving Methods and Applications. Micromachines 2023, 14, 1710. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Yang, X.; Liang, W.; Wang, Y. Construction of micro-nano robots: Living cells and functionalized biological cell membranes. Front. Bioeng. Biotechnol. 2023, 11, 1277964. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, H.; Liu, C.; Zhang, S.; Du, R.; Deng, Y.; Zou, X. Biomembrane-inspired design of medical micro/nanorobots: From cytomembrane stealth cloaks to cellularized Trojan horses. Aggregate 2023, 4, e359. [Google Scholar] [CrossRef]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef]
- Villa, C.H.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef]
- Li, T.; Yu, S.; Sun, B.; Li, Y.; Wang, X.; Pan, Y.; Song, C.; Ren, Y.; Zhang, Z.; Grattan, K.T.V.; et al. Bioinspired claw-engaged and biolubricated swimming microrobots creating active retention in blood vessels. Sci. Adv. 2023, 9, eadg4501. [Google Scholar] [CrossRef]
- Ihler, G.M.; Glew, R.H.; Schnure, F.W. Enzyme Loading of Erythrocytes. Proc. Natl. Acad. Sci. USA 1973, 70, 2663–2666. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Li, J.; Gao, W.; Xu, T.; Christianson, C.; Gao, W.; Galarnyk, M.; He, Q.; Zhang, L.; et al. Turning Erythrocytes into Functional Micromotors. ACS Nano 2014, 8, 12041–12048. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, M.; Cam, N.; Georgelin, T.; Clement, O.; Autret, G.; Siaugue, J.M.; Menager, C. Human erythrocytes covered with magnetic core-shell nanoparticles for multimodal imaging. Adv. Healthc. Mater. 2013, 2, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Agola, J.O.; Serda, R.; Franco, S.; Lei, Q.; Wang, L.; Minster, J.; Croissant, J.G.; Butler, K.S.; Zhu, W.; et al. Biomimetic Rebuilding of Multifunctional Red Blood Cells: Modular Design Using Functional Components. ACS Nano 2020, 14, 7847–7859. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Esteban-Fernandez de Avila, B.; Martin, A.; Christianson, C.; Gao, W.; Thamphiwatana, S.K.; Escarpa, A.; He, Q.; Zhang, L.; Wang, J. RBC micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale 2015, 7, 13680–13686. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lin, Z.; Wang, D.; Wu, Z.; Xie, H.; He, Q. Red Blood Cell-Mimicking Micromotor for Active Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23392–23400. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Gao, W.; Xu, T.; Jurado-Sánchez, B.; Li, J.; Gao, W.; He, Q.; Zhang, L.; Wang, J. Cell-Membrane-Coated Synthetic Nanomotors for Effective Biodetoxification. Adv. Funct. Mater. 2015, 25, 3881–3887. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Zhao, J.; Li, Q.; Dai, L.; Li, J. Magnetic Mesoporous Silica Nanoparticles Cloaked by Red Blood Cell Membranes: Applications in Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 6049–6053. [Google Scholar] [CrossRef]
- Ak, G.; Yilmaz, H.; Güneş, A.; Hamarat Sanlier, S. In vitro and in vivo evaluation of folate receptor-targeted a novel magnetic drug delivery system for ovarian cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–937. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Q.; Wu, S.; Qin, H.; Zhang, T.; Zheng, X.; Li, B. Optically Manipulated Neutrophils as Native Microcrafts In Vivo. ACS Cent. Sci. 2022, 8, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, Y.; Zhang, M.; Li, Y.; Tang, B.Z. Neutrophil-like Biomimic AIE Nanoparticles with High-Efficiency Inflammatory Cytokine Targeting Enable Precise Photothermal Therapy and Alleviation of Inflammation. ACS Nano 2023, 17, 7394–7405. