Mendelian Randomisation Analysis of Causal Association between Lifestyle, Health Factors, and Keratoconus
Abstract
1. Introduction
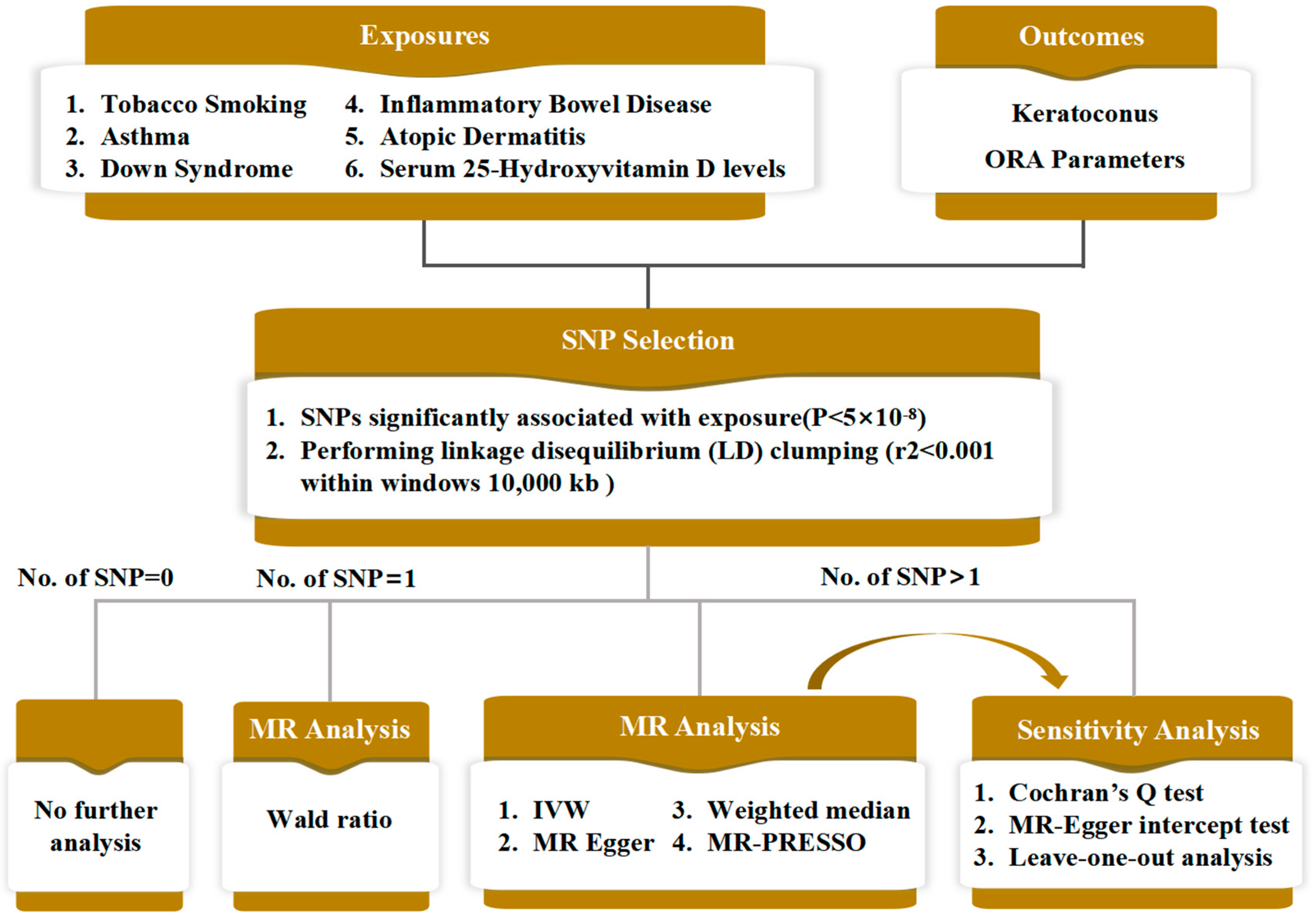
2. Methods
2.1. Study Design and Data Source
2.2. Data Analysis
3. Results
3.1. Mendelian Randomisation Analysis with ORA Parameters as the Outcomes
3.2. Mendelian Randomisation Analysis with Keratoconus as the Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KC | Keratoconus |
| MR | Mendelian randomisation |
| IVW | Inverse variance weighted |
| SNP | Single-nucleotide polymorphism |
| ORA | Ocular Response Analyzer |
| CVS | Corvis ST |
| CH | Corneal hysteresis |
| CRF | Corneal resistance factor |
| RCT | Randomised control trial |
| GWAS | Genome-wide association study |
| SSI | Stress–stain index |
| CBI | Sorneal biomechanical index |
| CEU | Cardiovascular Epidemiology Unit |
| IV | Instrumental variables |
| LD | Linkage disequilibrium |
| MR Egger | Mendelian randomisation–Egger |
| CI | Confidence interval |
| OR | Odds ratio |
| ECM | Extracellular matrix |
| MMPs | Matrix metalloproteinases |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| SP-A1 | Corneal stiffness parameter at first applanation time |
References
- Gordon-Shaag, A.; Millodot, M.; Shneor, E.; Liu, Y. The Genetic and Environmental Factors for Keratoconus. BioMed Res. Int. 2015, 2015, 795738. [Google Scholar] [CrossRef]
- Veerappa, A.M. Cascade of interactions between candidate genes reveals convergent mechanisms in keratoconus disease pathogenesis. Ophthalmic Genet. 2021, 42, 114–131. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Hanada, K.; Muramatsu, O.; Ishibazawa, A.; Nishikawa, N.; Yoshida, A. Wound Healing Process After Corneal Stromal Thinning Observed With Anterior Segment Optical Coherence Tomography. Cornea 2014, 33, 1056–1060. [Google Scholar] [CrossRef]
- Godefrooij, D.A.; de Wit, G.A.; Uiterwaal, C.S.; Imhof, S.M.; Wisse, R.P.L. Age-specific Incidence and Prevalence of Keratoconus: A Nationwide Registration Study. Am. J. Ophthalmol. 2017, 175, 169–172. [Google Scholar] [CrossRef]
- Hashemi, H.; Heydarian, S.; Hooshmand, E.; Saatchi, M.; Yekta, A.; Aghamirsalim, M.; Valadkhan, M.; Mortazavi, M.; Hashemi, A.; Khabazkhoob, M. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2020, 39, 263–270. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Roberts, C.J. Biomechanics of corneal ectasia and biomechanical treatments. J. Cataract Refract. Surg. 2014, 40, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Esporcatte, L.P.G.; Salomão, M.Q.; Lopes, B.T.; Sena, N.; Ferreira, É.; Filho, J.B.R.F.; Machado, A.P.; Ambrósio, R. Biomechanics in Keratoconus Diagnosis. Curr. Eye Res. 2023, 48, 130–136. [Google Scholar] [CrossRef]
