Bio-Inspired Magnetically Responsive Silicone Cilia: Fabrication Strategy and Interaction with Biological Mucus
Abstract
1. Introduction
2. Experimental Section
2.1. Fabrication of Magnetic Cilia
2.2. Structural Characterization of the Cilia
2.3. Qualitative Analysis of Cilia Interaction with Mucus
3. Results and Discussion
3.1. Fabrication of the Magnetic Cilia
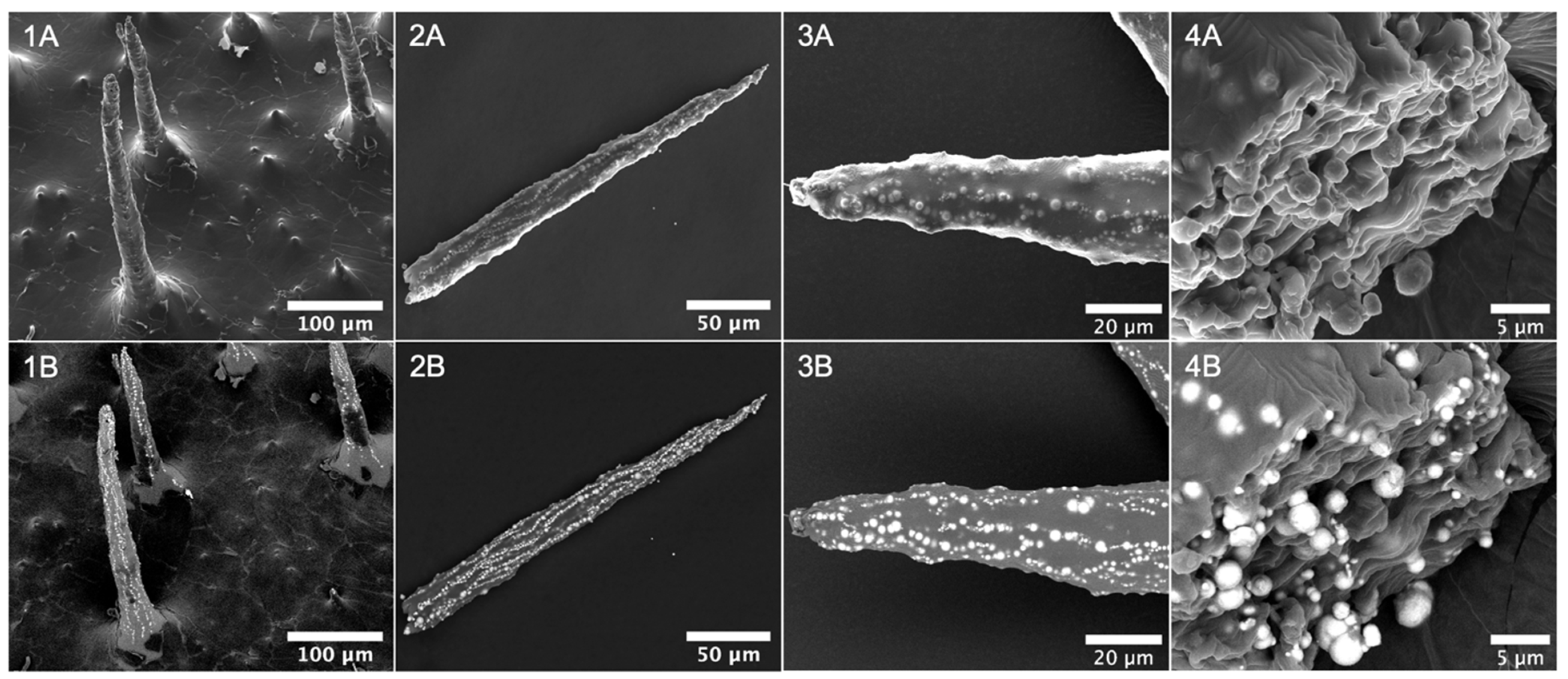
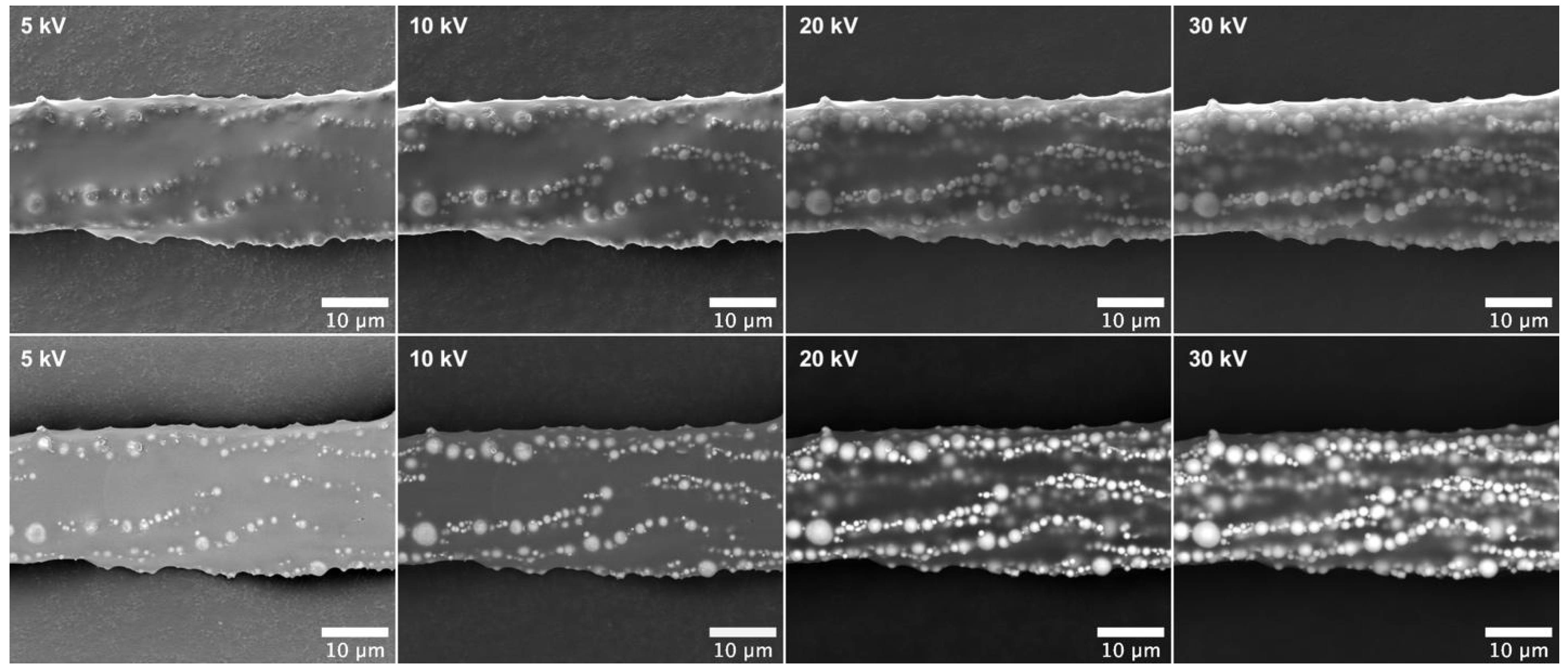
3.2. Characterization of Magnetic Cilia
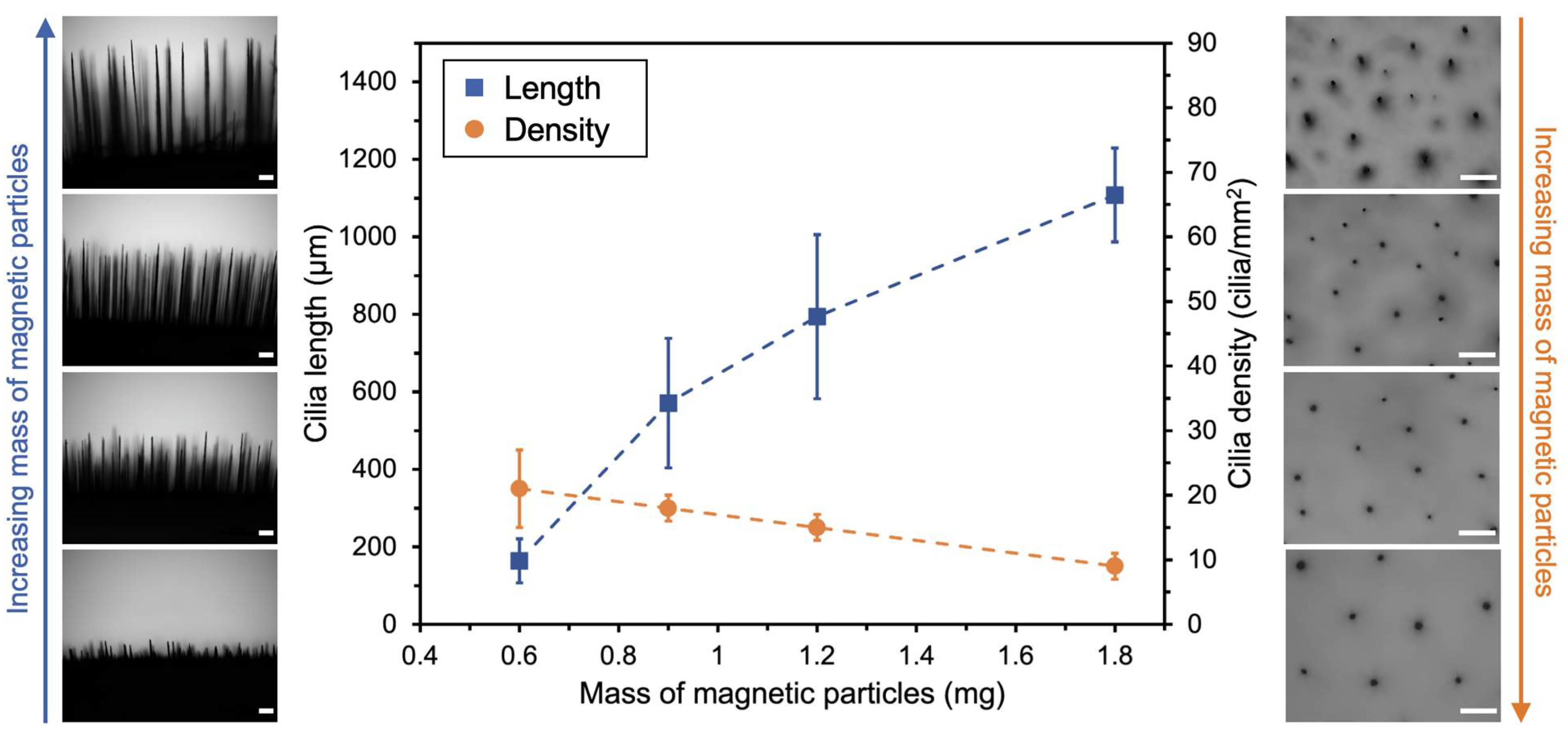
3.3. Fine-Tuning Cilia Dimensions
3.4. Rationale for the Magnetic Cilia, and Qualitative Analysis of Their Interaction with Mucus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilpin, W.; Bull, M.S.; Prakash, M. The Multiscale Physics of Cilia and Flagella. Nat. Rev. Phys. 2020, 2, 74–88. [Google Scholar] [CrossRef]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef]
- Grillo, H.C.; Donahue, D.M.; Mathisen, D.J.; Wain, J.C.; Wright, C.D. Postintubation Tracheal Stenosis: Treatment and Results. J. Thorac. Cardiovasc. Surg. 1995, 109, 486–493. [Google Scholar] [CrossRef]
- Piazza, C.; Filauro, M.; Dikkers, F.G.; Nouraei, S.A.R.; Sandu, K.; Sittel, C.; Amin, M.R.; Campos, G.; Eckel, H.E.; Peretti, G. Long-Term Intubation and High Rate of Tracheostomy in COVID-19 Patients Might Determine an Unprecedented Increase of Airway Stenoses: A Call to Action from the European Laryngological Society. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.T.; Guerreiro Cardoso, P.F.; Minamoto, H.; Bibas, B.J.; Salati, M.; Pego-Fernandes, P.M.; Cecconello, I.; Nasi, A.; Aissar Sallum, R.A. Impact of Fundoplication for Gastroesophageal Reflux in the Outcome of Benign Tracheal Stenosis. J. Thorac. Cardiovasc. Surg. 2019, 158, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.F.G.; Minamoto, H.; Bibas, B.J.; Pego-Fernandes, P.M. Impact of Gastroesophageal Reflux in the Pathogenesis of Tracheal Stenosis. Transl. Cancer Res. 2020, 9, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Bibas, B.J.; Cardoso, P.F.G.; Salati, M.; Minamoto, H.; Luiz Tamagno, M.F.; Terra, R.M.; Pêgo-Fernandes, P.M. Health-Related Quality of Life Evaluation in Patients with Non-Surgical Benign Tracheal Stenosis. J. Thorac. Dis. 2018, 10, 4782–4788. [Google Scholar] [CrossRef] [PubMed]