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, e1706245. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-Guided Hybrid Neutrophil Micromotors for Targeted Drug Transport. Angew. Chem. Int. Ed. 2017, 56, 12935–12939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Gao, C.; Fan, X.; Pang, Y.; Li, T.; Wu, Z.; Xie, H.; He, Q. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 2021, 6, eaaz9519. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, H. Neutrobots smuggle drugs across biological barriers. Sci. Robot. 2021, 6, eabh0286. [Google Scholar] [CrossRef]
- Xiong, K.; Wei, W.; Jin, Y.; Wang, S.; Zhao, D.; Wang, S.; Gao, X.; Qiao, C.; Yue, H.; Ma, G.; et al. Biomimetic Immuno-Magnetosomes for High-Performance Enrichment of Circulating Tumor Cells. Adv. Mater. 2016, 28, 7929–7935. [Google Scholar] [CrossRef]
- Zheng, J.; Qi, R.; Dai, C.; Li, G.; Sang, M. Enzyme Catalysis Biomotor Engineering of Neutrophils for Nanodrug Delivery and Cell-Based Thrombolytic Therapy. ACS Nano 2022, 16, 2330–2344. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Lu, G.H.; Nie, W.; Zhang, L.; Lv, Y.; Bao, W.; Gao, X.; Wei, W.; Pu, K.; et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano 2019, 13, 5662–5673. [Google Scholar] [CrossRef]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef]
- Li, Y.; Cong, Z.; Xie, L.; Tang, S.; Ren, C.; Peng, X.; Tang, D.; Wan, F.; Han, H.; Zhang, X.; et al. Magnetically Powered Immunogenic Macrophage Microrobots for Targeted Multimodal Cancer Therapy. Small 2023, 19, e2301489. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jia, L.; Wang, L.; Sun, H.; Ji, Y.; Wang, C.; Song, L.; Liang, S.; Chen, D.; Feng, Y.; et al. Magnetically Actuated Cell-Robot System: Precise Control, Manipulation, and Multimode Conversion. Small 2022, 18, e2105414. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, R.; Luo, Y.; Guo, X.; Yang, G.; Li, X.; Zhou, S. A Hierarchical Structured Fiber Device Remodeling the Acidic Tumor Microenvironment for Enhanced Cancer Immunotherapy. Adv. Mater. 2023, 35, e2300216. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wei, Y.; Zhao, Z.; Han, W.; Zhou, R.; Zhou, Y.; Zheng, Y.; Yin, L. Immuno-Engineered Nanodecoys for the Multi-Target Anti-Inflammatory Treatment of Autoimmune Diseases. Adv. Mater. 2022, 34, e2108817. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qian, R.; Li, T.; Fu, W.; Fang, L.; Cai, Y.; Guo, H.; Xi, L.; Cheang, U.K. Imaging-Guided Biomimetic M1 Macrophage Membrane-Camouflaged Magnetic Nanorobots for Photothermal Immunotargeting Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 56548–56559. [Google Scholar] [CrossRef]
- Sitti, M. Voyage of the microrobots. Nature 2009, 458, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Sukhov, A.; Hauri, D.; Rodrigue, D.; Maranta, G.; Harting, J.; Nelson, B.J. Bioinspired acousto-magnetic microswarm robots with upstream motility. Nat. Mach. Intell. 2021, 3, 116–124. [Google Scholar] [CrossRef]
- Smith, D.J.; Gaffney, E.A.; Gadêlha, H.; Kapur, N.; Kirkman-Brown, J.C. Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity. Cell Motil. 2009, 66, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.F.; Diel, L.; Overstreet, J.W. Differences in the movement of morphologically normal and abnormal human seminal spermatozoa. Biol. Reprod. 1982, 26, 566–570. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, C., Jr. Mensuration of the heads of bull spermatozoa. Mikroskopie 1960, 14, 265–276. [Google Scholar] [PubMed]
