Off-Label Use of an External Hand Fixator for Craniomaxillofacial Fractures—An Anatomical Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Tests on Anatomical Specimens
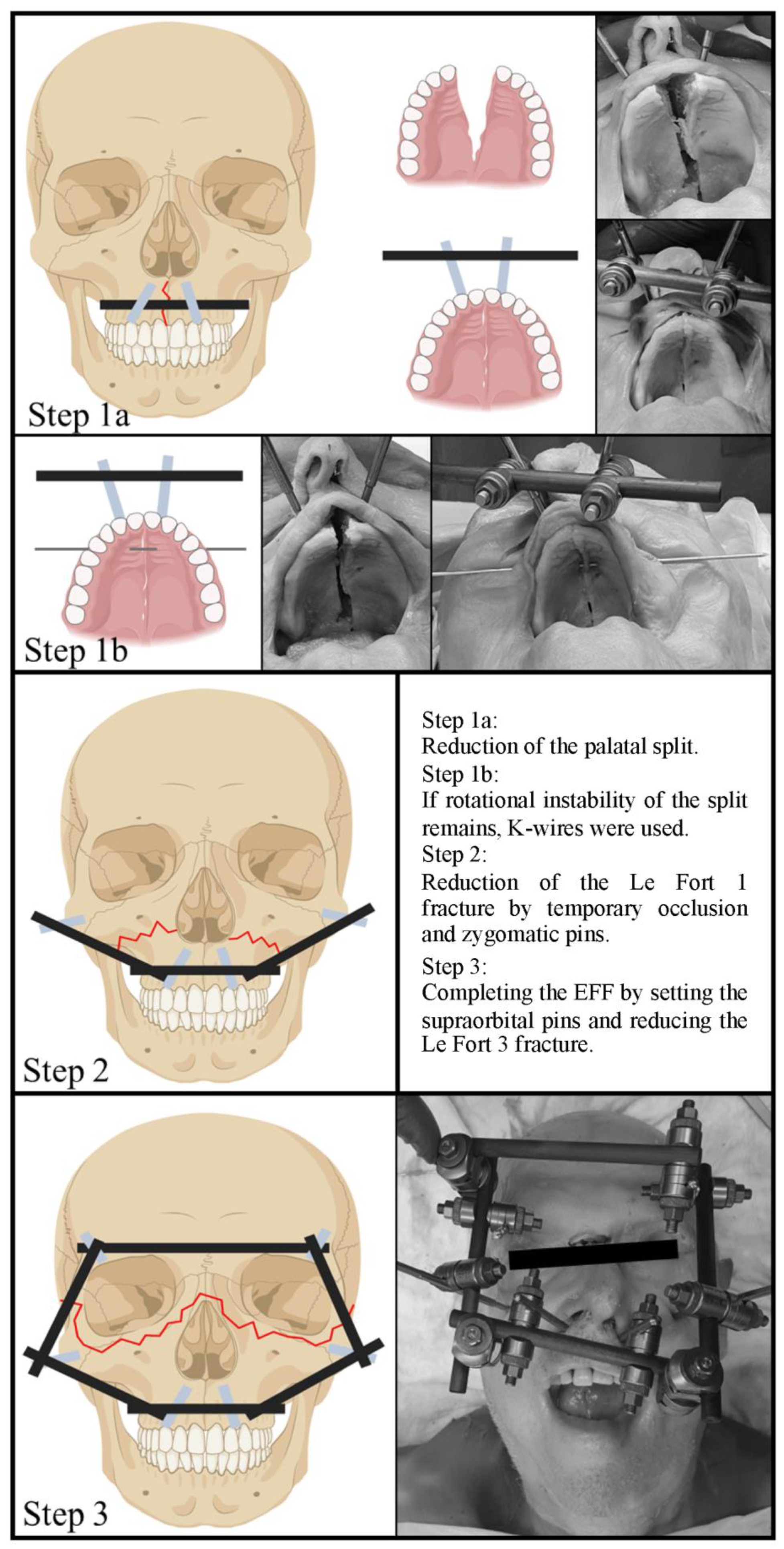
2.2. Application of the EFF Step by Step
2.2.1. Reduction and Fixation of the Palatal Split
2.2.2. Reduction and Fixation of the Le Fort 1 and 2 Fracture Components
2.2.3. Reduction and Fixation of the Le Fort 3 Fracture Component
2.3. Analysis of the Optimal Insertion Angle for the Longest Intraosseous Drilling Possibility
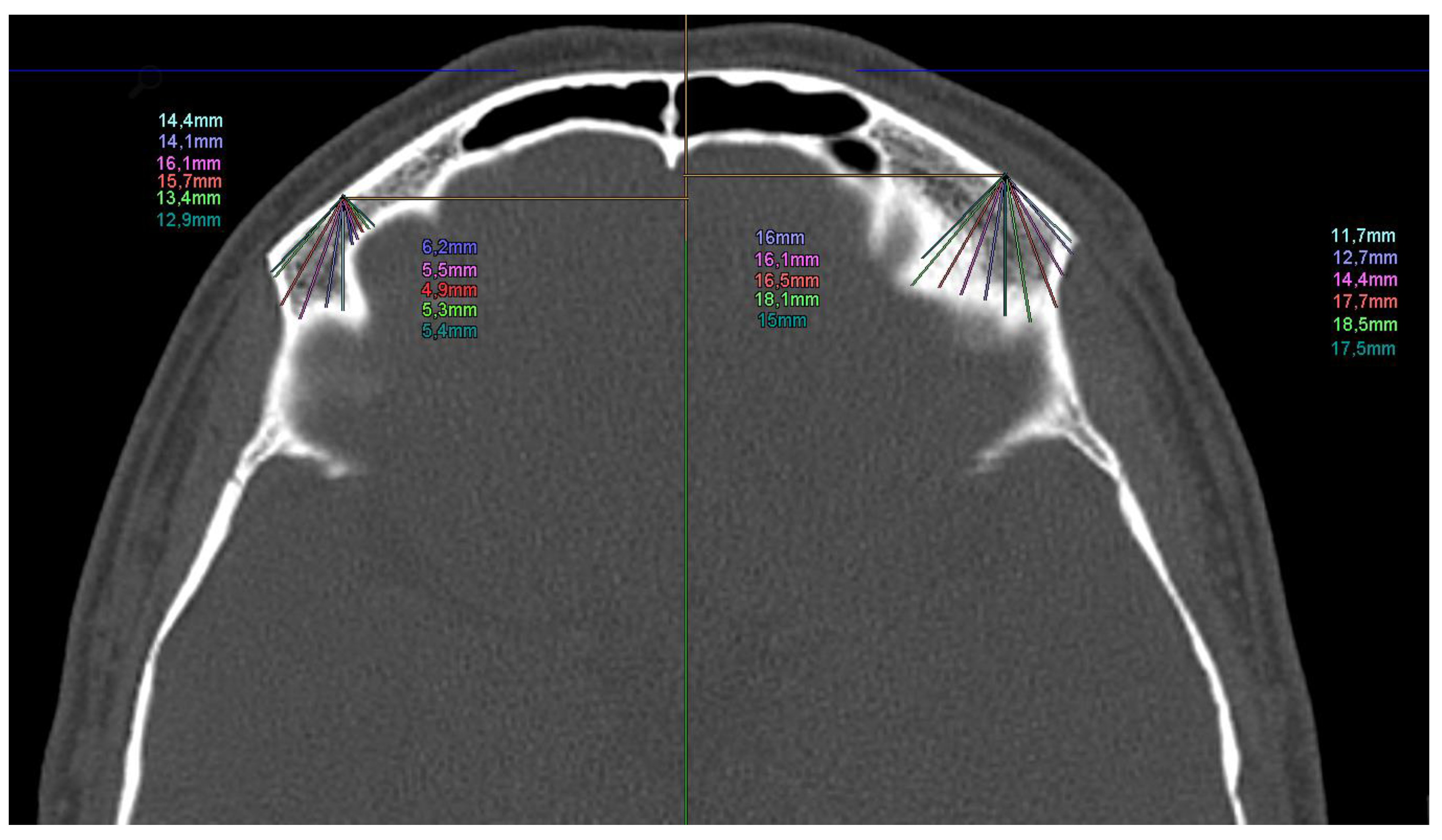
2.4. Digital Measurement of CT Scans for Analysis of Optimal Pin Insertion Angles
2.5. Statistical Methodology
3. Results
3.1. The Biomechanical Evaluation of the Pull-Out Force of the Pins
3.2. Results of the Digital Evaluation of the Possible Pin Insertion Areas of 100 CTs of the Skull
3.3. Clinical Evaluation of the Surgical Method
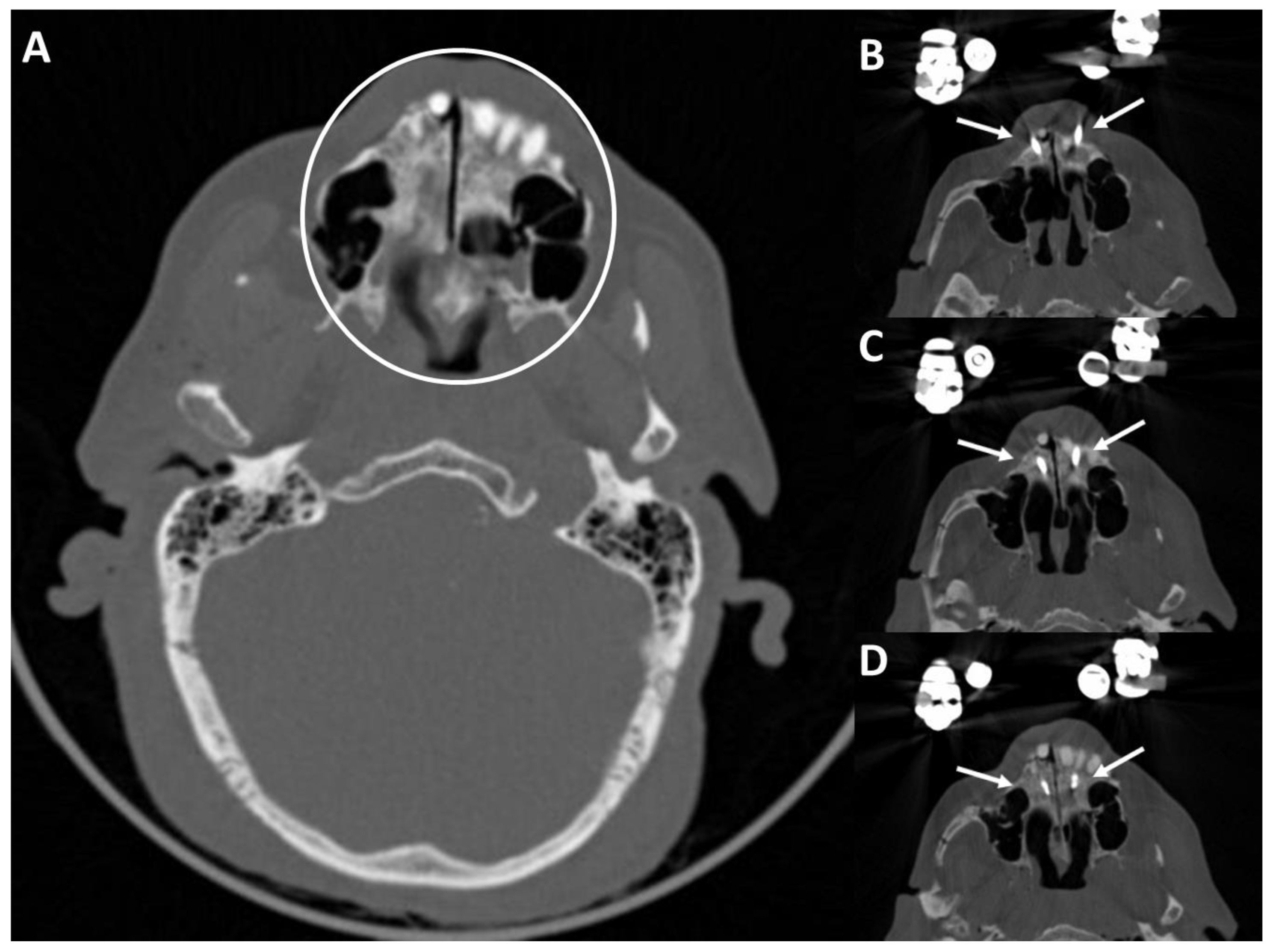
3.4. Postoperative Results in the CT Scans
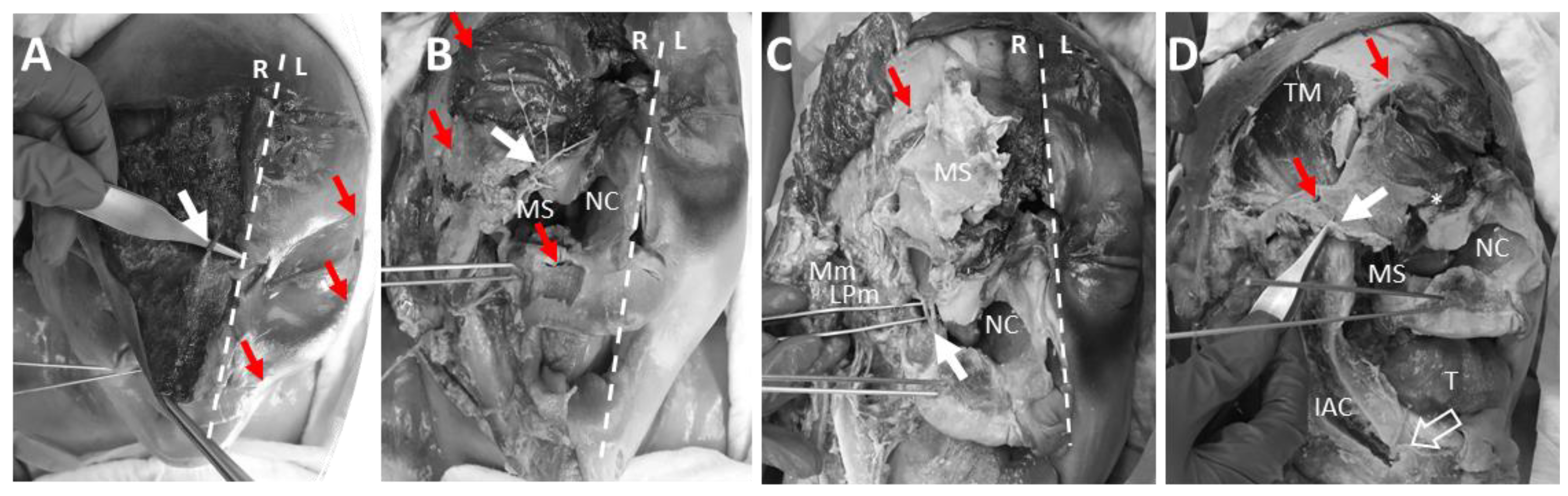
3.5. Anatomical Dissection of the Pin Insertion Areas and Description of Structures at Risk
4. Discussion
4.1. The Surgical Technique
4.2. Post-Operative CT Scans
