Biohydrogen Production from Waste Black Cumin (Nigella Sativa) Extract Liquid
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioreactors and Operation
2.1.1. Completely Stirred Tank Reactor (CSTR) Fed with Waste Black Cumin Extract Liquid
2.1.2. Fluidized Bed Reactor (FBR) Fed with Waste Black Cumin Extract Liquid
2.1.3. Batch Reactors Fed with Waste Black Cumin Extract Liquid
2.2. Analytical Methods
3. Results and Discussion
3.1. The Impact of Hydraulic Retention Time (HRT) and pH on Gas Manufacturing in a CSTR
3.2. The Impact of HRT and pH on Gas Manufacturing in an FBR
3.3. Batch Reactors
3.4. Inoculum Content in the CSTR and FBR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddik, A.; Islam, T.; Zaman, A.K.M.M.; Hasan, M. Current status and correlation of fossil fuels consumption and greenhouse gas emissions. Int. J. Energy Environ. Econ. 2021, 28, 103–119. [Google Scholar]
- Das, D.; Veziroglu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Demirbaş, A. Hydrogen production from biomass by the gasification process. Energy Source 2002, 24, 59–68. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Yields of hydrogen-rich gaseous products via pyrolysis from selected biomass samples. Fuel 2001, 80, 1885–1891. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Kothari, R.; Singh, D.P.; Tyagi, V.V.; Tyagi, S.K. Fermentative hydrogen production—An alternative clean energy source. Renew. Sustain. Energy Rev. 2012, 16, 2337–2346. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.-H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Toth, G.; Nemestothy, N.; Belafi-Bako, K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrogen Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Mota, V.T.; Ferraz Júnior, A.D.N.; Trably, E.; Zaiat, M. Biohydrogen production at pH below 3.0: Is it possible? Water Res. 2018, 128, 350–361. [Google Scholar] [CrossRef]
- Ren, N.-Q.; Guo, W.-Q.; Wang, X.-J.; Xiang, W.-S.; Liu, B.-F.; Wang, X.-Z.; Ding, J.; Chen, Z.-B. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Khanna, N.; Das, D. Biohydrogen production by dark fermentation. Wires Energy Environ. 2013, 2, 401–421. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, C.; Fan, C. Enhancement of solubility and biohydrogen production from sewage sludge with lime mud filtrate. Water Air Soil Poll. 2018, 229, 129. [Google Scholar] [CrossRef]
- Cheng, J.-R.; Zhu, M.-J. Biohydrogen production from pretreated lignocellulose by Clostridium thermocellum. Biotechnol. Bioproc. E 2016, 21, 87–94. [Google Scholar] [CrossRef]
