Near-Infrared Spectroscopy Regional Oxygen Saturation Based Cerebrovascular Reactivity Assessments in Chronic Traumatic Neural Injury versus in Health: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Ethical Consideration
2.3. Data Processing
2.4. Statistical Analysis
3. Results
3.1. Subject Demographics
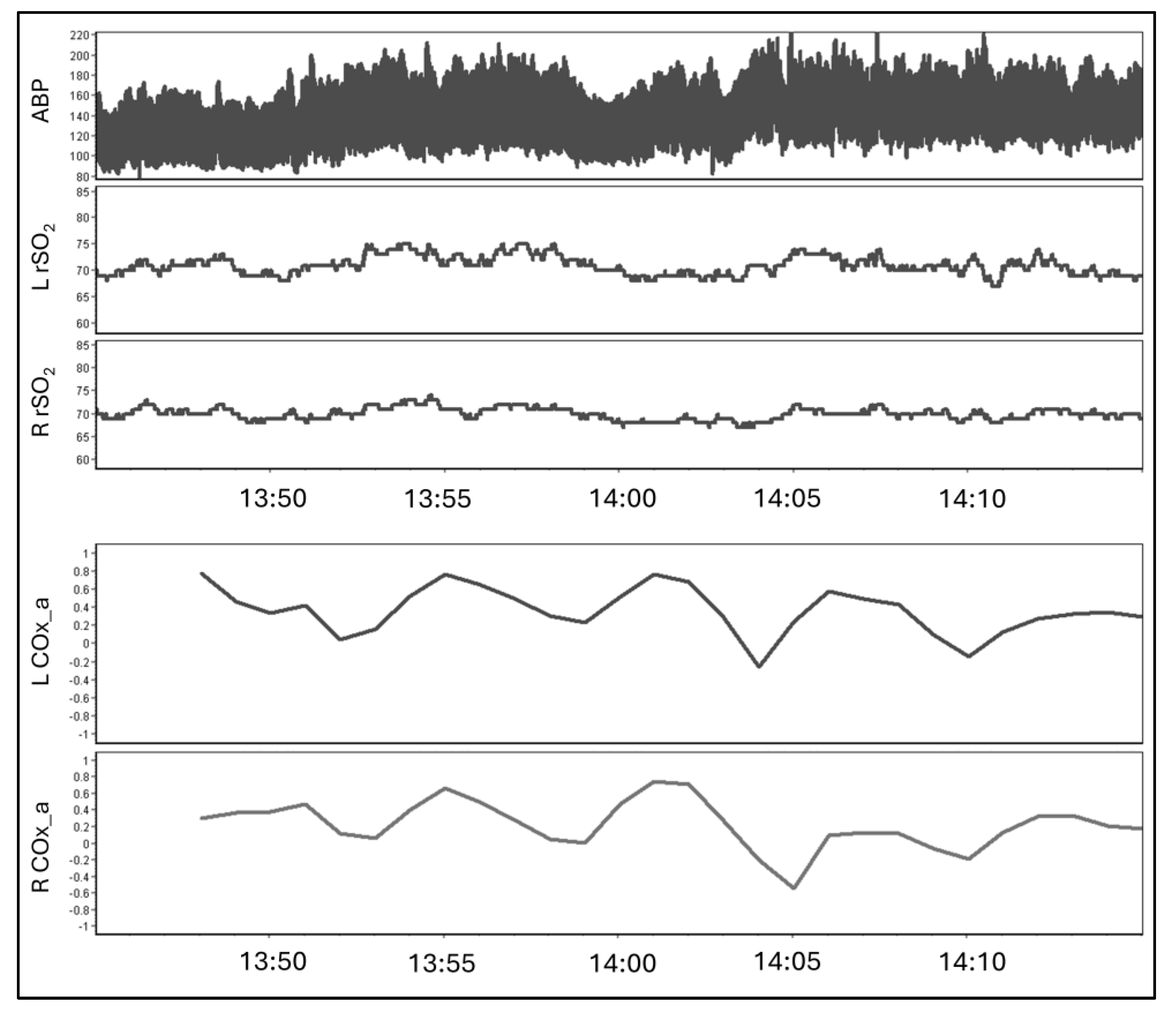
3.2. Healthy Volunteers
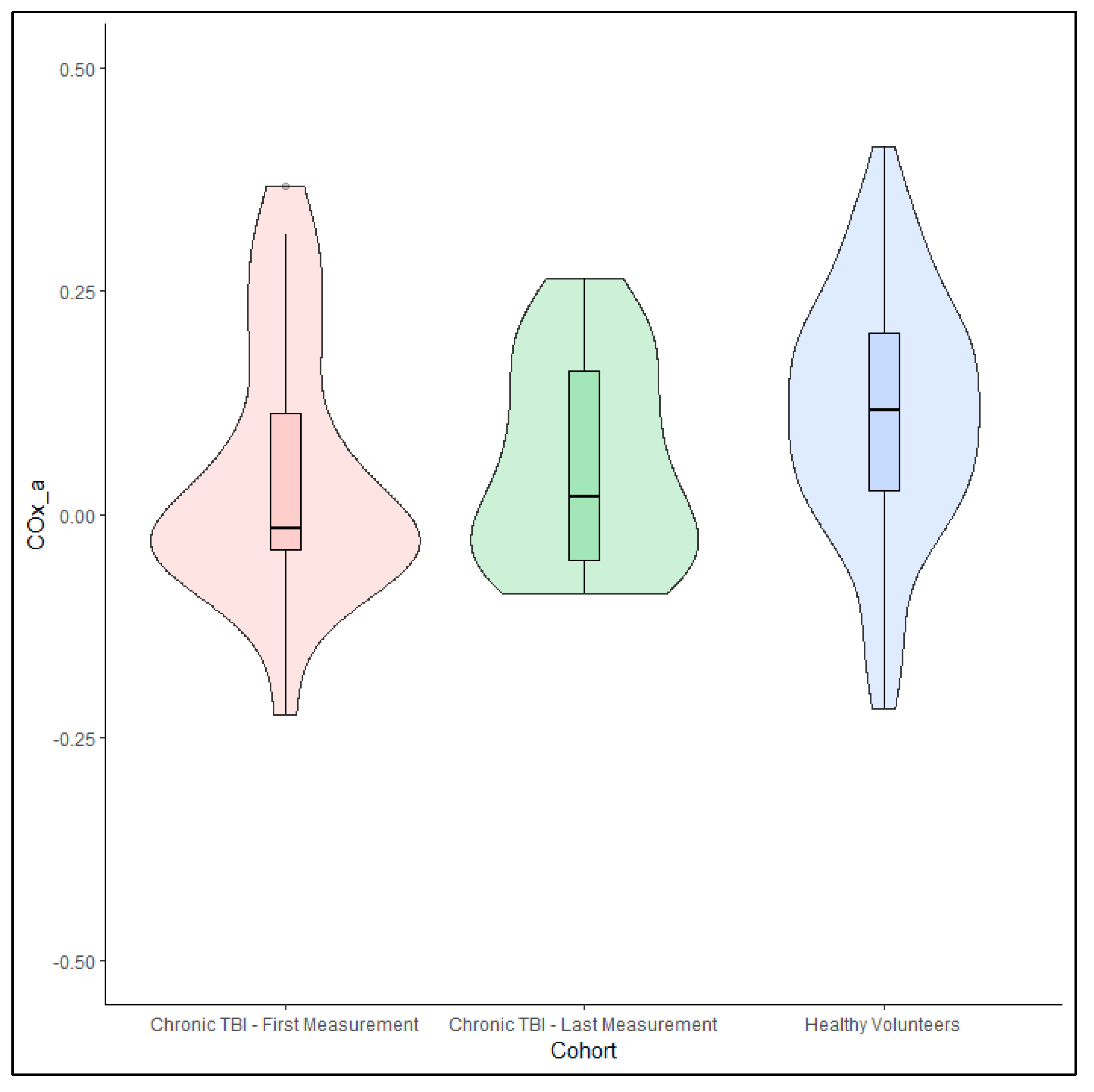
3.3. Chronic Traumatic Brain Injury Patients
4. Discussion
4.1. Study Limitations
4.2. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A Management Algorithm for Adult Patients with Both Brain Oxygen and Intracranial Pressure Monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castilla, L.; Gasco, J.; Nauta, H.J.W.; Okonkwo, D.O.; Robertson, C.S. Cerebral Pressure Autoregulation in Traumatic Brain Injury. Neurosurg. Focus. 2008, 25, E7. [Google Scholar] [CrossRef]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity after Traumatic Brain Injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Piechnik, S.; Steiner, L.A.; Pickard, J.D. Cerebral Autoregulation Following Head Injury. J. Neurosurg. 2001, 95, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Budohoski, K.P.; Czosnyka, M.; de Riva, N.; Smielewski, P.; Pickard, J.D.; Menon, D.K.; Kirkpatrick, P.J.; Lavinio, A. The Relationship Between Cerebral Blood Flow Autoregulation and Cerebrovascular Pressure Reactivity after Traumatic Brain Injury. Neurosurgery 2012, 71, 652–661. [Google Scholar] [CrossRef]
- Steiner, L.A.; Czosnyka, M.; Piechnik, S.K.; Smielewski, P.; Chatfield, D.; Menon, D.K.; Pickard, J.D. Continuous Monitoring of Cerebrovascular Pressure Reactivity Allows Determination of Optimal Cerebral Perfusion Pressure in Patients with Traumatic Brain Injury. Crit. Care Med. 2002, 30, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Petkus, V.; Preiksaitis, A.; Chaleckas, E.; Chomskis, R.; Zubaviciute, E.; Vosylius, S.; Rocka, S.; Rastenyte, D.; Aries, M.J.; Ragauskas, A.; et al. Optimal Cerebral Perfusion Pressure: Targeted Treatment for Severe Traumatic Brain Injury. J. Neurotrauma 2020, 37, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tas, J.; Beqiri, E.; van Kaam, R.C.; Czosnyka, M.; Donnelly, J.; Haeren, R.H.; van der Horst, I.C.C.; Hutchinson, P.J.; van Kuijk, S.M.J.; Liberti, A.L.; et al. Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury (COGiTATE): A Feasibility Randomized Controlled Clinical Trial. J. Neurotrauma 2021, 38, 2790–2800. [Google Scholar] [CrossRef]
- Gomez, A.; Dian, J.; Froese, L.; Zeiler, F.A. Near-Infrared Cerebrovascular Reactivity for Monitoring Cerebral Autoregulation and Predicting Outcomes in Moderate to Severe Traumatic Brain Injury: Proposal for a Pilot Observational Study. JMIR Res. Protoc. 2020, 9, e18740. [Google Scholar] [CrossRef]
- Gomez, A.; Dian, J.; Zeiler, F.A. Continuous and Entirely Non-Invasive Method for Cerebrovascular Reactivity Assessment: Technique and Implications. J. Clin. Monit. Comput. 2020, 35, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using near-Infrared Spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Froese, L.; Bergmann, T.J.G.; Sainbhi, A.S.; Vakitbilir, N.; Islam, A.; Stein, K.Y.; Marquez, I.; Ibrahim, Y.; Zeiler, F.A. Non-Invasive Estimation of Intracranial Pressure-Derived Cerebrovascular Reactivity Using Near-Infrared Spectroscopy Sensor Technology in Acute Neural Injury: A Time-Series Analysis. Sensors 2024, 24, 499. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Sainbhi, A.S.; Stein, K.Y.; Vakitbilir, N.; Froese, L.; Zeiler, F.A. Statistical Properties of Cerebral near Infrared and Intracranial Pressure-Based Cerebrovascular Reactivity Metrics in Moderate and Severe Neural Injury: A Machine Learning and Time-Series Analysis. Intensiv. Care Med. Exp. 2023, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.J.; Su, Z.; Clancy, M.T.; Lucas, S.J.E.; Dehghani, H.; Logan, A.; Belli, A. Near-Infrared Spectroscopy in the Monitoring of Adult Traumatic Brain Injury: A Review. J. Neurotrauma 2015, 32, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Moerman, A.; Vandenplas, G.; Bové, T.; Wouters, P.F.; De Hert, S.G. Relation between Mixed Venous Oxygen Saturation and Cerebral Oxygen Saturation Measured by Absolute and Relative Near-Infrared Spectroscopy during off-Pump Coronary Artery Bypass Grafting. Br. J. Anaesth. 2013, 110, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W.; Levine, A.; Hudson, A.; Lewis, C. Clinical Applications of Near-Infrared Spectroscopy Monitoring in Cardiovascular Surgery. Anesthesiology 2021, 134, 784–791. [Google Scholar] [CrossRef]