4.3. Biomechanical Analysis
4.4. Evaluation of the 100 CT Scans of Healthy Subjects
4.5. Anatomical Dissection of the Pin Insertion Sites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMF | Craniomaxillofacial fracture |
| CT | Computed tomography |
| EFF | External face fixator |
| K-wire | Kirschner wire |
| LIC | Low-income country |
| HIC | High-income country |
References
- Taiwo, A.; Godwin, N.; Ibikunle, A.; Soyele, O. Facial fracture management in Northwest Nigeria. J. Surg. Tech. Case Rep. 2013, 5, 65–71. [Google Scholar] [CrossRef][Green Version]
- Adeyemo, W.L.; Taiwo, O.A.; Ladeinde, A.L.; Ogunlewe, M.O.; Adeyemi, M.O.; Adepoju, A.A. Mid-facial fractures: A 5-year retrospective review in a Nigerian teaching hospital. Niger. J. Med. 2012, 21, 31–35. [Google Scholar]
- Zafar, S.N.; Canner, J.K.; Nagarajan, N.; Kushner, A.L.; Gupta, S.; Tran, T.M.; Stewart, B.T.; Kamara, T.B.; Kyamanywa, P.; Amatya, K.S.; et al. Road traffic injuries: Cross-sectional cluster randomized countrywide population data from 4 low-income countries. Int. J. Surg. 2018, 52, 237–242. [Google Scholar] [CrossRef]