- Skonieczny, M.T.; Yargeau, V. Biohydrogen production from wastewater by Clostridium beijerinckii: Effect of pH and substrate concentration. Int. J. Hydrogen Energy 2009, 34, 3288–3294. [Google Scholar] [CrossRef]
- Dada, O.; Yusoff, W.M.W.; Kalil, M.S. Biohydrogen production from ricebran using Clostridium saccharoperbutylacetonicum N1-4. Int. J. Hydrogen Energy 2013, 38, 15063–15073. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Tibin, E.-M.; Ali, E.; Hamid, A.A.; Yusoff, W.M.W.; Kalil, M.S. Biohydrogen production from agroindustrial wastes via Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). Clean Technol. Environ. 2014, 16, 11–21. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate wastewater dark fermentation: The effect of temperature and initial pH on biohydrogen production and microbial community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Wong, Y.M.; Show, P.L.; Wu, T.Y.; Leong, H.Y.; Ibrahim, S.; Juan, J.C. Production of bio-hydrogen from dairy wastewater using pretreated landfill leachate sludge as an inoculum. J. Biosci. Bioeng. 2019, 127, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Lin, C.-Y. Biohydrogen production from beverage wastewater using selectively enriched mixed culture. Waste Biomass Valori. 2020, 11, 1049–1058. [Google Scholar] [CrossRef]
- Kirankumar, P.; Krishna, S.V.; Chaitanya, N.; Bhagawan, D.; Himabindu, V.; Narasu, M.L. Effect of operational parameters on biohydrogen production from dairy wastewater in batch and continuous reactors. Biofuels 2017, 8, 693–699. [Google Scholar] [CrossRef]
- Krishna, S.V.; Kiran Kumar, P.; Chaitanya, N.; Bhagawan, D.; Himabindu, V.; Narasu, M.L. Biohydrogen production from brewery effluent in a batch and continuous reactor with anaerobic mixed microbial consortia. Biofuels 2017, 8, 701–707. [Google Scholar] [CrossRef]
- Wongthanate, J.; Chinnacotpong, K.; Khumpong, M. Impacts of pH, temperature, and pretreatment method on biohydrogen production from organic wastes by sewage microflora. Int. J. Energy Environ. Eng. 2014, 5, 6. [Google Scholar] [CrossRef]
- Sivaramakrishna, D.; Sreekanth, D.; Sivaramakrishnan, M.; Kumar, B.S.; Himabindu, V.; Narasu, M.L. Effect of system optimizing conditions on biohydrogen production from herbal wastewater by slaughterhouse sludge. Int. J. Hydrogen Energy 2014, 39, 7526–7533. [Google Scholar] [CrossRef]
- Pachiega, R.; Rodrigues, M.F.; Rodrigues, C.V.; Sakamoto, I.K.; Varesche, M.B.A.; De Oliveira, J.E.; Maintinguer, S.I. Hydrogen bioproduction with anaerobic bacteria consortium from brewery wastewater. Int. J. Hydrogen Energy 2019, 44, 155–163. [Google Scholar] [CrossRef]
