Validation of a New and Straightforward Algorithm to Evaluate Signal Quality during ECG Monitoring with Wearable Devices Used in a Clinical Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Background
- Enhance post-processing by extracting relevant signal segments selectively, improving signal processing efficiency;
- Implement real-time algorithms that provide acoustic or haptic feedback when noise compromises the signal. This possibility will empower users to take prompt corrective actions, such as adjusting electrode placement, thus potentially preventing the recording of hours of noisy signals.
- Clean/good quality/acceptable: All primary waves constituting the ECG (P, QRS, and T) are visible.
- Compromised/bad/unacceptable: The signal is partially unreadable, making it impossible to correctly identify the QRS complex, and P and Q waves.
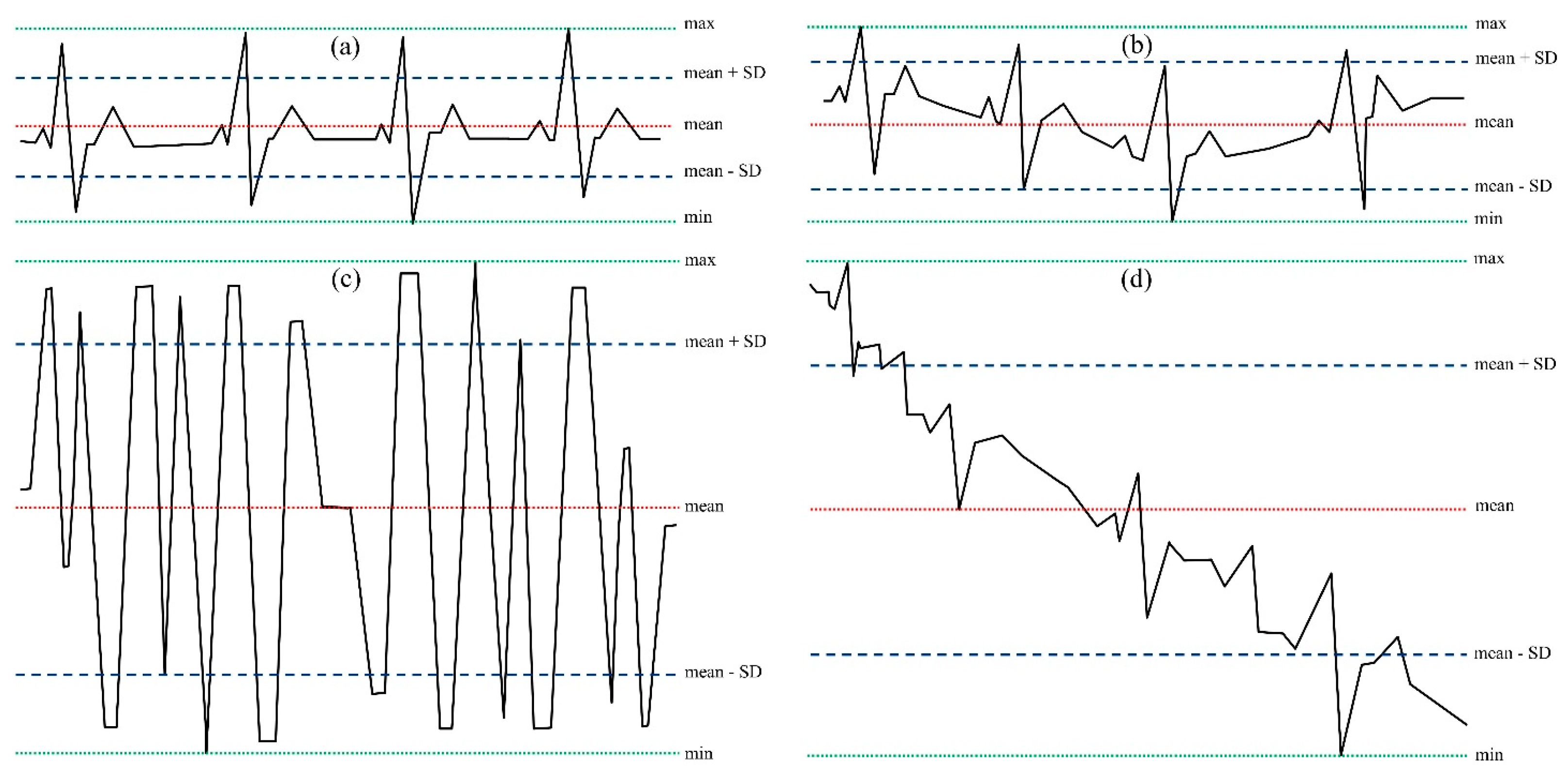
- Limited variability in the isoelectric line;
- A limited range of signal variation (maximum (max)–minimum (min) values);
- A limited statistical variability (quantified through the standard deviation, SD).