- Nantulya, V.M.; Reich, M.R. The neglected epidemic: Road traffic injuries in developing countries. BMJ 2002, 324, 1139–1141. [Google Scholar] [CrossRef]
- Menon, S.; Sham, M.; Kumar, V.; Archana, S.; Nath, P.; Shivakotee, S.; Hoda, M. Maxillofacial fracture patterns in road traffic accidents. Ann. Maxillofac. Surg. 2019, 9, 345–348. [Google Scholar] [CrossRef]
- Schuknecht, B.; Graetz, K. Radiologic assessment of maxillofacial, mandibular, and skull base trauma. Eur. Radiol. 2005, 15, 560–568. [Google Scholar] [CrossRef]
- Mast, G.; Ehrenfeld, M.; Cornelius, C.P. Maxillofacial fractures: Midface and internal orbit. Part 2: Therapeu-tic options. Unfallchirurg 2012, 115, 145–163, quiz 164. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R. The Search for the Ideal Fixation of Palatal Fractures: Innovative Experience with a Mini-Locking Plate. Craniomaxillofacial Trauma Reconstr. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bamjee, Y.; Noffke, C.E.E. A comparative study of three imaging modalities currently used in the assessment of patients for maxillofacial surgery. SADJ 2013, 68, 106–112. [Google Scholar]