- Sahebjada, S.; Chan, E.; Xie, J.; Snibson, G.R.; Daniell, M.; Baird, P.N. Risk factors and association with severity of keratoconus: The Australian study of Keratoconus. Int. Ophthalmol. 2021, 41, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Mathan, J.J.; Simkin, S.K.; Gokul, A.; McGhee, C.N.J. Down syndrome and the eye: Ocular characteristics and ocular assessment. Surv. Ophthalmol. 2022, 67, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Kristianslund, O.; Drolsum, L. Prevalence of keratoconus in persons with Down syndrome: A review. BMJ Open Ophth. 2021, 6, e000754. [Google Scholar] [CrossRef] [PubMed]
- Tréchot, F.; Angioi, K.; Latarche, C.; Conroy, G.; Beaujeux, P.; Andrianjafy, C.; Portier, M.; Batta, B.; Conart, J.-B.; Cloché, V.; et al. Keratoconus in Inflammatory Bowel Disease Patients: A Cross-sectional Study. J Crohns Colitis. 2015, 9, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.A.; Blachley, T.S.; Stein, J.D. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus. Ophthalmology 2016, 123, 457–465.e2. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Weng, S.; Wang, J.; Tseng, S.; Wang, J.; Jan, H.; Ko, S.; Tsai, W.; Chen, H.; Liou, C.; et al. Association between keratoconus and the risk of adolescent- or adult-onset atopic dermatitis. Allergy 2020, 75, 2946–2948. [Google Scholar] [CrossRef]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Cornish, A.J.; Tomlinson, I.P.M.; Houlston, R.S. Mendelian randomisation: A powerful and inexpensive method for identifying and excluding non-genetic risk factors for colorectal cancer. Mol. Asp. Med. 2019, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Huang, Y.; Guan, P.; Huang, D.; Yu, H.; Yang, X.; Liu, L. Mendelian randomization analyses in ocular disease: A powerful approach to causal inference with human genetic data. J. Transl. Med. 2022, 20, 621. [Google Scholar] [CrossRef]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Statist. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef]
- The International IBD Genetics Consortium (IIBDGC); Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Sheehan, N.A.; Didelez, V.; Burton, P.R.; Tobin, M.D. Mendelian Randomisation and Causal Inference in Observational Epidemiology. PLoS Med. 2008, 5, e177. [Google Scholar] [CrossRef]
- Yavorska, O.O.; Burgess, S. MendelianRandomization: An R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 2017, 46, 1734–1739. [Google Scholar] [CrossRef]
- Li, P.; Guo, M.; Wang, C.; Liu, X.; Zou, Q. An overview of SNP interactions in genome-wide association studies. Brief. Funct. Genom. 2015, 14, 143–155. [Google Scholar] [CrossRef]