2.2. Experimental Design
- (1)
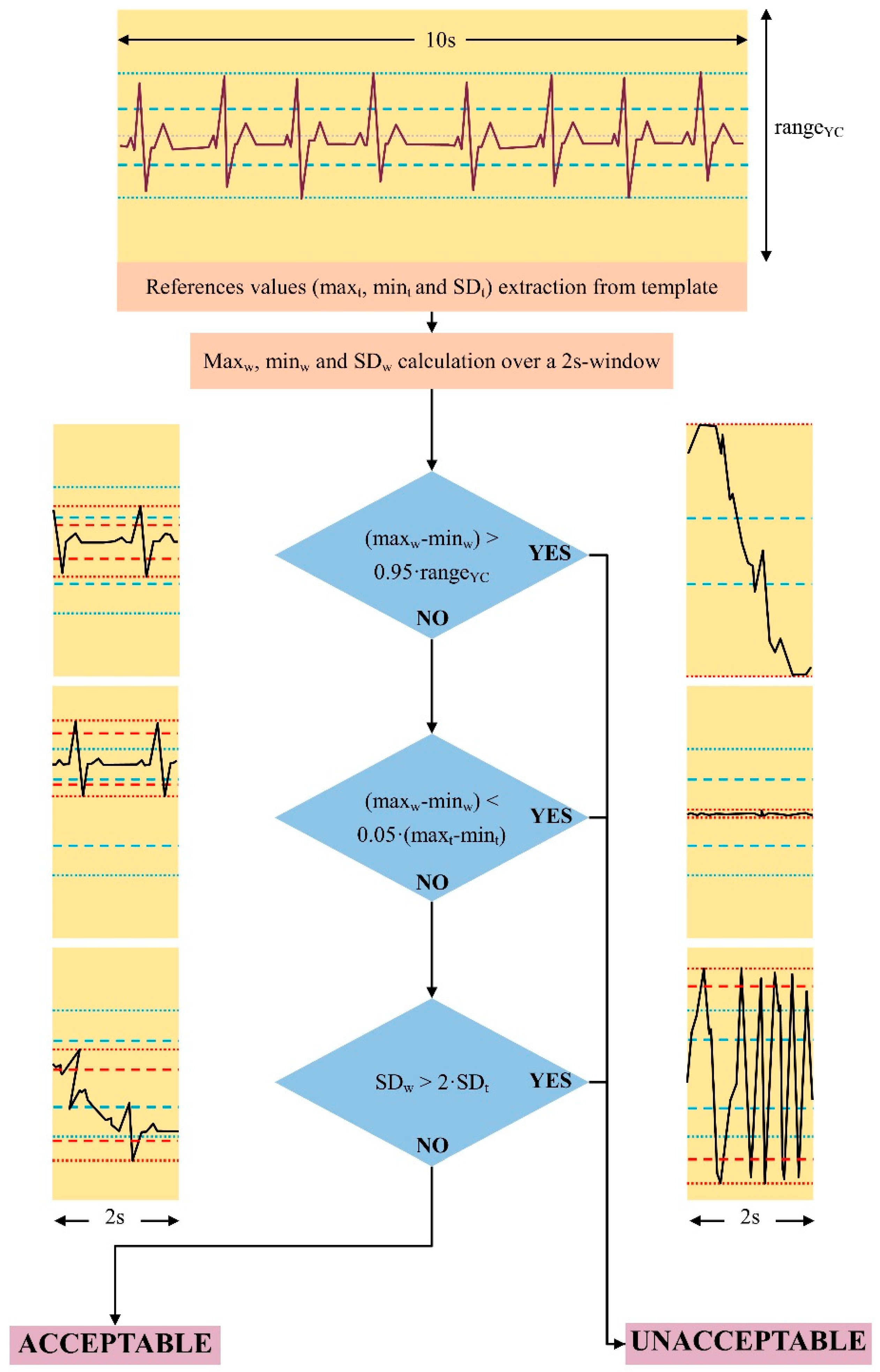
- Reference values calculation: search for the first stable signal period (constant isoelectric line and amplitude variability less than 70% of the maximal acquisition range for at least 10 s). Maximum (maxt), minimum (mint), and standard deviation (SDt) values were used as a reference for the subsequent steps.