- Torquato, L.D.M.; Pachiega, R.; Crespi, M.S.; Nespeca, M.G.; de Oliveira, J.E.; Maintinguer, S.I. Potential of biohydrogen production from effluents of citrus processing industry using anaerobic bacteria from sewage sludge. Waste Manag. 2017, 59, 181–193. [Google Scholar] [CrossRef]
- Ali, M.A.; Sayeed, M.A.; Alam, M.S.; Yeasmin, M.S.; Khan, A.M.; Muhamad, I.I. Characteristics of oils and nutrient contents of Nigella sativa Lınn. and Trigonella Foenum-Graecum seeds. Bull. Chem. Soc. Ethiop. 2012, 26, 55–64. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical characteristics of Nigella Seed (Nigella sativa L.) oil as affected by different extraction methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Javed, S.; Shahid, A.A.; Haider, M.S.; Umeera, A.; Ahmad, R.; Mushtaq, S. Nutritional, phytochemical potential and pharmacological evaluation of Nigella sativa (Kalonji) and Trachyspermum ammi (Ajwain). J. Med. Plants Res. 2012, 6, 768–775. [Google Scholar]
- Fang, H.H.P.; Li, C.; Zhang, T. Acidophilic biohydrogen production from rice slurry. Int. J. Hydrogen Energy 2006, 31, 683–692. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Dursun, N.; Gülşen, H. Evaluation of industrial waste black cumin (Nigella sativa) for biohydrogen production without pretreatment. Environ. Dev. Sustain. 2022, 24, 12182–12202. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skıadas, I.V.; Lyberatos, G. Influence of pH on fermentative hydrogen production from sweet sorghum extract. Int. J. Hydrogen Energy 2010, 35, 1921–1928. [Google Scholar] [CrossRef]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of pre-treatment and hydraulic retention time on biohydrogen production from organic wastes. Int. J. Hydrogen Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Fan, K.-S.; Kan, N.-R.; Lay, J.-J. Effect of hydraulic retention time on anaerobic hydrogenesis in CSTR. Bioresour. Technol. 2006, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hung, C.-H.; Lin, C.-Y.; Lin, P.-J.; Lee, K.-S.; Lin, C.-N.; Chang, F.-Y.; Chang, J.-S. HRT-dependent hydrogen production and bacterial community structure of mixed anaerobic microflora in suspended, granular and immobilized sludge systems using glucose as the carbon substrate. Int. J. Hydrogen Energy 2008, 33, 1542–1549. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef]
- Han, H.; Wei, L.; Liu, B.; Yang, H.; Shen, J. Optimization of biohydrogen production from soybean straw using anaerobic mixed bacteria. Int. J. Hydrogen Energy 2012, 37, 13200–13208. [Google Scholar] [CrossRef]