- Leung, M.R.; Zeng, J.; Wang, X.; Roelofs, M.C.; Huang, W.; Zenezini Chiozzi, R.; Hevler, J.F.; Heck, A.J.R.; Dutcher, S.K.; Brown, A.; et al. Structural specializations of the sperm tail. Cell 2023, 186, 2880–2896.e2817. [Google Scholar] [CrossRef] [PubMed]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sanchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular Cargo Delivery: Toward Assisted Fertilization by Sperm-Carrying Micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Q.; Yu, J.; Xu, T.; Deng, Y.; Tang, T.; Feng, Q.; Bian, L.; Zhang, Y.; Ferreira, A.; et al. Magnetite Nanostructured Porous Hollow Helical Microswimmers for Targeted Delivery. Adv. Funct. Mater. 2015, 25, 5333–5342. [Google Scholar] [CrossRef]
- Magdanz, V.; Gebauer, J.; Sharan, P.; Eltoukhy, S.; Voigt, D.; Simmchen, J. Sperm-Particle Interactions and Their Prospects for Charge Mapping. Adv. Biosyst. 2019, 3, e1900061. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Fatih Tabak, A.; Klingner, A.; Sitti, M. Magnetic propulsion of robotic sperms at low-Reynolds number. Appl. Phys. Lett. 2016, 109, 033701. [Google Scholar] [CrossRef]
- Magdanz, V.; Vivaldi, J.; Mohanty, S.; Klingner, A.; Vendittelli, M.; Simmchen, J.; Misra, S.; Khalil, I.S.M. Impact of Segmented Magnetization on the Flagellar Propulsion of Sperm-Templated Microrobots. Adv. Sci. 2021, 8, 2004037. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sanchez, M.; Magdanz, V.; Schwarz, L.; Hebenstreit, F.; Schmidt, O.G. Sperm-Hybrid Micromotor for Targeted Drug Delivery. ACS Nano 2018, 12, 327–337. [Google Scholar] [CrossRef]
- Magdanz, V.; Khalil, I.S.M.; Simmchen, J.; Furtado, G.P.; Mohanty, S.; Gebauer, J.; Xu, H.; Klingner, A.; Aziz, A.; Medina-Sánchez, M.; et al. IRONSperm: Sperm-templated soft magnetic microrobots. Sci. Adv. 2020, 6, eaba5855. [Google Scholar] [PubMed]
- Thoré, E.S.J.; Muylaert, K.; Bertram, M.G.; Brodin, T. Microalgae. Curr. Biol. 2023, 33, R91–R95. [Google Scholar] [CrossRef]
- Li, C.; Schramma, N.; Wang, Z.; Qari, N.F.; Jalaal, M.; Latz, M.I.; Cai, S. Ultrasensitive and robust mechanoluminescent living composites. Sci. Adv. 2023, 9, eadi8643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Yin, L.; Zhang, Q.; Askarinam, N.; Mundaca-Uribe, R.; Tehrani, F.; Karshalev, E.; Gao, W.; Zhang, L.; et al. ACE2 Receptor-Modified Algae-Based Microrobot for Removal of SARS-CoV-2 in Wastewater. J. Am. Chem. Soc. 2021, 143, 12194–12201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhong, D.; Ouyang, J.; He, J.; Qi, Y.; Chen, W.; Zhang, X.; Tao, W.; Zhou, M. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat. Commun. 2022, 13, 1413. [Google Scholar] [CrossRef]
- Qi, Y.D.; Zhuang, Z.N.; Zeng, S.M.; Tang, Y.; Cheng, H.; Cheng, S.X.; Zhang, X.Z. In Situ Self-Aggregation of Spirulina Skeleton Fibers Enhances the Efficacy of Anti-Tumor Thermal Immunotherapy. Adv. Funct. Mater. 2024. [Google Scholar] [CrossRef]
- Zhong, D.; Li, W.; Qi, Y.; He, J.; Zhou, M. Photosynthetic Biohybrid Nanoswimmers System to Alleviate Tumor Hypoxia for FL/PA/MR Imaging-Guided Enhanced Radio-Photodynamic Synergetic Therapy. Adv. Funct. Mater. 2020, 30, 1910395. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.-X.J.; et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2017, 2, eaaq1155. [Google Scholar] [CrossRef]
- Terzopoulou, A.; Palacios-Corella, M.; Franco, C.; Sevim, S.; Dysli, T.; Mushtaq, F.; Romero-Angel, M.; Martí-Gastaldo, C.; Gong, D.; Cai, J.; et al. Biotemplating of Metal–Organic Framework Nanocrystals for Applications in Small-Scale Robotics. Adv. Funct. Mater. 2021, 32, 2107421. [Google Scholar] [CrossRef]