- Van As, A.; van Loghem, A.; Biermans, B.; Douglas, T.; Wieselthaler, N.; Naidoo, S. Causes and distribution of facial fractures in a group of South African children and the value of computed tomography in their assessment. Int. J. Oral Maxillofac. Surg. 2006, 35, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Cuddy, K.; Dierks, E.J.; Cheng, A.; Patel, A.; Amundson, M.; Bell, R.B. Management of Zygomaticomaxillary Complex Fractures Utilizing Intraoperative 3-Dimensional Imaging: The ZYGOMAS Protocol. J. Oral Maxillofac. Surg. 2021, 79, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cuddy, K.; Khatib, B.; Bell, R.B.; Cheng, A.; Patel, A.; Amundson, M.; Dierks, E.J. Use of Intraoperative Computed Tomography in Craniomaxillofacial Trauma Surgery. J. Oral Maxillofac. Surg. 2018, 76, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Alibhai, A.; Hendrikse, C.; Bruijns, S.R. Poor access to acute care resources to treat major trauma in low- and middle-income settings: A self-reported survey of acute care providers. Afr. J. Emerg. Med. 2019, 9, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.A.B.; Morris, J.M.; Sharaf, B.A.M. Craniomaxillofacial Trauma: The Past, Present and the Future. J. Craniofacial Surg. 2023, 34, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marin, F. Access to oral & maxillofacial surgery in Sub-Saharan African countries. J. Oral Biol. Craniofacial Res. 2021, 11, 608–611. [Google Scholar] [CrossRef]
- Shah, I.; Gadkaree, S.K.; Tollefson, T.T.; Shaye, D.A. Update on the management of craniomaxillofacial trauma in low-resource settings. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, V.; Tsoucalas, G.; Fiska, A. The Hippocratic Method for the Reduction of the Mandibular Disloca-tion, an Ancient Greek Procedure Still in Use in Maxillofacial Surgery. Acta Med. Acad. 2018, 47, 139–143. [Google Scholar] [CrossRef]
- Béogo, R.; Bouletreau, P.; Konsem, T.; Traoré, I.; Coulibaly, A.T.; Ouédraogo, D. Wire internal fixation: An obsolete, yet valuable method for surgical management of facial fractures. Pan Afr. Med. J. 2014, 17, 219. [Google Scholar] [CrossRef]
- Deininger, C.; Hofmann, V.; Necchi, M.; Deininger, S.; Wichlas, F. Off-Label Treatment for Severe Craniomaxillofacial Fractures in Low-Income Countries—A Novel Operation Method with the External Face Fixator. J. Clin. Med. 2022, 11, 1488. [Google Scholar] [CrossRef]
- SQUIRE 2.0 Guidlines. Available online: https://www.squire-statement.org/ (accessed on 2 November 2022).