- (2)
- Starting from the trace starting point, selection of consecutive signal 2 s windows for quality evaluation. Calculation of maximum (maxw), minimum (minw), and standard deviation (SDw) over the 2 s window;
- (3)
- Comparison between the maximum (maxw) and minimum (minw) values of the unknown signal and the reference analog to digital conversion range of the YouCare system (±500 mV, rangeYC);
- (4)
- Comparison of the standard deviation (SDw) and the variability range (maxw–minw) of the signal to be classified with the reference values (maxt–mint and SDt).
- (a)
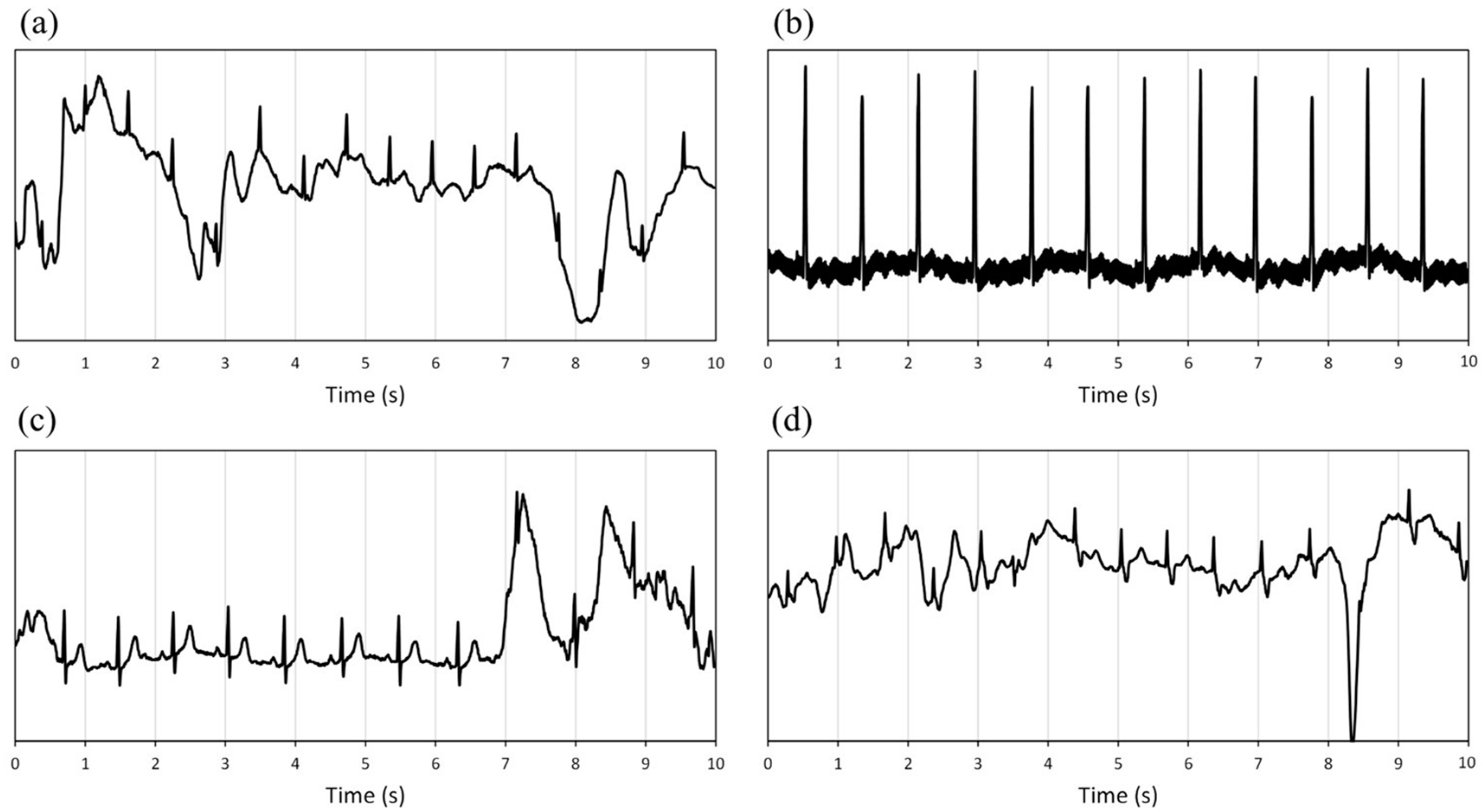
- If the trace oscillates between values close to saturation ((maxw–minw) > 0.95 × (rangeYC)) (e.g., Figure 1c in a short period (2 s), a short-time significant isoelectric line’s oscillation compromises the reading and the signal is classified as unacceptable;
- (b)
- If the signal’s range (maxw–minw) is lower than 5% of the template’s range (maxt–mint), QRS, P, and T waves are very low or absent. The signal is classified as unacceptable.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huhn, S.; Axt, M.; Gunga, H.-C.; Maggioni, M.A.; Munga, S.; Obor, D.; Sié, A.; Boudo, V.; Bunker, A.; Sauerborn, R.; et al. The Impact of Wearable Technologies in Health Research: Scoping Review. JMIR Mhealth Uhealth 2022, 10, e34384. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.D.; Naik, G.R. (Eds.) Wearable/Personal Monitoring Devices Present to Future; Springer: Singapore, 2022; ISBN 9789811653230. [Google Scholar]
- Statistics & Facts on Wearable Technology | Statista. Available online: https://www.statista.com/topics/1556/wearable-technology/#topicOverview (accessed on 27 December 2023).
- Prieto-Avalos, G.; Cruz-Ramos, N.A.; Alor-Hernández, G.; Sánchez-Cervantes, J.L.; Rodríguez-Mazahua, L.; Guarneros-Nolasco, L.R. Wearable Devices for Physical Monitoring of Heart: A Review. Biosensors 2022, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Oberdier, M.T.; van Abeelen, K.C.J.; Menghini, L.; Tumarkin, E.; Tripathi, H.; Jaipalli, S.; Orro, A.; Paolocci, N.; Gallelli, I.; et al. Electrocardiogram Monitoring Wearable Devices and Artificial-Intelligence-Enabled Diagnostic Capabilities: A Review. Sensors 2023, 23, 4805. [Google Scholar] [CrossRef] [PubMed]
- van der Bijl, K.; Elgendi, M.; Menon, C. Automatic ECG Quality Assessment Techniques: A Systematic Review. Diagnostics 2022, 12, 2578. [Google Scholar] [CrossRef] [PubMed]