- ul Islam, T.; Wang, Y.; Aggarwal, I.; Cui, Z.; Eslami Amirabadi, H.; Garg, H.; Kooi, R.; Venkataramanachar, B.B.; Wang, T.; Zhang, S.; et al. Microscopic Artificial Cilia—A Review. Lab Chip 2022, 22, 1650–1679. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, G.; Kang, M.; Kim, W.; Kim, J.; Jeong, H.E. Bioinspired Magnetic Cilia: From Materials to Applications. Microsyst. Nanoeng. 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Tierno, P. Recent Advances in Anisotropic Magnetic Colloids: Realization, Assembly and Applications. Phys. Chem. Chem. Phys. 2014, 16, 23515–23528. [Google Scholar] [CrossRef]
- Evans, B.A.; Shields, A.R.; Carroll, R.L.; Washburn, S.; Falvo, M.R.; Superfine, R. Magnetically Actuated Nanorod Arrays as Biomimetic Cilia. Nano Lett. 2007, 7, 1428–1434. [Google Scholar] [CrossRef]
- Zhu, Y.; Antao, D.S.; Xiao, R.; Wang, E.N. Real-Time Manipulation with Magnetically Tunable Structures. Adv. Mater. 2014, 26, 6442–6446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Onck, P.R.; den Toonder, J.M.J. Removal of Microparticles by Ciliated Surfaces—An Experimental Study. Adv. Funct. Mater. 2019, 29, 1806434. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, X.A.; Evans, B.A.; Chang, C.-H. Active Periodic Magnetic Nanostructures with High Aspect Ratio and Ultrahigh Pillar Density. ACS Appl. Mater. Interfaces 2020, 12, 11135–11143. [Google Scholar] [CrossRef] [PubMed]
- ul Islam, T.; Bellouard, Y.; den Toonder, J.M.J. Highly Motile Nanoscale Magnetic Artificial Cilia. Proc. Natl. Acad. Sci. USA 2021, 118, e2104930118. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, S.; Wang, Y.; Tormey, L.; Kanies, O.S.; Spero, R.C.; Fisher, J.K.; den Toonder, J.M.J. Self-Cleaning Surfaces Realized by Biologically Sized Magnetic Artificial Cilia. Adv. Mater. Interfaces 2022, 9, 2102016. [Google Scholar] [CrossRef]