- Hung, C.-H.; Lee, K.-S.; Cheng, L.-H.; Huang, Y.-H.; Lin, P.-J.; Chang, J.-S. Quantitative analysis of a high-rate hydrogen-producing microbial community in anaerobic agitated granular sludge bed bioreactors using glucose as substrate. Appl. Microbiol. Biot. 2007, 75, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Peintner, C.; Zeidan, A.A.; Schnitzhofer, W. Bioreactor systems for thermophilic fermentative hydrogen production: Evaluation and comparison of appropriate systems. J. Clean. Prod. 2010, 18, S15–S22. [Google Scholar] [CrossRef]
- Barros, A.R.; Adorno, M.A.T.; Sakamoto, I.K.; Maintinguer, S.I.; Varesche, M.B.A.; Silva, E.L. Performance evaluation and phylogenetic characterization of anaerobic fluidized bed reactors using ground tire and pet as support materials for biohydrogen production. Bioresour. Technol. 2011, 102, 3840–3847. [Google Scholar] [CrossRef]
- Silva-Illanes, F.; Tapia-Venegas, E.; Schiappacasse, M.C.; Trably, E.; Ruiz-Filippi, G. Impact of hydraulic retention time (HRT) and pH on dark fermentative hydrogen production from glycerol. Energy 2017, 141, 358–367. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skıadas, I.V.; Lyberatos, G. Effect of substrate concentration on fermentative hydrogen production from sweet sorghum extract. Int. J. Hydrogen Energy 2011, 36, 4843–4851. [Google Scholar] [CrossRef]
- Buitrón, G.; Carvajal, C. Biohydrogen production from Tequila vinasses in an anaerobic sequencing batch reactor: Effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 2010, 101, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Amorim, N.C.S.; Alves, I.; Martins, J.S.; Amorim, E.L.C. Biohydrogen Production from Cassava Wastewater in an Anaerobic Fluidized Bed Reactor. Braz. J. Chem. Eng. 2014, 31, 603–612. [Google Scholar] [CrossRef]
- Wadjeam, P.; Reungsang, A.; Imai, T.; Plangklang, P. Co-digestion of cassava starch wastewater with buffalo dung for bio-hydrogen production. Int. J. Hydrogen Energy 2019, 44, 14694–14706. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Tawfik, A.; Qelish, M.E.; Salem, A. Efficient anaerobic co-digestion of municipal food waste and kitchen wastewater for bio-hydrogen production. Int. J. Green Energy 2015, 12, 1301–1308. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, S.Y.; Khanal, S.K.; Sung, S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Gomez, X.; Moran, A.; Cuetos, M.J.; Sanchez, M.E. The production of hydrogen by dark fermentation of municipal solid wastes and slaughterhouse waste: A two-phase process. J. Power Sources 2006, 157, 727–732. [Google Scholar] [CrossRef]
- Guo, W.Q.; Ren, N.Q.; Chen, Z.B.; Liu, B.F.; Wang, X.J.; Xiang, W.S.; Ding, J. Simultaneous biohydrogen production and starch wastewater treatment in an acidogenic expanded granular sludge bed reactor by mixed culture for long-term operation. Int. J. Hydrogen Energy 2008, 33, 7397–7404. [Google Scholar] [CrossRef]