- Xie, L.; Pang, X.; Yan, X.; Dai, Q.; Lin, H.; Ye, J.; Cheng, Y.; Zhao, Q.; Ma, X.; Zhang, X.; et al. Photoacoustic Imaging-Trackable Magnetic Microswimmers for Pathogenic Bacterial Infection Treatment. ACS Nano 2020, 14, 2880–2893. [Google Scholar] [CrossRef]
- Higashiyama, T. Pollen tube navigation can inspire microrobot design. Sci. Robot. 2017, 2, eaao1891. [Google Scholar] [CrossRef]
- Cai, L.; Cao, X.; Zhao, C.; Luo, Z.; Zhao, Y. Near-Infrared-II-Driven Pollen Micromotors for Inflammatory Bowel Disease Treatment. ACS Nano 2023, 17, 19993–20001. [Google Scholar] [CrossRef]
- Huang, D.; Wang, J.; Nie, M.; Chen, G.; Zhao, Y. Pollen-Inspired Adhesive Multilobe Microparticles from Microfluidics for Intestinal Drug Delivery. Adv. Mater. 2023, 35, 2301192. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.; Zhang, Z.; Xu, T.; Ye, C.; Shi, T.; Wang, Y. Extraordinary microcarriers derived from spores and pollens. Mater. Horiz. 2023, 10, 1121–1139. [Google Scholar] [CrossRef]
- Schouten, P.J.C.; Soto-Aguilar, D.; Aldalbahi, A.; Ahamad, T.; Alzahly, S.; Fogliano, V. Design of sporopollenin-based functional ingredients for gastrointestinal tract targeted delivery. Curr. Opin. Food Sci. 2022, 44, 100809. [Google Scholar] [CrossRef]
- Wang, H.; Potroz, M.G.; Jackman, J.A.; Khezri, B.; Marić, T.; Cho, N.J.; Pumera, M. Bioinspired Spiky Micromotors Based on Sporopollenin Exine Capsules. Adv. Funct. Mater. 2017, 27, 1702338. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Rosli, N.F.; Budanović, M.; Webster, R.D.; Cho, N.J.; Pumera, M. Microrobots Derived from Variety Plant Pollen Grains for Efficient Environmental Clean Up and as an Anti-Cancer Drug Carrier. Adv. Funct. Mater. 2020, 30, 2000112. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Fojtů, M.; Vyskočil, J.; Cho, N.J.; Pumera, M. Pollen-Based Magnetic Microrobots are Mediated by Electrostatic Forces to Attract, Manipulate, and Kill Cancer Cells. Adv. Funct. Mater. 2022, 32, 2207272. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, T.; Guo, Y.; Liao, Y.; Wu, Z.; Jiang, H.; Lu, Y. Biohybrid Magnetic Microrobots for Tumor Assassination and Active Tissue Regeneration. ACS Appl. Bio. Mater. 2022, 5, 5933–5942. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chan, K.F.; Zhang, Z.; Wang, L.; Wang, Q.; Yang, S.; Chan, S.M.; Chiu, P.W.Y.; Sung, J.J.Y.; Zhang, L. Magnetic Microswarm and Fluoroscopy-Guided Platform for Biofilm Eradication in Biliary Stents. Adv. Mater. 2022, 34, e2201888. [Google Scholar] [CrossRef]
- Kolipaka, T.; Khairnar, P.; Phatale, V.; Pandey, G.; Famta, P.; Shah, S.; Asthana, A.; Nanduri, S.; Raghuvanshi, R.S.; Srivastava, S. Multifaceted roles of pollen in the management of cancer. Int. J. Pharm. 2023, 643, 123278. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Gong, H.; Wei, F.; Zhuang, J.; Karshalev, E.; Esteban-Fernández de Ávila, B.; Huang, C.; Zhou, Z.; Li, Z.; et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 2020, 5, eaba6137. [Google Scholar] [CrossRef]
- Martín, M.; Carmona, F.; Cuesta, R.; Rondón, D.; Gálvez, N.; Domínguez-Vera, J.M. Magnets: Artificial Magnetic Bacteria: Living Magnets at Room Temperature. Adv. Funct. Mater. 2014, 24, 3488. [Google Scholar] [CrossRef]
- Contreras-Llano, L.E.; Liu, Y.-H.; Henson, T.; Meyer, C.C.; Baghdasaryan, O.; Khan, S.; Lin, C.-L.; Wang, A.; Hu, C.-M.J.; Tan, C. Engineering Cyborg Bacteria Through Intracellular Hydrogelation. Adv. Sci. 2023, 10, 2204175. [Google Scholar] [CrossRef]