- Timonen, J.V.I.; Johans, C.; Kontturi, K.; Walther, A.; Ikkala, O.; Ras, R.H.A. A Facile Template-Free Approach to Magnetodriven, Multifunctional Artificial Cilia. ACS Appl. Mater. Interfaces 2010, 2, 2226–2230. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, S.M.; Lee, B.J.; Ko, H.; Bae, W.-G.; Suh, K.Y.; Kwak, M.K.; Jeong, H.E. Remote Manipulation of Droplets on a Flexible Magnetically Responsive Film. Sci. Rep. 2015, 5, 17843. [Google Scholar] [CrossRef]
- Huang, Y.; Stogin, B.B.; Sun, N.; Wang, J.; Yang, S.; Wong, T.-S. A Switchable Cross-Species Liquid Repellent Surface. Adv. Mater. 2017, 29, 1604641. [Google Scholar] [CrossRef]
- Sohn, S.; Lee, H.; Kee, H.; Park, S. Reprogrammable Magnetically Actuated Self-Assembled Cilia Array. Adv. Intell. Syst. 2023, 5, 2200227. [Google Scholar] [CrossRef]
- Liu, J.A.-C.; Evans, B.A.; Tracy, J.B. Photothermally Reconfigurable Shape Memory Magnetic Cilia. Adv. Mater. Technol. 2020, 5, 2000147. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Fu, Y.-F.; Li, Y.-Q.; Huang, P.; Xu, C.-H.; Hu, N.; Fu, S.-Y. Bio-Inspired Highly Flexible Dual-Mode Electronic Cilia. J. Mater. Chem. B 2018, 6, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Chen, R.; Lin, W.; Yuan, D.; Geng, D.; Luo, T.; Zhang, J.; Wu, L.; Zhou, W. Ordered Magnetic Cilia Array Induced by the Micro-Cavity Effect for the In Situ Adjustable Pressure Sensor. ACS Appl. Mater. Interfaces 2022, 14, 38291–38301. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zheng, Y. Bio-Inspired Artificial Cilia with Magnetic Dynamic Properties. Front. Mater. Sci. 2015, 9, 178–184. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Wyss, H.; Anderson, P.; den Toonder, J. Out of the Cleanroom, Self-Assembled Magnetic Artificial Cilia. Lab Chip 2013, 13, 3360–3366. [Google Scholar] [CrossRef]
- Ni, K.; Wang, Z. Recent Progress on the Development of Magnetically-Responsive Micropillars: Actuation, Fabrication, and Applications. Adv. Funct. Mater. 2023, 33, 2213350. [Google Scholar] [CrossRef]