- Mohan, S.V.; Babu, V.L.; Sarma, P.N. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.M.; Zeng, G.M.; Zhou, Y. Effective hydrogen production using waste sludge and its filtrate. Energy 2010, 35, 3557–3562. [Google Scholar] [CrossRef]
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium Butyricum. Int. J. Hydrogen Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Mars, A.E.; Veuskens, T.; Budde, M.A.W.; Van Doeveren, P.F.N.M.; Lips, S.J.; Bakker, R.R.; Vrije, T.D.; Claassen, P.A.M. Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme Thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 7730–7737. [Google Scholar] [CrossRef]
- Show, K.-Y.; Zhang, Z.-P.; Tay, J.-H.; Liang, D.T.; Lee, D.-J.; Jiang, W.-J. Production of hydrogen in a granular sludge-based anaerobic continuous stirred tank reactor. Int. J. Hydrogen Energy 2007, 32, 4744–4753. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Razo-Flores, E.; Celis, L.B.; Alatriste-Mondragón, F. Sequential hydrolysis of oat straw and hydrogen production from hydrolysates: Role of hydrolysates constituents. Int. J. Hydrogen Energy 2015, 40, 10756–10765. [Google Scholar] [CrossRef]
- Poontaweegeratigarn, T.; Chavadej, S.; Rangsunvigit, P. Hydrogen production from alcohol wastewater by upflow anaerobic sludge blanket reactors under mesophilic temperature. Int. J. Chem. Biol. Eng. 2012, 6, 305–308. [Google Scholar]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Sohaili, J. Effect of organic loading rate on hydrogen (H2) and methane (CH4) production in two-stage fermentation under thermophilic conditions using palm oil mill effluent (POME). Energy Sustain. Dev. 2016, 34, 130–138. [Google Scholar] [CrossRef]
- Sattar, A.; Arslan, C.; Ji, C.; Sattar, S.; Umair, M.; Sattar, S.; Zia Bakht, M. Quantification of temperature effect on batch production of bio-hydrogen from rice crop wastes in an anaerobic bio reactor. Int. J. Hydrogen Energy 2016, 41, 11050–11061. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Sung, S.; Lay, J.-J. Biohydrogen production as a function of pH and substrate concentration. Environ. Sci. Technol. 2001, 35, 4726–4730. [Google Scholar] [CrossRef]
- Van Ginkel, S.V.; Oh, S.-E.; Logan, B.E. Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy 2005, 30, 1535–1542. [Google Scholar] [CrossRef]
- Lee, K.S.; Tseng, T.S.; Liu, Y.W.; Hsiao, Y.D. Enhancing the performance of dark fermentative hydrogen production using a reduced pressure fermentation strategy. Int. J. Hydrogen Energy 2012, 37, 15556–15562. [Google Scholar] [CrossRef]
