In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Preparation
2.3. Characterization
2.4. Cell Viability Studies
2.5. Wound Scratch
2.6. Wound Healing In Vivo
2.7. Statistical Analysis
3. Results
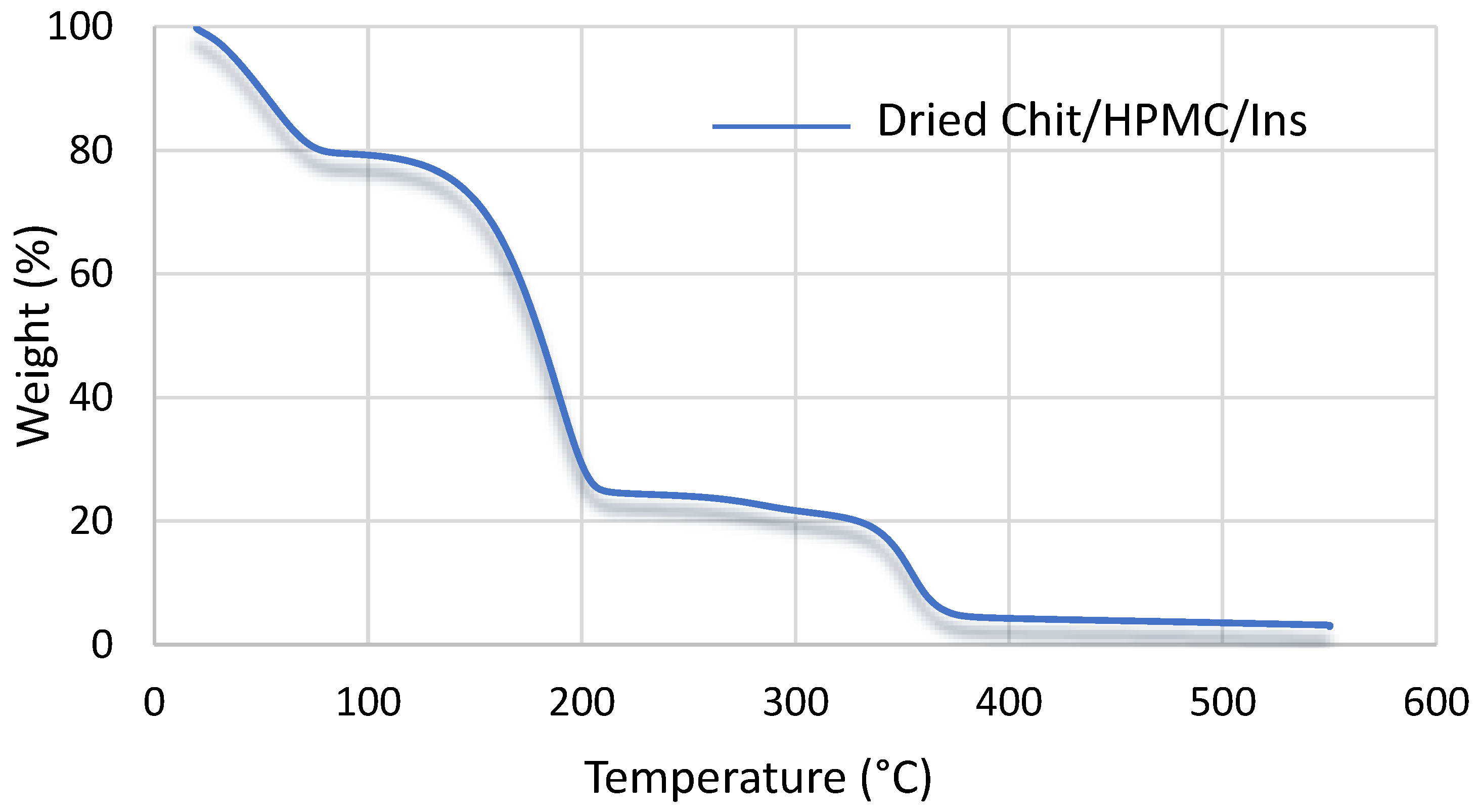
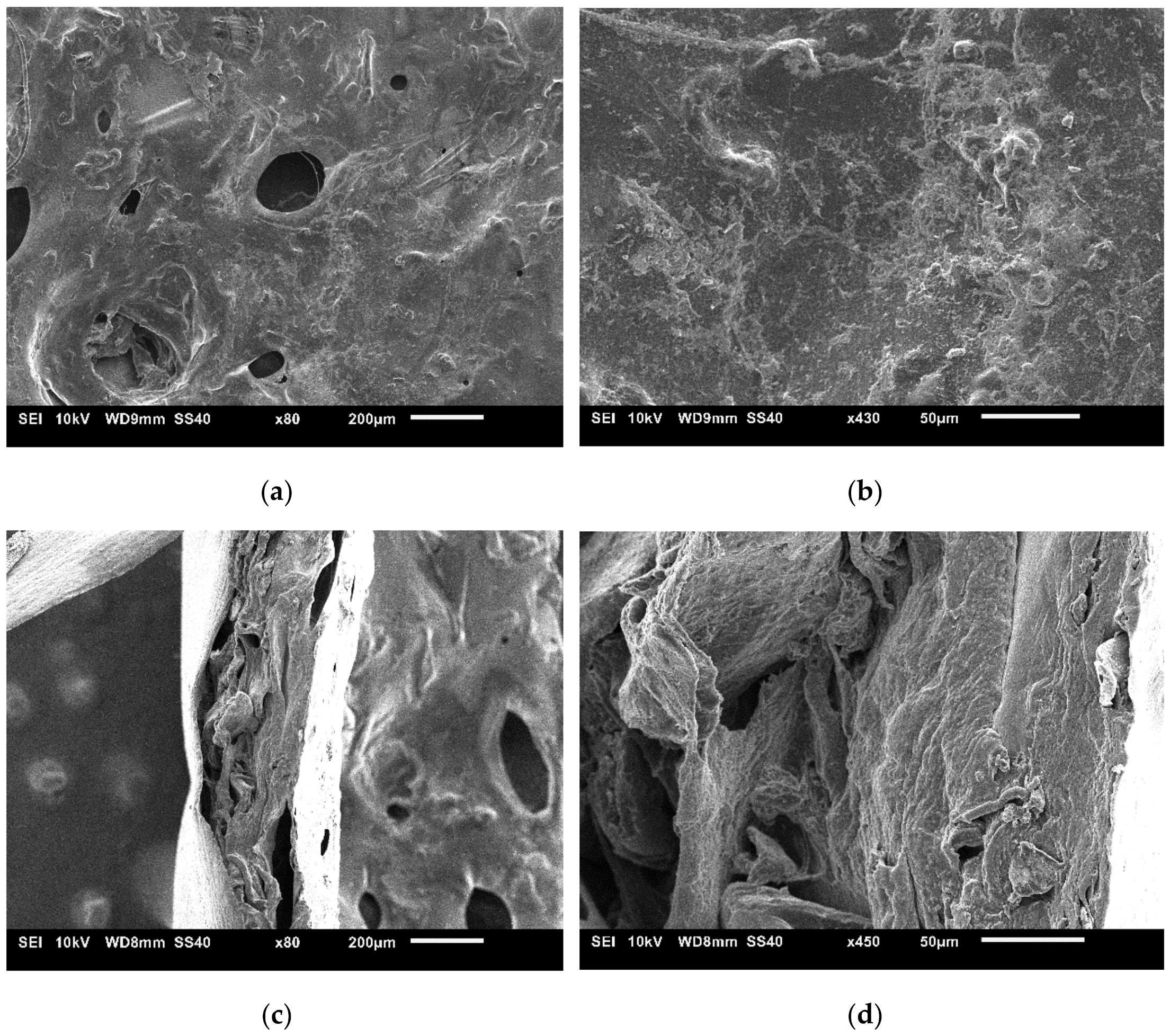
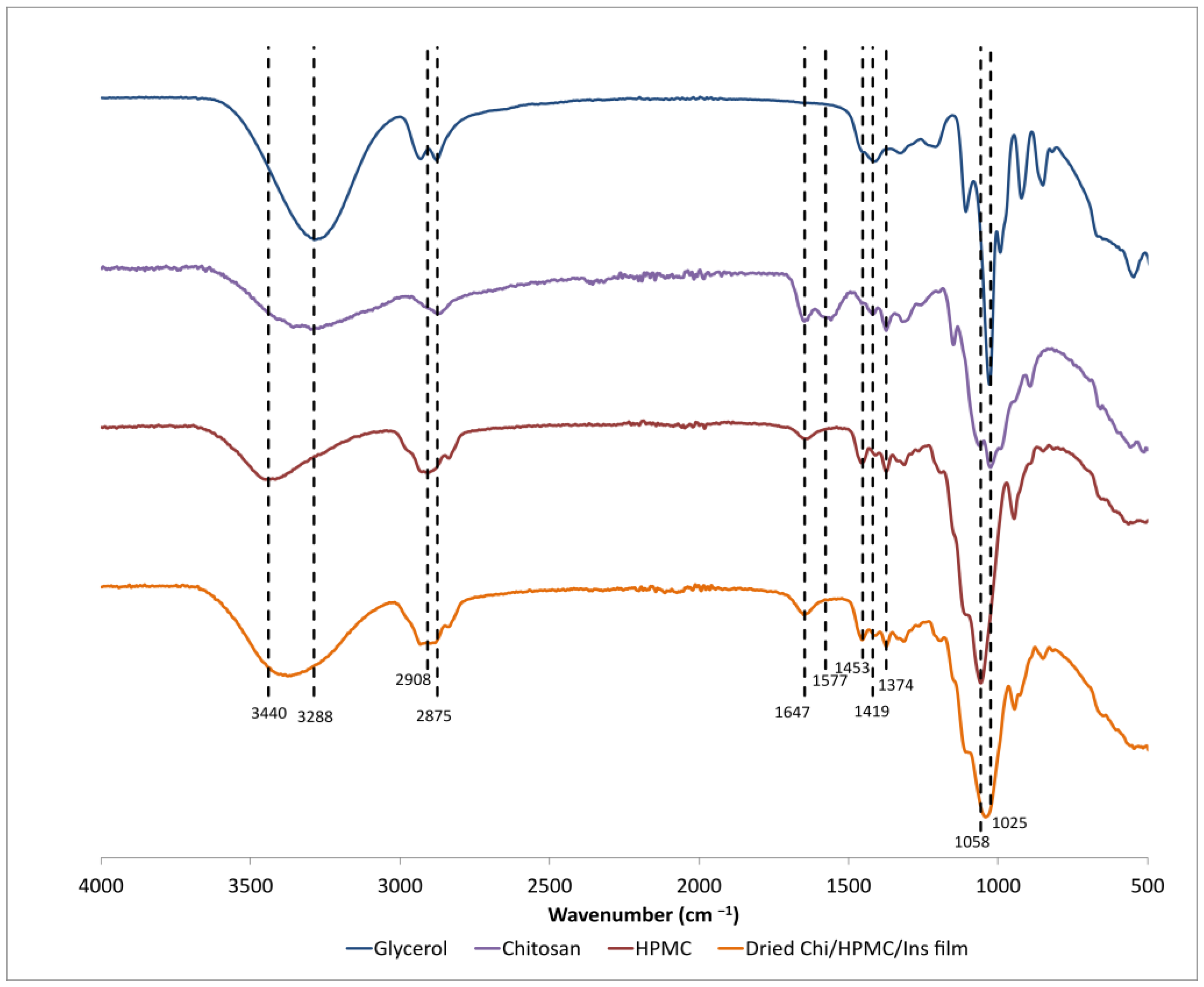
3.1. Characterization
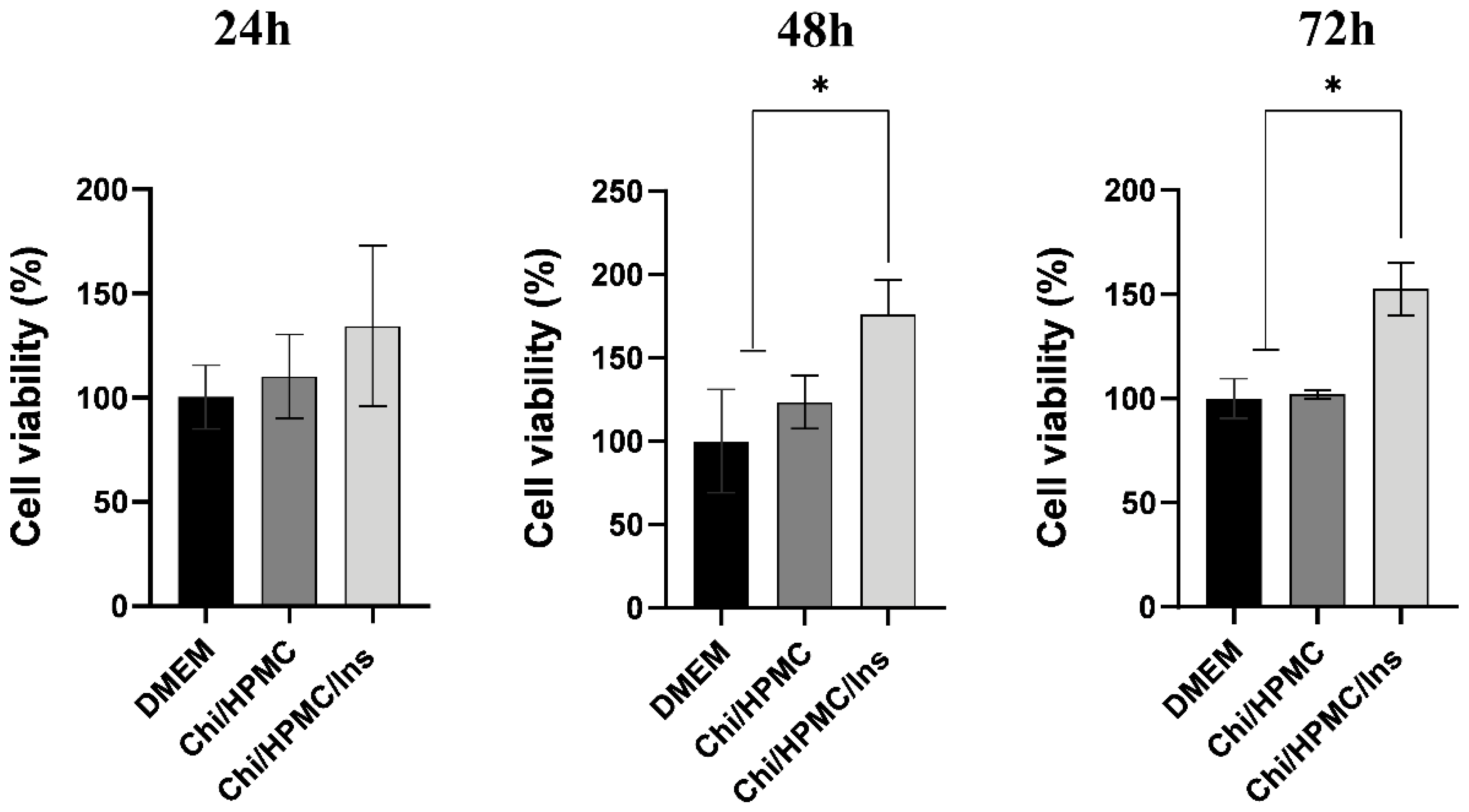
3.2. Chi/HPMC/Ins Hydrogel Enhances the Cellular Viability of Human Keratinocyte HaCaT
3.3. Chi/HPMC/Ins Hydrogel Stimulates Cell Proliferation and Migration according to the HaCaT Scratch Assay in Keratinocytes
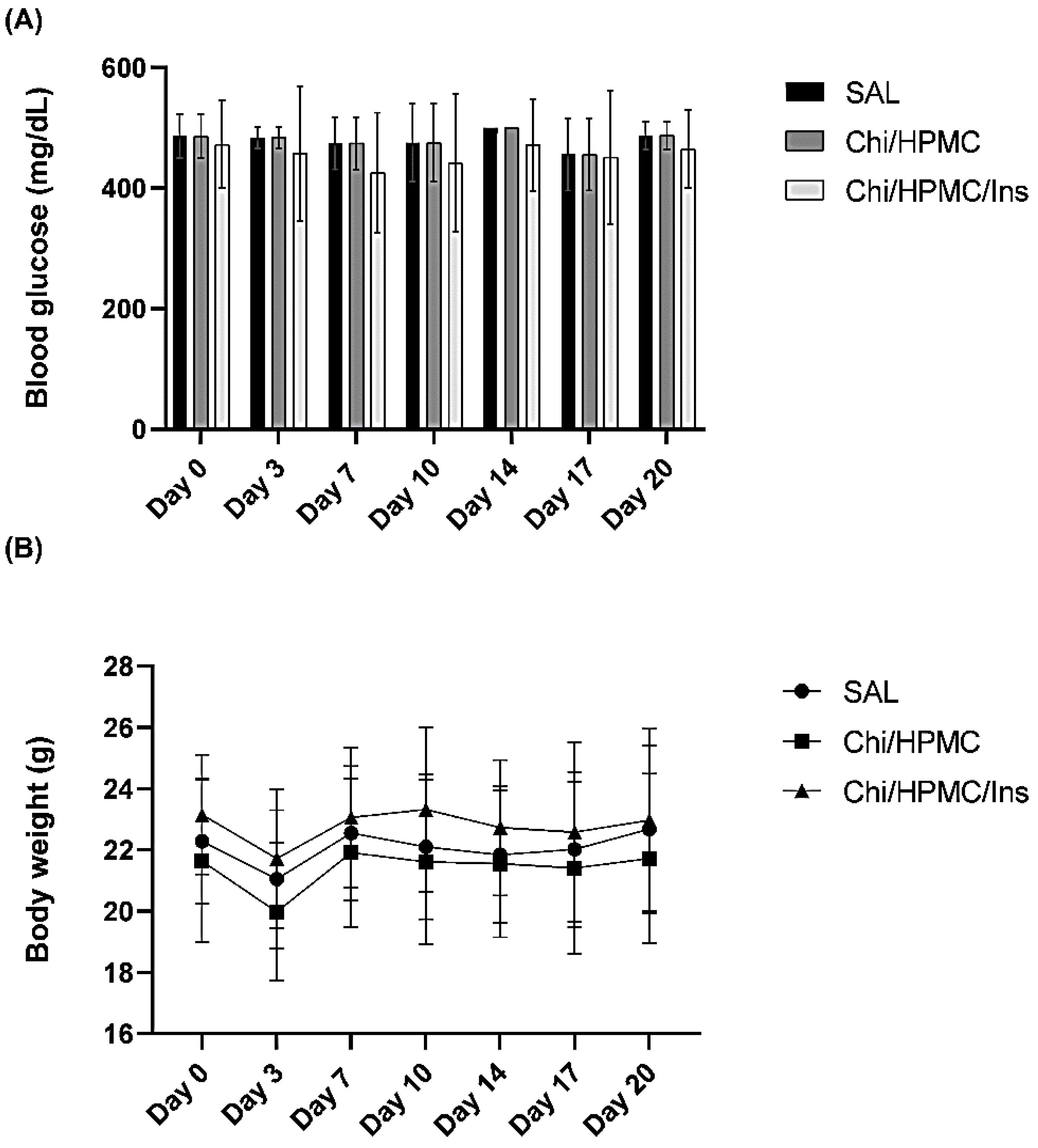
3.4. The Effects of Chi/HPMC/Ins on Body Weight and Fasting Blood Glucose
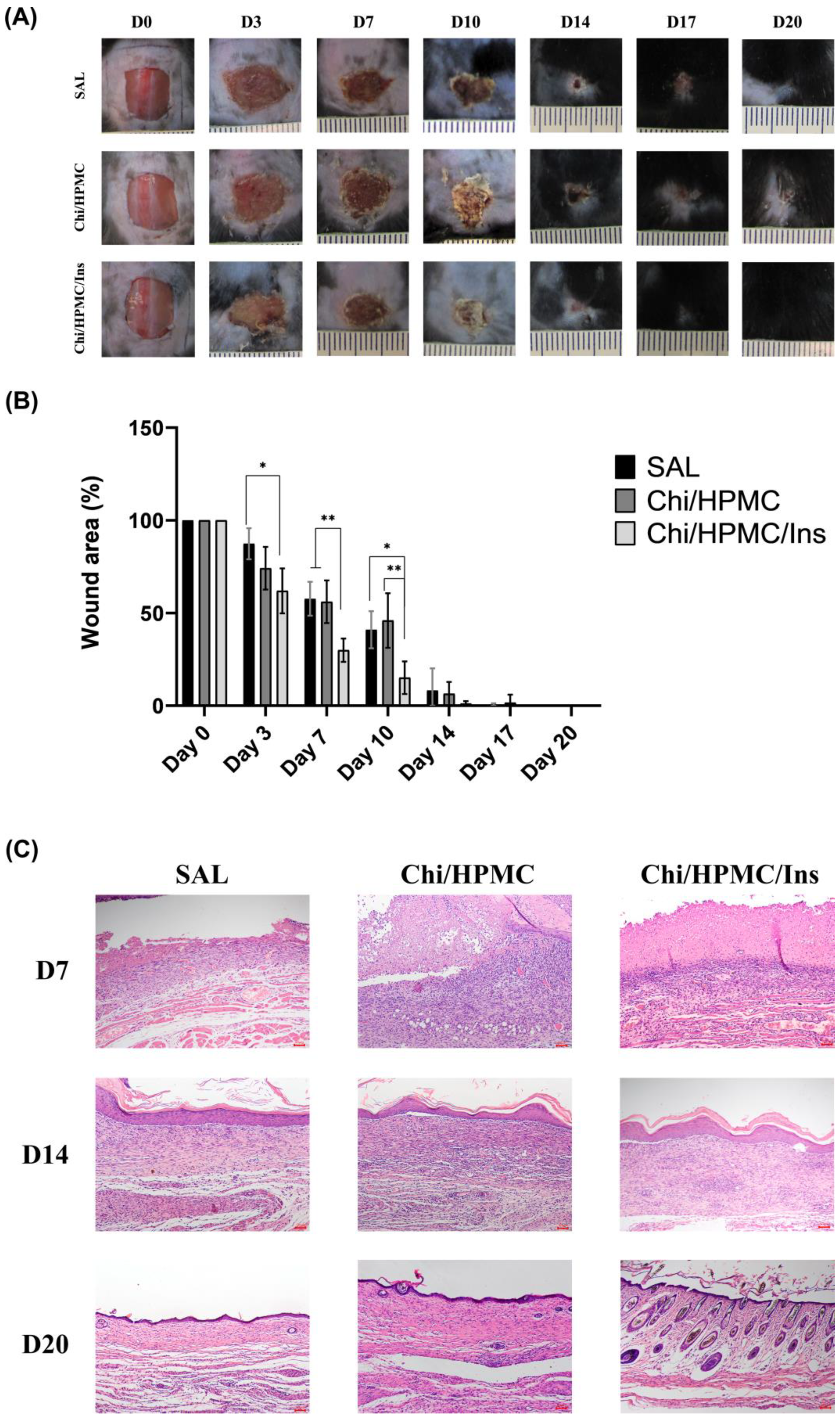
3.5. Chi/HPMC/Ins Hydrogel Reduces the Area of Full-Thickness Dorsal Skin Wound in Diabetic Mice