- Satija, U.; Ramkumar, B.; Manikandan, M.S. A Review of Signal Processing Techniques for Electrocardiogram Signal Quality Assessment. IEEE Rev. Biomed. Eng. 2018, 11, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.-F.; Nault, I.; Blier, L.; Roy, K.; et al. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, Z.; Al-Zaiti, S.S.; Bond, R.; Sejdić, E. Remote and Wearable ECG Devices with Diagnostic Abilities in Adults: A State-of-the-Science Scoping Review. Heart Rhythm 2022, 19, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Moodie, M.; Freedman, B.; Lam, C.; Tu, H.; Swift, C.; Ma, S.; Mok, V.C.T.; Sui, Y.; Sharpe, D.; et al. Cost-Effectiveness of Monitoring Patients Post-Stroke with Mobile ECG During the Hospital Stay. J. Am. Heart Assoc. 2022, 11, e022735. [Google Scholar] [CrossRef]
- Liverani, M.; Ir, P.; Perel, P.; Khan, M.; Balabanova, D.; Wiseman, V. Assessing the Potential of Wearable Health Monitors for Health System Strengthening in Low- and Middle-Income Countries: A Prospective Study of Technology Adoption in Cambodia. Health Policy Plan. 2022, 37, 943–951. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, S.; Hou, J.; Ma, G.; Liu, Y.; Fan, Y.; Luo, L.; Shi, Z. Nurses’ Willingness and Demand for Internet+Home Care Services and the Associated Factors in Municipal Hospitals in China: Cross-Sectional Survey. J. Med. Internet. Res. 2023, 25, e45602. [Google Scholar] [CrossRef]
- Teng, X.-F.; Zhang, Y.-T.; Poon, C.C.Y.; Bonato, P. Wearable Medical Systems for P-Health. IEEE Rev. Biomed. Eng. 2008, 1, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Vasan, A.; Friend, J. Medical Devices for Low- and Middle-Income Countries: A Review and Directions for Development. J. Med. Device 2020, 14, 010803. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Ho, S.H.; Bae, Y.-H.; Lee, M.; Kim, J.H.; Kim, J.H.; Lee, J. Digital Health Equity and Tailored Health Care Service for People With Disability: User-Centered Design and Usability Study. J. Med. Internet. Res. 2023, 25, e50029. [Google Scholar] [CrossRef] [PubMed]
- Investing in the Radical Reorientation of Health Systems towards Primary Health Care: The Best and Only Choice to Achieve Universal Health Coverage. Available online: https://www.who.int/news/item/09-11-2023-investing-in-the-radical-reorientation-of-health-systems-towards-primary-health-care--the-best-and-only-choice-to-achieve-universal-health-coverage (accessed on 27 December 2023).