- Gwisai, T.; Mirkhani, N.; Christiansen, M.G.; Nguyen, T.T.; Ling, V.; Schuerle, S. Magnetic torque–driven living microrobots for increased tumor infiltration. Sci. Robot. 2022, 7, eabo0665. [Google Scholar] [CrossRef]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.-W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Zhong Xu, Y.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yin, T.; Li, S.; Xu, T.; Ma, A.; Chen, Z.; Luo, Y.; Lai, Z.; Lv, Y.; Pan, H.; et al. Sequential Magneto-Actuated and Optics-Triggered Biomicrorobots for Targeted Cancer Therapy. Adv. Funct. Mater. 2020, 31, 2008262. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.; Feng, X.; Yu, C.; Feng, F.; Chai, Y.; Lu, P.; Song, T.; Wang, X.; Yao, L. Nanoparticle-Regulated Semiartificial Magnetotactic Bacteria with Tunable Magnetic Moment and Magnetic Sensitivity. Small 2019, 15, e1900427. [Google Scholar] [CrossRef]
- Sonnenborn, U.; Schulze, J. The non-pathogenicEscherichia colistrain Nissle 1917—features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar]
- Singh, A.V.; Hosseinidoust, Z.; Park, B.W.; Yasa, O.; Sitti, M. Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery. ACS Nano 2017, 11, 9759–9769. [Google Scholar] [CrossRef] [PubMed]
- Akolpoglu, M.B.; Alapan, Y.; Dogan, N.O.; Baltaci, S.F.; Yasa, O.; Aybar Tural, G.; Sitti, M. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci. Adv. 2022, 8, eabo6163. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Wang, Y.; Ning, P.; Shen, Y.; Wei, X.; Feng, Q.; Liu, Y.; Li, Z.; Xu, C.; et al. An Engineered Bacteria-Hybrid Microrobot with the Magnetothermal Bioswitch for Remotely Collective Perception and Imaging-Guided Cancer Treatment. ACS Nano 2022, 16, 6118–6133. [Google Scholar] [CrossRef] [PubMed]
- Schauer, O.; Mostaghaci, B.; Colin, R.; Hurtgen, D.; Kraus, D.; Sitti, M.; Sourjik, V. Motility and chemotaxis of bacteria-driven microswimmers fabricated using antigen 43-mediated biotin display. Sci. Rep. 2018, 8, 9801. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, T.; Li, S.; Feng, J.; Li, W.; Li, L.; Zhou, X.; Wang, M.; Li, F.; Zhao, X.; et al. Living Magnetotactic Microrobots Based on Bacteria with a Surface-Displayed CRISPR/Cas12a System for Penaeus Viruses Detection. ACS Appl. Mater. Interfaces 2023, 15, 47930–47938. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Aida, T. Swallowing a Surgeon: Toward Clinical Nanorobots. Acc. Chem. Res. 2017, 50, 492–497. [Google Scholar] [CrossRef]
- Kim, Y.; Genevriere, E.; Harker, P.; Choe, J.; Balicki, M.; Regenhardt, R.W.; Vranic, J.E.; Dmytriw, A.A.; Patel, A.B.; Zhao, X. Telerobotic neurovascular interventions with magnetic manipulation. Sci. Robot. 2022, 7, eabg9907. [Google Scholar] [CrossRef]
- Kim, Y.; Zhao, X. Magnetic Soft Materials and Robots. Chem. Rev. 2022, 122, 5317–5364. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yin, T.; Zhang, B.; Ma, A.; Kang, T.; He, Y.; Long, Y.; Zheng, S.; Pan, H.; Cai, L. Cell-based intelligent micro/nanorobots for precise regulation and active biotherapy. Matter 2023, 6, 4158–4194. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zhou, Q.; Geng, P.; Wang, B.; Zhou, Y.; Liu, K.; Peng, F.; Tu, Y. Biodegradability of Micro/Nanomotors: Challenges and Opportunities. Adv. Healthc. Mater. 2021, 10, e2100335. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Villa, K.; Krejčová, L.; Novotný, F.; Heger, Z.; Sofer, Z.; Pumera, M. Cooperative Multifunctional Self-Propelled Paramagnetic Microrobots with Chemical Handles for Cell Manipulation and Drug Delivery. Adv. Funct. Mater. 2018, 28, 1804343. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.; Gao, Y.; Cheng, X.; Liu, X.; Tang, S.; Peng, Y.; Wang, N.; Hu, D.; Peng, H.; et al. Biomimetic erythrocytes engineered drug delivery for cancer therapy. Chem. Eng. J. 2022, 433, 133498. [Google Scholar] [CrossRef]