- Grein-Iankovski, A.; Graillot, A.; Radiom, M.; Loh, W.; Berret, J.-F. Template-Free Preparation of Thermoresponsive Magnetic Cilia Compatible with Biological Conditions. J. Phys. Chem. C 2020, 124, 26068–26075. [Google Scholar] [CrossRef]
- Grein-Iankovski, A.; Graillot, A.; Loh, W.; Berret, J.-F. Stimuli-Responsive Assembly of Iron Oxide Nanoparticles into Magnetic Flexible Filaments. Emergent Mater. 2021, 4, 1351–1362. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Martín-Roca, J.; Ortega, F.; Valeriani, C.; Rubio, R.G.; Martínez-Pedrero, F. Magnetic Colloidal Currents Guided on Self-Assembled Colloidal Tracks. Adv. Funct. Mater. 2023, 33, 2306541. [Google Scholar] [CrossRef]
- Chandra, D.; Yang, S. Capillary-Force-Induced Clustering of Micropillar Arrays: Is It Caused by Isolated Capillary Bridges or by the Lateral Capillary Meniscus Interaction Force? Langmuir 2009, 25, 10430–10434. [Google Scholar] [CrossRef]
- Cao, M.; Ju, J.; Li, K.; Dou, S.; Liu, K.; Jiang, L. Facile and Large-Scale Fabrication of a Cactus-Inspired Continuous Fog Collector. Adv. Funct. Mater. 2014, 24, 3235–3240. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical Properties of Mucus and Their Impact on Transmucosal Drug Delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, M.; Luo, Y.; Deng, L.; Hu, Z.; Song, Y. Determination of Rheology and Surface Tension of Airway Surface Liquid: A Review of Clinical Relevance and Measurement Techniques. Respir. Res. 2019, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, W.W. T-Tube Tracheal Stent. Arch. Otolaryngol. 1965, 82, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Dumon, J.-F. A Dedicated Tracheobronchial Stent. CHEST 1990, 97, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.K.; Ramirez, O.; King, M. Mucus-Depleted Frog Palate as a Model for the Study of Mucociliary Clearance. J. Appl. Physiol. 1990, 69, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Braga, K.A.d.O.; Nepomuceno, N.A.; Correia, A.T.; Jatene, F.B.; Pêgo-Fernandes, P.M. The Effects on Mucociliary Clearance of Prednisone Associated with Bronchial Section. Clin. Sao Paulo Braz. 2012, 67, 647–652. [Google Scholar] [CrossRef]