- Mukhopadhyay, A.; D’Angelo, R.; Senser, E.; Whelan, K.; Wee, C.C.; Mukamal, K.J. Racial and Insurance Disparities among Patients Presenting with Chest Pain in the US: 2009–2015. Am. J. Emerg. Med. 2020, 38, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Ardeti, V.A.; Kolluru, V.R.; Varghese, G.T.; Patjoshi, R.K. An Overview on State-of-the-Art Electrocardiogram Signal Processing Methods: Traditional to AI-Based Approaches. Expert Syst. Appl. 2023, 217, 119561. [Google Scholar] [CrossRef]
- Xie, J.; Peng, L.; Wei, L.; Gong, Y.; Zuo, F.; Wang, J.; Yin, C.; Li, Y. A Signal Quality Assessment-Based ECG Waveform Delineation Method Used for Wearable Monitoring Systems. Med. Biol. Eng. Comput. 2021, 59, 2073–2084. [Google Scholar] [CrossRef]
- Holgado-Cuadrado, R.; Plaza-Seco, C.; Lovisolo, L.; Blanco-Velasco, M. Characterization of Noise in Long-Term ECG Monitoring with Machine Learning Based on Clinical Criteria. Med. Biol. Eng. Comput. 2023, 61, 2227–2240. [Google Scholar] [CrossRef]
- Li, Q.; Rajagopalan, C.; Clifford, G.D. A Machine Learning Approach to Multi-Level ECG Signal Quality Classification. Comput. Methods Programs Biomed. 2014, 117, 435–447. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, F.; Xia, S.; Shi, S.; Chen, L.; Wang, Z. Dynamic ECG Signal Quality Evaluation Based on Persistent Homology and GoogLeNet Method. Front. Neurosci. 2023, 17, 1153386. [Google Scholar] [CrossRef]
- Smital, L.; Haider, C.R.; Vitek, M.; Leinveber, P.; Jurak, P.; Nemcova, A.; Smisek, R.; Marsanova, L.; Provaznik, I.; Felton, C.L.; et al. Real-Time Quality Assessment of Long-Term ECG Signals Recorded by Wearables in Free-Living Conditions. IEEE Trans. Biomed. Eng. 2020, 67, 2721–2734. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J Cardiovasc Dev Dis 2023, 10, 175. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Hazra, A.; Gogtay, N. Biostatistics Series Module 7: The Statistics of Diagnostic Tests. Indian J. Dermatol. 2017, 62, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Witvliet, M.P.; Karregat, E.P.M.; Himmelreich, J.C.L.; de Jong, J.S.S.G.; Lucassen, W.A.M.; Harskamp, R.E. Usefulness, Pitfalls and Interpretation of Handheld Single-lead Electrocardiograms. J. Electrocardiol. 2021, 66, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Mannhart, D.; Lischer, M.; Knecht, S.; du Fay de Lavallaz, J.; Strebel, I.; Serban, T.; Vögeli, D.; Schaer, B.; Osswald, S.; Mueller, C.; et al. Clinical Validation of 5 Direct-to-Consumer Wearable Smart Devices to Detect Atrial Fibrillation: BASEL Wearable Study. JACC Clin. Electrophysiol. 