- Chu, C.F.; Li, Y.Y.; Xu, K.Q.; Ebie, Y.; Inamori, Y.; Kong, H.N. A pH-and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrogen Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Christine Santos, S.; Ferreira Rosa, P.R.; Sakamoto, I.K.; Amancio Varesche, M.B.; Silva, E.L. Continuous thermophilic hydrogen production and microbial community analysis from anaerobic digestion of diluted sugar cane stillage. Int. J. Hydrogen Energy 2014, 39, 9000–9011. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Cota-Navarro, C.B.; Rosales-Colunga, L.M.; León-Rodríguez, A.D.; Razo-Flores, E. Continuous biohydrogen production using cheese whey: Improving the hydrogen production rate. Int. J. Hydrogen Energy 2009, 34, 4296–4304. [Google Scholar] [CrossRef]
- Gadow, S.I.; Jiang, H.; Hojo, T.; Li, Y.Y. Cellulosic hydrogen production and microbial community characterization in hyper-thermophilic continuous bioreactor. Int. J. Hydrogen Energy 2013, 38, 7259–7267. [Google Scholar] [CrossRef]
- Zhu, G.-F.; Wu, P.; Wei, Q.-S.; Lin, J.-Y.; Gao, Y.-L.; Liu, H.-N. Biohydrogen production from Purified Terephthalic Acid (PTA) processing wastewater by anaerobic fermentation using mixed microbial communities. Int. J. Hydrogen Energy 2010, 35, 8350–8356. [Google Scholar] [CrossRef]
- Liu, C.-M.; Wu, S.-Y.; Chu, C.-Y.; Chou, Y.-P. Biohydrogen production from rice straw hydrolyzate in a continuously external circulating bioreactor. Int. J. Hydrogen Energy 2014, 39, 19317–19322. [Google Scholar] [CrossRef]



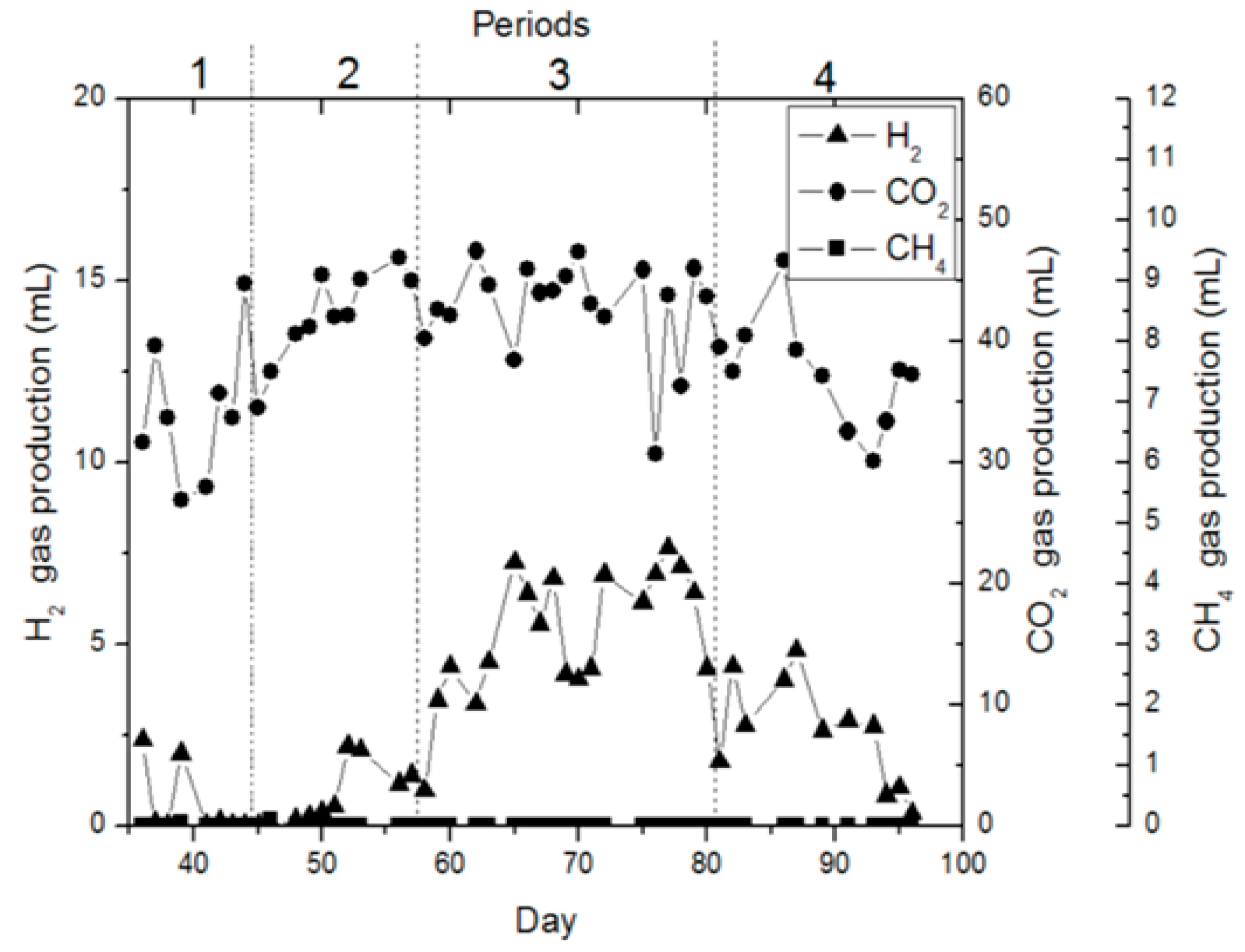
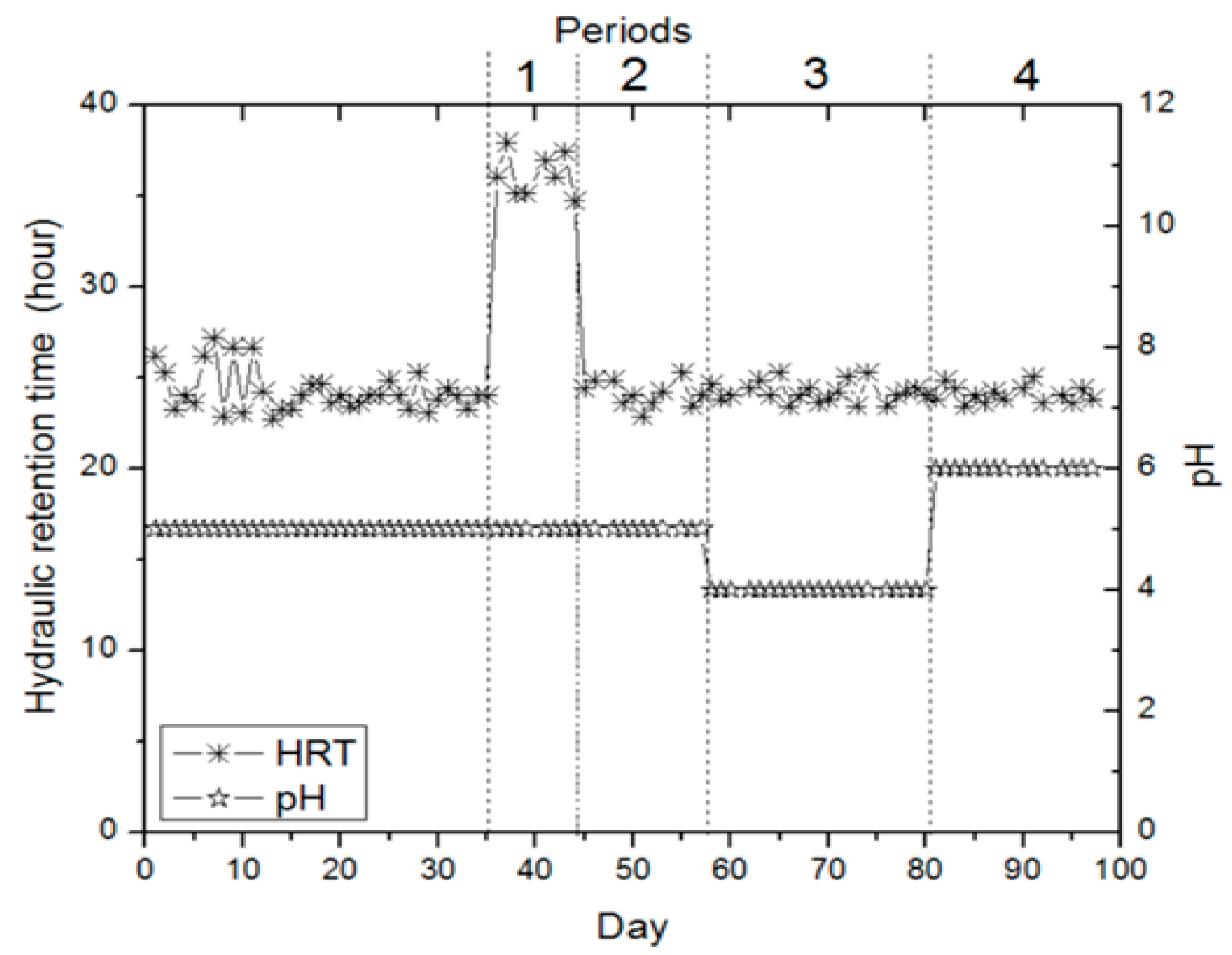
| Periods | Days | pH | HRT (h) | Loading * (gr. Black Cumin Extract/L) | Maximum Hydrogen * (mL/Day) |
|---|---|---|---|---|---|
| Acclimation phase | 0–35 | 5.0 | 23.9 | 2.22–4.44 (1–10) | - |
| 1 | 36–46 | 5.0 | 36.1 | 4.44 (36) | 3.1 (37) |
| 2 | 47–63 | 5.0 | 24.5 | 4.44 (47) | 5.1 (58) |
| 3 | 64–82 | 4.0 | 24.0 | 6.66–4.44 (64–76) | 20.8–14.2 (67–79) |
| 4 | 83–97 | 6.0 | 24.1 | 6.66–4.44 (83–93) | 14.2–2.4 (88–95) |
| Periods | Days | pH | HRT (h) | Loading * (gr. Black Cumin Extract/L) | Maximum Hydrogen * (mL/Day) |
|---|---|---|---|---|---|
| Acclimation phase | 0–35 | 5.0 | 24.1 | 2.22–4.44 (1–10) | - |
| 1 | 36–44 | 5.0 | 36.1 | 4.44 (36) | 2.3 (36) |
| 2 | 45–57 | 5.0 | 24.1 | 4.44 (45) | 2.1 (52) |
| 3 | 58–80 | 4.0 | 24.1 | 4.44–6.66 (58–66) | 4.5–7.6 (64–78) |
| 4 | 81–97 | 6.0 | 24.0 | 6.66–4.44 (81–90) | 4.8–2.8 (88–92) |
| pH-Day-Period | Acetic Acid (mg/mL) | Propionic Acid (mg/mL) | Butyric Acid (mg/mL) |
|---|---|---|---|