- AO Hand Fixator System. 2022. 10 May 2022. Available online: http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/DSEM-TRM-0416-0653a_LR.pdf (accessed on 2 November 2022).
- Panesar, K.; Susarla, S.M. Mandibular Fractures: Diagnosis and Management. Semin. Plast. Surg. 2021, 35, 238–249. [Google Scholar] [CrossRef]
- Ikeda, A.K.; Burke, A.B. LeFort Fractures. Semin. Plast. Surg. 2021, 35, 250–255. [Google Scholar] [CrossRef]
- Olasoji, H.; Tahir, A.; Arotiba, G. Changing picture of facial fractures in northern Nigeria. Br. J. Oral Maxillofac. Surg. 2002, 40, 140–143. [Google Scholar] [CrossRef]
- Mast, G.; Ehrenfeld, M.; Cornelius, C.P. Maxillofacial fractures: Midface and internal orbit: Part 1: Classifi-cation and diagnosis. Unfallchirurg 2011, 114, 1007–1017. [Google Scholar] [CrossRef]
- Rimell, F.; Marentette, L.J. Injuries of the hard palate and the horizontal buttress of the midface. Otolaryngol. Head Neck Surg. 1993, 109, 499–505. [Google Scholar] [CrossRef]
- Wu, C.A.; Dutta, R.; Virk, S.; Roy, N.; Ranganathan, K. The need for craniofacial trauma and oncologic reconstruction in global surgery. J. Oral Biol. Craniofacial Res. 2021, 11, 563–567. [Google Scholar] [CrossRef]
- Acero, J. International transfer of knowledge: Training and education in craniomaxillofacial surgery. Contributing to humanitarian aid by teaching the teachers. J. Oral Biol. Craniofacial Res. 2021, 11, 643–651. [Google Scholar] [CrossRef]
- Shaye, D.A.; Tollefson, T.; Shah, I.; Krishnan, G.; Matic, D.; Figari, M.; Lim, T.C.; Aniruth, S.; Schubert, W. Backward Planning a Craniomaxillofacial Trauma Curriculum for the Surgical Workforce in Low-Resource Settings. World J. Surg. 2018, 42, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Stanford-Moore, G.B.; Niyigaba, G.; Tuyishimire, G.; Yau, J.; Kulkrani, A.; Nyabyenda, V.; Ncogoza, I.; Shaye, D.A. Effect of Delay of Care for Patients with Craniomaxillofacial Trauma in Rwanda. OTO Open 2022, 6, 2473974X221096032. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Lownie, M.; Cleaton-Jones, P. Maxillofacial injury: A retrospective analysis of time lapse between injury and treatment in a South African academic maxillofacial and oral surgery unit. S. Afr. J. Surg. 2013, 51, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Cotton, M.; Henry, J.; Hasek, L. Value innovation: An important aspect of global surgical care. Glob. Health 2014, 10, 1. [Google Scholar] [CrossRef] [PubMed]






| Pin Insertion Areas: | Supraorbital | Mandibula | Hard Palate Medial | Zygomatic Bone | Hard Palate Lateral |
|---|---|---|---|---|---|
| Number of values | 21 | 16 | 14 | 13 | 8 |
| Force in Newton: | |||||
| Minimum | 210.0 | 179.0 | 125.0 | 139.8 | 15.00 |
| 25% Percentile | 386.8 | 362.0 | 192.5 | 183.3 | 20.05 |
| Median | 451.8 | 432.0 | 343.8 | 296.0 | 49.60 |