2023, 9, 232–242. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.-H.; Lee, W.; Kang, S.-H.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Comparison of Continuous ECG Monitoring by Wearable Patch Device and Conventional Telemonitoring Device. J. Korean Med. Sci. 2020, 35, e363. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in Cardiology: Here to Stay. Heart Rhythm 2020, 17, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Oberdier, M.T.; Augello, A.; Suzuki, M.; Tumarkin, E.; Jaipalli, S.; Geminiani, G.A.; Halperin, H.R.; Borghi, C. Algorithm for Mobile Platform-Based Real-Time QRS Detection. Sensors 2023, 23, 1625. [Google Scholar] [CrossRef]
| Classification by Cardiologists | ||||
|---|---|---|---|---|
| Classification by algorithm | Not acceptable | Acceptable | Total | |
| Not acceptable | 3150 (94.7%) | 177 (4.6%) | 3327 | |
| Acceptable | 175 (5.3%) | 3698 (95.4%) | 3873 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, L.; Gallelli, I.; Dall’Olio, M.; Lago, J.; Borghi, C.; Diemberger, I.; Corazza, I. Validation of a New and Straightforward Algorithm to Evaluate Signal Quality during ECG Monitoring with Wearable Devices Used in a Clinical Setting. Bioengineering 2024, 11, 222. https://doi.org/10.3390/bioengineering11030222
Neri L, Gallelli I, Dall’Olio M, Lago J, Borghi C, Diemberger I, Corazza I. Validation of a New and Straightforward Algorithm to Evaluate Signal Quality during ECG Monitoring with Wearable Devices Used in a Clinical Setting. Bioengineering. 2024; 11(3):222. https://doi.org/10.3390/bioengineering11030222
Chicago/Turabian StyleNeri, Luca, Ilaria Gallelli, Massimo Dall’Olio, Jessica Lago, Claudio Borghi, Igor Diemberger, and Ivan Corazza. 2024. "Validation of a New and Straightforward Algorithm to Evaluate Signal Quality during ECG Monitoring with Wearable Devices Used in a Clinical Setting" Bioengineering 11, no. 3: 222. https://doi.org/10.3390/bioengineering11030222
APA StyleNeri, L., Gallelli, I., Dall’Olio, M., Lago, J., Borghi, C., Diemberger, I., & Corazza, I. (2024). Validation of a New and Straightforward Algorithm to Evaluate Signal Quality during ECG Monitoring with Wearable Devices Used in a Clinical Setting. Bioengineering, 11(3), 222. https://doi.org/10.3390/bioengineering11030222