| 5.0-43-1 | 0.711 ± 0.006 | 0.025 ± 0.004 | 0.154 ± 0.001 |
| 5.0-50-2 | 0.861 ± 0.018 | 0.275 ± 0.017 | 0.109 ± 0.000 |
| 5.0-52-2 | 0.110 ± 0.007 | 0.079 ± 0.004 | 0.233 ± 0.001 |
| 4.0-67-3 | 2.279 ± 0.006 | 0.063 ± 0.001 | 1.129 ± 0.006 |
| 4.0-82-3 | 0.902 ± 0.004 | 0.008 ± 0.002 | 0.481 ± 0.002 |
| 6.0-85-4 | 0.556 ± 0.004 | 0.035 ± 0.000 | 0.034 ± 0.001 |
| 6.0-97-4 | 0.354 ± 0.004 | 0.024 ± 0.001 | 0.027 ± 0.001 |
| pH-Day-Period | Acetic Acid (mg/mL) | Propionic Acid (mg/mL) | Butyric Acid (mg/mL) |
|---|---|---|---|
| 5.0-49-2 | 0.369 ± 0.001 | 0.013 ± 0.002 | 0.014 ± 0.001 |
| 5.0-52-2 | 0.140 ± 0.004 | 0.000 | 0.012 ± 0.004 |
| 4.0-67-3 | 1.995 ± 0.008 | 0.057 ± 0.002 | 0.688 ± 0.000 |
| 4.0-80-3 | 1.881 ± 0.002 | 0.027 ± 0.001 | 0.851 ± 0.002 |
| 6.0-85-4 | 0.556 ± 0.002 | 0.032 ± 0.000 | 0.037 ± 0.001 |
| 6.0-97-4 | 0.346 ± 0.001 | 0.012 ± 0.000 | 0.028 ± 0.001 |
| Organic Loading Rate (OLR) | Measurement Time (Hour) | H2 (10−4 mL) | CO2 (10−2 mL) | CH4 (10−4 mL) |
|---|---|---|---|---|
| 2.22 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 15 | 7 | 65 | 0 | |
| 21 | 11 | 65 | 0 | |
| 43 | 11 | 65 | 0 | |
| 67 | 4 | 64 | 0 | |
| 4.44 g.nigella sativa extract/L | 2 | 0 | 64 | 0 |
| 14 | 74 | 65 | 0 | |
| 16 | 77 | 65 | 0 | |
| 19 | 210 | 65 | 0 | |
| 21 | 70 | 65 | 0 | |
| 37 | 59 | 65 | 0 | |
| 43 | 48 | 65 | 0 | |
| 6.66 g.nigella sativa extract/L | 2 | 0 | 64 | 0 |
| 15 | 37 | 65 | 0 | |
| 21 | 37 | 65 | 0 | |
| 39 | 30 | 65 | 0 | |
| 64 | 19 | 65 | 0 | |
| 86 | 17 | 65 | 0 |
| Organic Loading Rate (OLR) | Measurement Time (Hour) | H2 (10−4 mL) | CO2 (10−2 mL) | CH4 (10−4 mL) |
|---|---|---|---|---|
| 2.22 g.nigella sativa extract/L | 2 | 0 | 64 | 0 |
| 15 | 96 | 65 | 0 | |
| 19 | 96 | 65 | 0 | |
| 38 | 77 | 65 | 0 | |
| 64 | 26 | 65 | 0 | |
| 68 | 26 | 65 | 0 | |
| 4.44 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 15 | 236 | 65 | 0 | |
| 16 | 162 | 65 | 0 | |
| 20 | 225 | 65 | 0 | |
| 38 | 188 | 65 | 0 | |
| 43 | 166 | 65 | 0 | |
| 63 | 103 | 65 | 0 | |
| 68 | 70 | 64 | 0 | |
| 6.66 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 16 | 870 | 64 | 0 | |
| 21 | 977 | 64 | 0 | |
| 39 | 940 | 65 | 0 | |
| 64 | 652 | 65 | 0 | |
| 86 | 542 | 65 | 0 |
| Organic Loading Rate (OLR) | Measurement Time (Hour) | H2 (10−4 mL) | CO2 (10−2 mL) | CH4 (10−4 mL) |
|---|---|---|---|---|
| 2.22 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 17 | 107 | 65 | 0 | |
| 20 | 107 | 65 | 0 | |
| 38 | 70 | 65 | 0 | |
| 63 | 4 | 65 | 0 | |
| 4.44 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 17 | 136 | 65 | 0 | |
| 20 | 118 | 65 | 0 | |
| 38 | 100 | 65 | 0 | |
| 64 | 26 | 65 | 0 | |