- Pazetti, R.; Pêgo-Fernandes, P.M.; Jatene, F.B. Adverse Effects of Immunosuppressant Drugs upon Airway Epithelial Cell and Mucociliary Clearance: Implications for Lung Transplant Recipients. Drugs 2013, 73, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, Z.; Wang, Y.; den Toonder, J.M.J. Metachronal Actuation of Microscopic Magnetic Artificial Cilia Generates Strong Microfluidic Pumping. Lab Chip 2020, 20, 3569–3581. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, S.; Maldonado, F.; Dong, X. Wirelessly Actuated Ciliary Airway Stent for Excessive Mucus Transportation. Adv. Mater. Technol. 2023, 8, 2301003. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grein-Iankovski, A.; de Oliveira Braga, K.A.; Legendre, D.F.; Cardoso, P.F.G.; Loh, W. Bio-Inspired Magnetically Responsive Silicone Cilia: Fabrication Strategy and Interaction with Biological Mucus. Bioengineering 2024, 11, 261. https://doi.org/10.3390/bioengineering11030261
Grein-Iankovski A, de Oliveira Braga KA, Legendre DF, Cardoso PFG, Loh W. Bio-Inspired Magnetically Responsive Silicone Cilia: Fabrication Strategy and Interaction with Biological Mucus. Bioengineering. 2024; 11(3):261. https://doi.org/10.3390/bioengineering11030261
Chicago/Turabian StyleGrein-Iankovski, Aline, Karina Andrighetti de Oliveira Braga, Daniel Formariz Legendre, Paulo Francisco Guerreiro Cardoso, and Watson Loh. 2024. "Bio-Inspired Magnetically Responsive Silicone Cilia: Fabrication Strategy and Interaction with Biological Mucus" Bioengineering 11, no. 3: 261. https://doi.org/10.3390/bioengineering11030261
APA StyleGrein-Iankovski, A., de Oliveira Braga, K. A., Legendre, D. F., Cardoso, P. F. G., & Loh, W. (2024). Bio-Inspired Magnetically Responsive Silicone Cilia: Fabrication Strategy and Interaction with Biological Mucus. Bioengineering, 11(3), 261. https://doi.org/10.3390/bioengineering11030261