- Howells, T.; Johnson, U.; McKelvey, T.; Enblad, P. An Optimal Frequency Range for Assessing the Pressure Reactivity Index in Patients with Traumatic Brain Injury. J. Clin. Monit. Comput. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.D.; Brady, K.M.; Rhee, C.J.; Easley, R.B.; Kibler, K.; Smielewski, P.; Czosnyka, M.; Kaczka, D.W.; Andropoulos, D.B.; Rusin, C. The Frequency Response of Cerebral Autoregulation. J. Appl. Physiol. 2013, 115, 52–56. [Google Scholar] [CrossRef]
- Lang, E.W.; Yip, K.; Griffith, J.; Lagopoulos, J.; Mudaliar, Y.; Dorsch, N.W. Hemispheric Asymmetry and Temporal Profiles of Cerebral Pressure Autoregulation in Head Injury. J. Clin. Neurosci. 2003, 10, 670–673. [Google Scholar] [CrossRef]
- Vavilala, M.S.; Tontisirin, N.; Udomphorn, Y.; Armstead, W.; Zimmerman, J.J.; Chesnut, R.; Lam, A.M. Hemispheric Differences in Cerebral Autoregulation in Children with Moderate and Severe Traumatic Brain Injury. Neurocrit. Care 2008, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Hutchinson, P.J.; Balestreri, M.; Hiler, M.; Smielewski, P.; Pickard, J.D. Monitoring and Interpretation of Intracranial Pressure after Head Injury. Acta Neurochir. Suppl. 2006, 96, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Radolovich, D.; Balestreri, M.; Lavinio, A.; Hutchinson, P.; Timofeev, I.; Smielewski, P.; Pickard, J.D. Gender-Related Differences in Intracranial Hypertension and Outcome after Traumatic Brain Injury. Acta Neurochir. Suppl. 2008, 102, 25–28. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Donnelly, J.; Nourallah, B.; Thelin, E.P.; Calviello, L.; Smielewski, P.; Czosnyka, M.; Ercole, A.; Menon, D.K. Intracranial and Extracranial Injury Burden as Drivers of Impaired Cerebrovascular Reactivity in Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1569–1577. [Google Scholar] [CrossRef]