| 75% Percentile | 496.0 | 500.0 | 461.3 | 418.5 | 139.8 |
| Maximum | 512.0 | 500.0 | 500.0 | 460.0 | 146.0 |
| Mean | 428.4 | 415.3 | 326.6 | 293.8 | 72.23 |
| Std. Deviation | 80.38 | 94.99 | 139.8 | 115.8 | 59.26 |
| Std. Error | 17.54 | 23.75 | 37.37 | 32.12 | 20.95 |
| Lower 95% CI of mean | 391.8 | 364.7 | 245.9 | 223.8 | 22.68 |
| Upper 95% CI of mean | 464.9 | 465.9 | 407.3 | 363.8 | 121.8 |
| Kruskal–Wallis Test | |||
|---|---|---|---|
| p value | <0.0001 | ||
| Exact or approximate p value? | Gaussian Approximation | ||
| p value summary | **** | ||
| Do the medians vary signif. (p < 0.05) | Yes | ||
| Number of groups | 5 | ||
| Kruskal–Wallis statistic | 31.01 | ||
| Dunn’s multiple comparison test | Difference in rank sum | Significant? p < 0.05? | Summary |
| Supraorbital vs. Mandibula | 1.772 | No | ns |
| Supraorbital vs. Hard palate medial | 15.07 | No | ns |
| Supraorbital vs. Zygomatic bone | 21.31 | Yes | * |
| Supraorbital vs. Hard palate lateral | 42.80 | Yes | *** |
| Mandibula vs. Hard palate medial | 13.30 | No | ns |
| Mandibula vs. Zygomatic bone | 19.54 | No | ns |
| Mandibula vs. Hard palate lateral | 41.03 | Yes | *** |
| Hard palate medial vs. Zygomatic bone | 6.242 | No | ns |
| Hard palate medial vs. Hard palate lateral | 27.73 | Yes | * |
| Zygomatic bone vs. Hard palate lateral | 21.49 | No | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichlas, F.; Necchi, M.; Gruber, T.; Hofmann, V.; Deininger, S.; Deininger, S.H.M.; Deluca, A.; Steidle-Kloc, E.; Pruszak, J.; Wittig, J.; et al. Off-Label Use of an External Hand Fixator for Craniomaxillofacial Fractures—An Anatomical Feasibility Study. Bioengineering 2024, 11, 279. https://doi.org/10.3390/bioengineering11030279
Wichlas F, Necchi M, Gruber T, Hofmann V, Deininger S, Deininger SHM, Deluca A, Steidle-Kloc E, Pruszak J, Wittig J, et al. Off-Label Use of an External Hand Fixator for Craniomaxillofacial Fractures—An Anatomical Feasibility Study. Bioengineering. 2024; 11(3):279. https://doi.org/10.3390/bioengineering11030279
Chicago/Turabian StyleWichlas, Florian, Marco Necchi, Teresa Gruber, Valeska Hofmann, Susanne Deininger, Sebastian Hubertus Markus Deininger, Amelie Deluca, Eva Steidle-Kloc, Jan Pruszak, Jörn Wittig, and et al. 2024. "Off-Label Use of an External Hand Fixator for Craniomaxillofacial Fractures—An Anatomical Feasibility Study" Bioengineering 11, no. 3: 279. https://doi.org/10.3390/bioengineering11030279
APA StyleWichlas, F., Necchi, M., Gruber, T., Hofmann, V., Deininger, S., Deininger, S. H. M., Deluca, A., Steidle-Kloc, E., Pruszak, J., Wittig, J., & Deininger, C. (2024). Off-Label Use of an External Hand Fixator for Craniomaxillofacial Fractures—An Anatomical Feasibility Study. Bioengineering, 11(3), 279. https://doi.org/10.3390/bioengineering11030279