- Wood, A.T.A. An F Approximation to the Distribution of a Linear Combination of Chi-squared Variables. Commun. Stat.-Simul. Comput. 1989, 18, 1439–1456. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Y.; Zhong, X.; Meng, F.; Hemminki, K.; Qiu, J.; Shu, X. Association between intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid (n-3 PUFA DHA) and reduced risk of ovarian cancer: A systematic Mendelian Randomization study. Clin. Nutr. 2023, 42, 1379–1388. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization: A framework for two-sample summary data MR. Statist. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Han, X.; Ong, J.-S.; An, J.; Hewitt, A.W.; Gharahkhani, P.; MacGregor, S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur. J. Epidemiol. 2020, 35, 139–146. [Google Scholar] [CrossRef]
- Cuellar-Partida, G.; Lu, Y.; Kho, P.F.; Hewitt, A.W.; Wichmann, H.-E.; Yazar, S.; Stambolian, D.; Bailey-Wilson, J.E.; Wojciechowski, R.; Wang, J.J.; et al. Assessing the Genetic Predisposition of Education on Myopia: A Mendelian Randomization Study: Assessing the Genetic Predisposition of Education on Myopia. Genet. Epidemiol. 2016, 40, 66–72. [Google Scholar] [CrossRef]
- Cuellar-Partida, G.; Williams, K.M.; Yazar, S.; Guggenheim, J.A.; Hewitt, A.W.; Williams, C.; Wang, J.J.; Kho, P.-F.; Saw, S.M.; Cheng, C.-Y.; et al. Genetically low vitamin D concentrations and myopic refractive error: A Mendelian randomization study. Int. J. Epidemiol. 2017, 46, 1882–1890. [Google Scholar] [CrossRef]
- Hafezi, F. Smoking and Corneal Biomechanics. Ophthalmology 2009, 116, 2259.e1. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Shen, Y.; Yan, Z.; Tian, M.; Zhao, J.; Zhou, X. Relationship Among Corneal Stiffness, Thickness, and Biomechanical Parameters Measured by Corvis ST, Pentacam and ORA in Keratoconus. Front. Physiol. 2019, 10, 740. [Google Scholar] [CrossRef]
- Shah, S.; Laiquzzaman, M.; Bhojwani, R.; Mantry, S.; Cunliffe, I. Assessment of the Biomechanical Properties of the Cornea with the Ocular Response Analyzer in Normal and Keratoconic Eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3026. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Mohan, S.; Pye, D.C.; Willcox, M.D.P. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012, 90, e303–e309. [Google Scholar] [CrossRef]
- Claessens, J.L.J.; Godefrooij, D.A.; Vink, G.; Frank, L.E.; Wisse, R.P.L. Nationwide epidemiological approach to identify associations between keratoconus and immune-mediated diseases. Br. J. Ophthalmol. 2022, 106, 1350–1354. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Toft, P.B.; Halling-Overgaard, A.-S.; Gislason, G.H.; Skov, L.; Egeberg, A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J. Am. Acad. Dermatol. 2017, 77, 280–286.e1. [Google Scholar] [CrossRef]
- Zhou, W.; Cai, J.; Li, Z.; Lin, Y. Association of atopic dermatitis with conjunctivitis and other ocular surface diseases: A bidirectional two-sample Mendelian randomization study. Acad. Dermatol. Venereol. 2023, 37, 1642–1648. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Are Proteinases the Reason for Keratoconus? Curr. Eye Res. 2010, 35, 185–191. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 147, pp. 1–73. [Google Scholar] [CrossRef]