- Batson, C.; Froese, L.; Gomez, A.; Sainbhi, A.S.; Stein, K.Y.; Alizadeh, A.; Zeiler, F.A. Impact of Age and Biological Sex on Cerebrovascular Reactivity in Adult Moderate/Severe Traumatic Brain Injury: An Exploratory Analysis. Neurotrauma Rep. 2021, 2, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Batson, C.; Froese, L.; Sekhon, M.; Griesdale, D.; Gomez, A.; Thelin, E.P.; Raj, R.; Aries, M.; Gallagher, C.; Bernard, F.; et al. Impact of Chronological Age and Biological Sex on Cerebrovascular Reactivity in Moderate/Severe Traumatic Brain Injury: A CAnadian High-Resolution Traumatic Brain Injury (CAHR-TBI) Study. J. Neurotrauma 2022, 40, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Balestreri, M.; Steiner, L.; Smielewski, P.; Hutchinson, P.J.; Matta, B.; Pickard, J.D. Age, Intracranial Pressure, Autoregulation, and Outcome after Brain Trauma. J. Neurosurg. 2005, 102, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Donnelly, J.; Czosnyka, M.; Aries, M.J.H.; Brady, K.; Cardim, D.; Robba, C.; Cabeleira, M.; Kim, D.-J.; Haubrich, C.; et al. Cerebrovascular Pressure Reactivity Monitoring Using Wavelet Analysis in Traumatic Brain Injury Patients: A Retrospective Study. PLoS Med. 2017, 14, e1002348. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.J.H.; Czosnyka, M.; Budohoski, K.P.; Kolias, A.G.; Radolovich, D.K.; Lavinio, A.; Pickard, J.D.; Smielewski, P. Continuous Monitoring of Cerebrovascular Reactivity Using Pulse Waveform of Intracranial Pressure. Neurocrit. Care 2012, 17, 67–76. [Google Scholar] [CrossRef]
- Hiler, M.; Czosnyka, M.; Hutchinson, P.; Balestreri, M.; Smielewski, P.; Matta, B.; Pickard, J.D. Predictive Value of Initial Computerized Tomography Scan, Intracranial Pressure, and State of Autoregulation in Patients with Traumatic Brain Injury. J. Neurosurg. 2006, 104, 731–737. [Google Scholar] [CrossRef]
- Radolovich, D.K.; Aries, M.J.H.; Castellani, G.; Corona, A.; Lavinio, A.; Smielewski, P.; Pickard, J.D.; Czosnyka, M. Pulsatile Intracranial Pressure and Cerebral Autoregulation after Traumatic Brain Injury. Neurocrit. Care 2011, 15, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Yam, A.T.; Lang, E.W.; Lagopoulos, J.; Yip, K.; Griffith, J.; Mudaliar, Y.; Dorsch, N.W.C. Cerebral Autoregulation and Ageing. J. Clin. Neurosci. 2005, 12, 643–646. [Google Scholar] [CrossRef]