| 68 | 26 | 65 | 0 | |
| 6.66 g.nigella sativa extract/L | 2 | 0 | 65 | 0 |
| 17 | 254 | 64 | 0 | |
| 20 | 250 | 65 | 0 | |
| 38 | 181 | 65 | 0 | |
| 61 | 92 | 65 | 0 | |
| 85 | 63 | 65 | 0 |
| Substrate | Operating Conditions | H2 Production | References |
|---|---|---|---|
| Oat straw subjected to enzymatic treatment | Batch reactor, 4.7 g reducing sugars/L, 35 °C, 7.0 pH | 110 mL H2/L/h | [60] |
| Oat straw subjected to HCl pretreatment | Batch reactor, 4.7 g reducing sugars/L, 35 °C, 7.0 pH | 70 mL H2/L/h | [60] |
| Alcohol industry wastewater | Upflow Anaerobic Sludge Blanket Reactor, OLR 45 g COD/L, 37 °C, 5.5 pH, 0.96 d HRT | 125.1 mL H2/g COD | [61] |
| Palm oil mill effluent | Upflow Anaerobic Sludge Blanket (UASB)–Continuous Stirred Tank Reactor (CSTR), OLR 76.5 ± 0.3 g COD/L, 55 °C, 5.5 pH, 9 h HRT | 49.22 mL H2/g COD | [62] |
| Rice bran | An amount of 10% total solids in the batch reactor and completion of the remaining volume with water, 37 °C, 7.0 pH | 57.65 mL/h | [63] |
| Rice husk | An amount of 10% total solids in the batch reactor and completion of the remaining volume with water, 37 °C, 7.5 pH | 24.29 mL/h | [63] |
| Rice straw | An amount of 10% total solids in the batch reactor and completion of the remaining volume with water, 37 °C, 7.5 pH | 42.51 mL/h | [63] |
| Rice waste | An amount of 10% total solids in the batch reactor and completion of the remaining volume with water, 37 °C, 7.0 pH | 54.73 mL/h | [63] |
| Nigella sativa extract (liquid) | Completely Stirred Tank Reactor (CSTR), OLR 6.66 g.nigella sativa extract/L (275.5 mg COD L−1), 35 ± 2 °C, 4.0 pH, 24 h HRT | 20.8 mL H2/d | This study |
| Nigella sativa extract (liquid) | Fluidized Bed Reactor (FBR), OLR 6.66 g.nigella sativa extract/L (294.7 mg COD L−1), 35 ± 2 °C, 4.0 pH, 24 h HRT | 7.6 mL H2/d | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dursun, N.; Gülşen, H. Biohydrogen Production from Waste Black Cumin (Nigella Sativa) Extract Liquid. Bioengineering 2024, 11, 282. https://doi.org/10.3390/bioengineering11030282
Dursun N, Gülşen H. Biohydrogen Production from Waste Black Cumin (Nigella Sativa) Extract Liquid. Bioengineering. 2024; 11(3):282. https://doi.org/10.3390/bioengineering11030282
Chicago/Turabian StyleDursun, Nesrin, and Hakki Gülşen. 2024. "Biohydrogen Production from Waste Black Cumin (Nigella Sativa) Extract Liquid" Bioengineering 11, no. 3: 282. https://doi.org/10.3390/bioengineering11030282
APA StyleDursun, N., & Gülşen, H. (2024). Biohydrogen Production from Waste Black Cumin (Nigella Sativa) Extract Liquid. Bioengineering, 11(3), 282. https://doi.org/10.3390/bioengineering11030282