- Lema, I.; Duran, J. Inflammatory Molecules in the Tears of Patients with Keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef]
- Lema, I.; Sobrino, T.; Duran, J.A.; Brea, D.; Diez-Feijoo, E. Subclinical keratoconus and inflammatory molecules from tears. Br. J. Ophthalmol. 2009, 93, 820–824. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Desreumaux, P.; Sandborn, W.J.; Colombel, J.-F. Crohn’s disease: Beyond antagonists of tumour necrosis factor. Lancet 2008, 372, 67–81. [Google Scholar] [CrossRef]
- Akkaya, S.; Ulusoy, D.M. Serum Vitamin D Levels in Patients with Keratoconus. Ocul. Immunol. Inflamm. 2020, 28, 348–353. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: Its role and uses in immunology. FASEB J. 2001, 15, 2579–2585. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal Morphologic Characteristics in Patients With Down Syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef]
- Kristianslund, O.; Drolsum, L. Prevalence of Keratoconus in Persons With Down Syndrome in a National Registry in Norway. JAMA Netw. Open 2021, 4, e210814. [Google Scholar] [CrossRef]
- Haugen, O.H.; Høvding, G.; Eide, G.E. Biometric measurements of the eyes in teenagers and young adults with Down syndrome. Acta Ophthalmol. Scand. 2001, 79, 616–625. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Sex Hormones, Growth Hormone, and the Cornea. Cells 2022, 11, 224. [Google Scholar] [CrossRef]
- Sharif, R.; Bak-Nielsen, S.; Hjortdal, J.; Karamichos, D. Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Prog. Retin. Eye Res. 2018, 67, 150–167. [Google Scholar] [CrossRef]
- Mazzotta, C.; Ferrise, M.; Gabriele, G.; Gennaro, P.; Meduri, A. Chemically-Boosted Corneal Cross-Linking for the Treatment of Keratoconus through a Riboflavin 0.25% Optimized Solution with High Superoxide Anion Release. JCM 2021, 10, 1324. [Google Scholar] [CrossRef]
- Esporcatte, L.P.G. Biomechanical diagnostics of the cornea. Eye Vis. 2020, 7, 9. [Google Scholar] [CrossRef]
- Tejwani, S.; Shetty, R.; Kurien, M.; Dinakaran, S.; Ghosh, A.; Roy, A.S. Biomechanics of the Cornea Evaluated by Spectral Analysis of Waveforms from Ocular Response Analyzer and Corvis-ST. PLoS ONE 2014, 9, e97591. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Chernyak, D. Brillouin microscopy: Assessing ocular tissue biomechanics. Curr. Opin. Ophthalmol. 2018, 29, 299–305. [Google Scholar] [CrossRef]
| Outcome | MR Methods | No. of SNPs | OR (95%CI) | p-Value | P-Heterogeneity | P-Pleiotropy |
|---|---|---|---|---|---|---|
| CH | 20 | 0.360 | 0.192 | |||
| IVW | 1.572 (1.216–2.033) | <0.001 * | ||||
| Weighted median | 1.345 (0.955–1.893) | 0.090 | ||||
| MR-Egger | 0.874 (0.360–2.121) | 0.769 | ||||