- Ding, K.; Tarumi, T.; Tomoto, T.; Mccolloster, M.; Le, T.; Dieppa, M.; Diaz-Arrastia, R.; Bell, K.; Madden, C.; Cullum, C.M.; et al. Impaired Cerebral Blood Flow Regulation in Chronic Traumatic Brain Injury. Brain Res. 2020, 1743, 146924. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.S. A Review of Wavelet Transform Time-Frequency Methods for NIRS-Based Analysis of Cerebral Autoregulation. IEEE Rev. Biomed. Eng. 2015, 8, 78–85. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Piechnik, S.; Laing, R.; Pickard, J.D. Continuous Monitoring of Cerebrovascular Pressure-Reactivity in Head Injury. Acta Neurochir. Suppl. 1998, 71, 74–77. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Froese, L.; Gomez, A.; Marquez, I.; Amenta, F.; Batson, C.; Stein, K.Y.; Zeiler, F.A. High Spatial and Temporal Resolution Cerebrovascular Reactivity for Humans and Large Mammals: A Technological Description of Integrated fNIRS and niABP Mapping System. Front. Physiol. 2023, 14, 1124268. [Google Scholar] [CrossRef]



| Demographic Parameter | Median or Number of Patients N = 101 | |
|---|---|---|
| Age (IQR) | 26 (22 to 31) | |
| Biologic Sex | Male subjects (%) | 41 (40.6) |
| Female subjects (%) | 60 (59.4) | |
| Handedness | Right (%) | 92 (91.8) |
| Left (%) | 9 (8.9) | |
| Subject Average COx_a (IQR) | 0.12 (0.03 to 0.20) | |
| Subject Average ABP (IQR) | 101.5 (83.8 to 105.5) mmHg | |
| Subject Average rSO2 (IQR) | Right | 72.6 (66.2 to 79.1)% |
| Left | 73.7 (69.6 to 79.0)% | |
| Demographic Parameter | Median or Number of Patients N = 29 | |
|---|---|---|
| Age (IQR) | 40 (25 to 50) | |
| Biologic Sex | Male subjects (%) | 24 (82.8) |
| Female subjects (%) | 5 (17.2) | |
| Admission GCS (IQR) | 7 (4 to 8) | |
| Admission Pupils | Bilaterally reactive (%) | 22 (81.5) |
| Unilaterally reactive (%) | 5 (17.2) | |
| Bilaterally unreactive (%) | 1 (3.4) | |
| Cranial Surgery Side | Right | 7 |
| Left | 6 | |
| None | 16 | |
| Marshall CT Classification | I (%) | 0 (0) |
| II (%) | 1 (3.4) | |
| III (%) | 11 (37.9) | |
| IV (%) | 6 (20.7) | |