- Heunis, C.; Sikorski, J.; Misra, S. Flexible Instruments for Endovascular Interventions: Improved Magnetic Steering, Actuation, and Image-Guided Surgical Instruments. IEEE Robot. Autom. Mag. 2018, 25, 71–82. [Google Scholar] [CrossRef]
- Koepele, C.A.; Guix, M.; Bi, C.; Adam, G.; Cappelleri, D.J. 3D-Printed Microrobots with Integrated Structural Color for Identification and Tracking. Adv. Intell. Syst. 2020, 2, 1900147. [Google Scholar] [CrossRef]
- Heunis, C.M.; Wotte, Y.P.; Sikorski, J.; Furtado, G.P.; Misra, S. The ARMM System—Autonomous Steering of Magnetically-Actuated Catheters: Towards Endovascular Applications. IEEE Robot. Autom. Lett. 2020, 5, 705–712. [Google Scholar] [CrossRef]
- Chen, P.M.; Pan, W.Y.; Luo, P.K.; Phung, H.N.; Liu, Y.M.; Chiang, M.C.; Chang, W.A.; Tien, T.L.; Huang, C.Y.; Wu, W.W.; et al. Pollen-Mimetic Metal-Organic Frameworks with Tunable Spike-Like Nanostructures That Promote Cell Interactions to Improve Antigen-Specific Humoral Immunity. ACS Nano 2021, 15, 7596–7607. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Potroz, M.G.; Park, S.; Shirahama, H.; Lee, J.H.; Seo, J.; Cho, N.J. Natural Sunflower Pollen as a Drug Delivery Vehicle. Small 2016, 12, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Chessa, L.; Leuzzi, V.; Plebani, A.; Soresina, A.; Micheli, R.; D’Agnano, D.; Venturi, T.; Molinaro, A.; Fazzi, E.; Marini, M.; et al. Intra-Erythrocyte Infusion of Dexamethasone Reduces Neurological Symptoms in Ataxia Teleangiectasia Patients: Results of a Phase 2 Trial. Orphanet J. Rare Dis. 2014, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, V.; Micheli, R.; D’Agnano, D.; Molinaro, A.; Venturi, T.; Plebani, A.; Soresina, A.; Marini, M.; Ferremi Leali, P.; Quinti, I.; et al. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e98. [Google Scholar] [CrossRef]


| Bio-Hybrid Magnetic Robots | Size | Drugs | Driven Force | Magnetic Decoration | Application | Velocity | Ref. |
|---|---|---|---|---|---|---|---|
| Rebuilt red blood cells (RRBCs) | ~20 μm | Mn-TPPS4, DOX | Permanent magnet | MNPs loading | MRI contrast imaging, therapeutic drug delivery | - | [35] |
| RBC-based micromotors | ~6–8 μm | Quantum dots/doxorubicin | Ultrasound | MNPs loading | Therapeutic and diagnostic agents | 15 ± 2 μm/s | [36] |
| Magnetically navigated red blood cell-mimicking (RBCM) micromotor | 2 μm | Photosensitizers | Ultrasound | MNPs loading | Photodynamic cancer therapy | 56.5 μm/s | [37] |
| RBC@magnetic mesoporous silica nanoparticles (MMSNs) | ~100 nm | Hypocrellin B | Permanent magnet | MNPs loading | Cancer therapy | - | [39] |
| DOX-loaded glucose/gluconic acid-coated magnetic nanoparticles | 91.2 ± 20.8 nm | Doxorubicin/glucose/gluconic acid | Permanent magnet | MNPs loading | Cancer therapy | - | [40] |
| Dual-responsive biohybrid neutrobots | ~105 nm | Paclitaxel | Electromagnetic system | MNPs loading | Active target delivery | 16.4 μm/s | [46] |
| Engineered magnetosomes | ~100 nm | PD-1 antibody/TGF- β inhibitor | Permanent magnet | No | Cancer therapy | - | [51] |
| Magnetic-propelled macrophage-based microrobots | ~90 nm | - | Electromagnetic system | MNPs loading | Cancer therapy | 25.9 μm/s | [53] |