| CRF | 20 | 0.525 | 0.624 | |||
| IVW | 1.380 (1.086–1.755) | 0.009 * | ||||
| Weighted median | 1.251 (0.881–1.777) | 0.211 | ||||
| MR-Egger | 1.122 (0.481–2.616) | 0.792 |
| Outcome | MR Methods | No. of SNPs | OR (95%CI) | p-Value | P-Heterogeneity | P-Pleiotropy |
|---|---|---|---|---|---|---|
| CH (Outlier removed) | 110 | <0.001 * | 0.722 | |||
| IVW | 0.989 (0.978–0.999) | 0.032 * | ||||
| Weighted median | 0.994 (0.980–1.009) | 0.446 | ||||
| MR-Egger | 0.984 (0.960–1.010) | 0.226 | ||||
| CRF (Outlier removed) | 108 | <0.001 * | 0.879 | |||
| IVW | 0.982 (0.971–0.993) | 0.001 * | ||||
| Weighted median | 0.983 (0.971–0.996) | 0.010 * | ||||
| MR-Egger | 0.980 (0.955–1.006) | 0.137 |
| Exposures | MR Methods | No. of SNPs | OR (95%CI) | p-Value | P-Heterogeneity | P-Pleiotropy |
|---|---|---|---|---|---|---|
| Current tobacco smoking | Wald ratio | 1 | 0.055 (0.004–0.677) | 0.024 * | NA | NA |
| Down syndrome | Wald ratio | 1 | 3.276 (1.453–7.388) | 0.004 * | NA | NA |
| Asthma | 98 | 0.683 | 0.255 | |||
| IVW | 39.901 (2.522–631.169) | 0.009 * | ||||
| Weighted median | 3.273 (0.043–251.786) | 0.593 | ||||
| MR-Egger | 1.020 (0.001–966.600) | 0.996 | ||||
| Inflammatory bowel disease | 111 | 0.303 | 0.723 | |||
| IVW | 1.206 (1.034–1.407) | 0.017 * | ||||
| Weighted median | 1.163 (0.921–1.469) | 0.203 | ||||
| MR-Egger | 1.132 (0.770–1.663) | 0.531 | ||||
| Atopic dermatitis | 20 | 0.606 | 0.504 | |||
| IVW | 1.452 (1.085–1.944) | 0.012 * | ||||
| Weighted median | 1.302 (0.864–1.961) | 0.207 | ||||
| MR-Egger | 1.142 (0.539–2.417) | 0.733 | ||||
| Serum 25-Hydroxyvitamin D levels | 104 | 0.327 | 0.930 | |||
| IVW | 2.146 (1.040–4.429) | 0.039 * | ||||
| Weighted median | 2.365 (0.770–7.264) | 0.142 | ||||
| MR-Egger | 2.218 (0.673–7.317) | 0.195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Yang, L.; Ye, Y.; He, L.; Chen, S.; Wang, J. Mendelian Randomisation Analysis of Causal Association between Lifestyle, Health Factors, and Keratoconus. Bioengineering 2024, 11, 221. https://doi.org/10.3390/bioengineering11030221
Cheng J, Yang L, Ye Y, He L, Chen S, Wang J. Mendelian Randomisation Analysis of Causal Association between Lifestyle, Health Factors, and Keratoconus. Bioengineering. 2024; 11(3):221. https://doi.org/10.3390/bioengineering11030221
Chicago/Turabian StyleCheng, Jiaxuan, Lanting Yang, Yishan Ye, Lvfu He, Shihao Chen, and Junjie Wang. 2024. "Mendelian Randomisation Analysis of Causal Association between Lifestyle, Health Factors, and Keratoconus" Bioengineering 11, no. 3: 221. https://doi.org/10.3390/bioengineering11030221
APA StyleCheng, J., Yang, L., Ye, Y., He, L., Chen, S., & Wang, J. (2024). Mendelian Randomisation Analysis of Causal Association between Lifestyle, Health Factors, and Keratoconus. Bioengineering, 11(3), 221. https://doi.org/10.3390/bioengineering11030221