| V (%) | 11 (37.9) | |
| VI (%) | 0 (0) | |
| Follow-up GOSE | 3-Month | 6 (5 to6) |
| 6-Month | 7 (5 to7) | |
| 12-Month | 1 (5) | |
| Recording Sessions | 3-Month | 23 |
| 6-Month | 24 | |
| 12-Month | 18 | |
| Subject Average COx_a (IQR) | 3-Month | −0.02 (−0.04 to 0.06) |
| 6-Month | 0.05 (−0.06 to 0.17) | |
| 12-Month | 0.02 (−0.04 to 0.11) | |
| Subject Average ABP (IQR) | 93.5 (83.8 to105.5) mmHg | |
| Subject Average rSO2 (IQR) | Right | 69.0 (64.6 to73.9)% |
| Left | 70.1 (65.5 to 73.7)% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, A.; Marquez, I.; Froese, L.; Bergmann, T.; Sainbhi, A.S.; Vakitbilir, N.; Islam, A.; Stein, K.Y.; Ibrahim, Y.; Zeiler, F.A. Near-Infrared Spectroscopy Regional Oxygen Saturation Based Cerebrovascular Reactivity Assessments in Chronic Traumatic Neural Injury versus in Health: A Prospective Cohort Study. Bioengineering 2024, 11, 310. https://doi.org/10.3390/bioengineering11040310
Gomez A, Marquez I, Froese L, Bergmann T, Sainbhi AS, Vakitbilir N, Islam A, Stein KY, Ibrahim Y, Zeiler FA. Near-Infrared Spectroscopy Regional Oxygen Saturation Based Cerebrovascular Reactivity Assessments in Chronic Traumatic Neural Injury versus in Health: A Prospective Cohort Study. Bioengineering. 2024; 11(4):310. https://doi.org/10.3390/bioengineering11040310
Chicago/Turabian StyleGomez, Alwyn, Izabella Marquez, Logan Froese, Tobias Bergmann, Amanjyot Singh Sainbhi, Nuray Vakitbilir, Abrar Islam, Kevin Y. Stein, Younis Ibrahim, and Frederick A. Zeiler. 2024. "Near-Infrared Spectroscopy Regional Oxygen Saturation Based Cerebrovascular Reactivity Assessments in Chronic Traumatic Neural Injury versus in Health: A Prospective Cohort Study" Bioengineering 11, no. 4: 310. https://doi.org/10.3390/bioengineering11040310
APA StyleGomez, A., Marquez, I., Froese, L., Bergmann, T., Sainbhi, A. S., Vakitbilir, N., Islam, A., Stein, K. Y., Ibrahim, Y., & Zeiler, F. A. (2024). Near-Infrared Spectroscopy Regional Oxygen Saturation Based Cerebrovascular Reactivity Assessments in Chronic Traumatic Neural Injury versus in Health: A Prospective Cohort Study. Bioengineering, 11(4), 310. https://doi.org/10.3390/bioengineering11040310