| Macrophage template-based microrobots | 15–20 µm | - | Electromagnetic system | MNPs loading | Object transportation | - | [54] |
| M1 macrophage membrane-camouflaged magnetic nanorobots | 182 ± 3 nm | Doxorubicin/black phosphorus quantum dots | Electromagnetic system | MNPs loading | Cancer chemo-phototherapy | 10 μm/s | [58] |
| Micro-bio-robot | 50 μm × 5–8 μm | - | Permanent magnet | Loading | Micromanipulation | 10 μm/s | [65] |
| Sperm-Templated Microrobots | ~65 μm | - | ATP driven force | Electrostatic attraction | - | 15.6 ± 3.6 μm/s | [70] |
| Sperm-hybrid micromotor | 10 μm | Doxorubicin | ATP driven force | Loading | Targeted drug delivery | 41 ± 10 μm/s | [71] |
| IRONSperm | ~60 μm | Doxorubicin | Electromagnetic system | Electrostatic attraction | Targeted drug delivery | 6.8 ± 4.1 µm/s | [72] |
| Biohybrid nanoswimmers system (PBNs) | ~150 μm × 5 μm | - | Permanent magnet | Dip-coating | Radio-photodynamic therapy | 78.3 μm/s | [78] |
| Biohybrid magnetic robot (BMR) | ~150 μm × 5 μm | - | Electromagnetic system | Dip-coating | Fluorescence and MR imaging-guided therapy | 90 μm/s | [79] |
| MOF-based microrobot (MOFBOT) | - | Doxorubicin | Electromagnetic system | Dip-coating | photocatalytic degradation | - | [80] |
| Magnetic microswimmers | ~100 μm × 5 μm | - | Electromagnetic system | Dip-coating | Antibacterial therapy | - | [81] |
| Sunflower pollen-based BioBot (SFPµP-BioBots) | ~25 μm | Doxorubicin | Rotating magnetic field | Electrostatic attraction | Cancer therapy | 24.9 μm/s | [89] |
| Chrysanthemum pollen-derived biohybrid magnetic microrobots (CDBMRs) | ~30 μm | Doxorubicin | Rotating magnetic field | In situ deposition | Active drug delivery | - | [90] |
| Magnetic urchin-like capsule robots (MUCRs) | ~25 μm | L-aspartic acid | Electromagnetic system | Loading | Biofilm eradication | 2 mm/s | [91] |
| Magnetococcus marinus strain MC-1 | 1–2 μm | Drug-containing nanoliposomes | Electromagnetic system | No | Active drug delivery | - | [100] |
| Magnetospirillum magneticum (AMB-1) | ~2 μm × 0.5 μm | Indocyanine green nanoparticles | Electromagnetic system | No | Targeted cancer therapy | 13.3 ± 4.5 μm/s | [101] |
| Semiartificial magnetotactic bacteria (SAMTB) | ~3 μm × 0.5 μm | - | Electromagnetic system | In situ deposition | Cargo-transportation | - | [102] |
| Bacterial biohybrid microrobots | - | Doxorubicin and indocyanine green | Electromagnetic system | Biotin-streptavidin binding | Stimuli-responsive cargo delivery | 18.5 μm/s | [105] |
| Biohybrid microrobot | ~5 μm | - | Rotating magnetic field | Amide bonds | Imaging-guided cancer treatment | 25 μm/s | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zeng, Y.; Zhao, Y.; Peng, X.; Ren, E.; Liu, G. Bio-Hybrid Magnetic Robots: From Bioengineering to Targeted Therapy. Bioengineering 2024, 11, 311. https://doi.org/10.3390/bioengineering11040311
Zhang Q, Zeng Y, Zhao Y, Peng X, Ren E, Liu G. Bio-Hybrid Magnetic Robots: From Bioengineering to Targeted Therapy. Bioengineering. 2024; 11(4):311. https://doi.org/10.3390/bioengineering11040311
Chicago/Turabian StyleZhang, Qian, Yun Zeng, Yang Zhao, Xuqi Peng, En Ren, and Gang Liu. 2024. "Bio-Hybrid Magnetic Robots: From Bioengineering to Targeted Therapy" Bioengineering 11, no. 4: 311. https://doi.org/10.3390/bioengineering11040311
APA StyleZhang, Q., Zeng, Y., Zhao, Y., Peng, X., Ren, E., & Liu, G. (2024). Bio-Hybrid Magnetic Robots: From Bioengineering to Targeted Therapy. Bioengineering, 11(4), 311. https://doi.org/10.3390/bioengineering11040311