3.6. Histological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Brocke, T.; Barr, J. The History of Wound Healing. Surg. Clin. N. Am. 2020, 100, 787–806. [Google Scholar] [CrossRef] [PubMed]
- González-Saldivar, G.; Rodríguez-Gutiérrez, R.; Ocampo-Candiani, J.; González-González, J.G.; Gómez-Flores, M. Skin Manifestations of Insulin Resistance: From a Biochemical Stance to a Clinical Diagnosis and Management. Dermatol. Ther. 2017, 7, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J. Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab. Syndr. Obes. 2020, 13, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, T.; Burgeiro, A.; Carvalho, E. Effects of Insulin on the Skin: Possible Healing Benefits for Diabetic Foot Ulcers. Arch. Dermatol. Res. 2016, 308, 677–694. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhang, X. Topical Insulin Application Improves Healing by Regulating the Wound Inflammatory Response. Wound Repair Regen. 2012, 20, 425–434. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Hydrogels for the Controlled Delivery of Insulin. Pharmaceutics 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Ostróżka-Cieślik, A.; Wilczyński, S.; Dolińska, B. Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane. Polymers 2023, 15, 3639. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Aliabadi, H.A.M.; Sukhtezari, S.; Tahmasebi, B.; Maleki, A.; Madanchi, H. Chitosan Hydrogel/Silk Fibroin/Mg(OH)2 Nanobiocomposite as a Novel Scaffold with Antimicrobial Activity and Improved Mechanical Properties. Sci. Rep. 2021, 11, 650. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.-L.; Han, J.; Si, R.-J.; Liu, P.-P.; Zhang, Z.-R.; Wang, A.-M.; Zhang, J. Chitosan Hydrogel Encapsulated with LL-37 Peptide Promotes Deep Tissue Injury Healing in a Mouse Model. Mil. Med. Res. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalil, S.; Ayub, A.; Banat, I.M. Recent Developments in Chitosan Encapsulation of Various Active Ingredients for Multifunctional Applications. Carbohydr. Res. 2020, 492, 108004. [Google Scholar] [CrossRef]
- Guyot, C.; Cerruti, M.; Lerouge, S. Injectable, Strong and Bioadhesive Catechol-Chitosan Hydrogels Physically Crosslinked Using Sodium Bicarbonate. Mater. Sci. Eng. C 2021, 118, 111529. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-Based Hydrogels with Injectable, Self-Healing and Antibacterial Properties for Wound Healing. Carbohydr. Polym. 2022, 276, 118718. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar]
- Varshosaz, J.; Taymouri, S.; Minaiyan, M.; Rastegarnasab, F.; Baradaran, A. Development and In Vitro/In Vivo Evaluation of HPMC/Chitosan Gel Containing Simvastatin Loaded Self-Assembled Nanomicelles as a Potent Wound Healing Agent. Drug Dev. Ind. Pharm. 2018, 44, 276–288. [Google Scholar] [CrossRef]
- Chen, C.-P.; Hsieh, C.-M.; Tsai, T.; Yang, J.-C.; Chen, C.-T. Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef]
- Negrini, J.; Mozos, E.; Escamilla, A.; Pérez, J.; Lucena, R.; Guerra, R.; Ginel, P.J. Effects of Topical Insulin on Second-Intention Wound Healing in the Red-Eared Slider Turtle (Trachemys Scripta Elegans)—A Controlled Study. BMC Vet. Res. 2017, 13, 160. [Google Scholar] [CrossRef]
- Hardman, D.; George Thuruthel, T.; Iida, F. Self-Healing Ionic Gelatin/Glycerol Hydrogels for Strain Sensing Applications. NPG Asia Mater. 2022, 14, 11. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Enache, A.A.; Rimbu, C.M.; Badea, G.I.; Aflori, M. Chitosan Membranes Containing Plant Extracts: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2023, 24, 8673. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl Methylcellulose-Based Hydrogel Copolymeric for Controlled Delivery of Galantamine Hydrobromide in Dementia. Processes 2020, 8, 1350. [Google Scholar] [CrossRef]
- Akhlaq, M.; Maryam, F.; Elaissari, A.; Ullah, H.; Adeel, M.; Hussain, A.; Ramzan, M.; Ullah, O.; Zeeshan Danish, M.; Iftikhar, S.; et al. Pharmacokinetic Evaluation of Quetiapine Fumarate Controlled Release Hybrid Hydrogel: A Healthier Treatment of Schizophrenia. Drug Deliv. 2018, 25, 916–927. [Google Scholar] [CrossRef]
- Prusty, A.K.; Sahu, S.K. Development and Evaluation of Insulin Incorporated Nanoparticles for Oral Administration. ISRN Nanotechnol. 2013, 2013, 591751. [Google Scholar] [CrossRef]
- Azevedo, J.R.; Sizilio, R.H.; Brito, M.B.; Costa, A.M.B.; Serafini, M.R.; Araújo, A.A.S.; Santos, M.R.V.; Lira, A.A.M.; Nunes, R.S. Physical and Chemical Characterization Insulin-Loaded Chitosan-TPP Nanoparticles. J. Therm. Anal. Calorim. 2011, 106, 685–689. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer Chitosan/Montmorillonite Nanocomposites: Preparation and Characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A.-C. Effect of Lactic/Glycolic Acid Side Chains on the Thermal Degradation Kinetics of Chitosan Derivatives. Polymer 2000, 41, 4841–4847. [Google Scholar] [CrossRef]
- Li, R.; Pan, Y.; Chen, D.; Xu, X.; Yan, G.; Fan, T. Design, Preparation and In Vitro Evaluation of Core–Shell Fused Deposition Modelling 3D-Printed Verapamil Hydrochloride Pulsatile Tablets. Pharmaceutics 2022, 14, 437. [Google Scholar] [CrossRef]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical Properties of Aqueous Glycerol Solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living Bacterial Hydrogels for Accelerated Infected Wound Healing. Adv. Sci. 2021, 8, 2102545. [Google Scholar] [CrossRef] [PubMed]
- Kushibiki, T.; Mayumi, Y.; Nakayama, E.; Azuma, R.; Ojima, K.; Horiguchi, A.; Ishihara, M. Photocrosslinked Gelatin Hydrogel Improves Wound Healing and Skin Flap Survival by the Sustained Release of Basic Fibroblast Growth Factor. Sci. Rep. 2021, 11, 23094. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Ma, F.; Liu, X.; Liu, Y.; Cao, Y.; Pei, R. A Low-Swelling and Toughened Adhesive Hydrogel with Anti-Microbial and Hemostatic Capacities for Wound Healing. J. Mater. Chem. B 2022, 10, 915–926. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Zhang, X.; Shang, Y.; Zhao, Y. Living Microecological Hydrogels for Wound Healing. Sci. Adv. 2023, 9, eadg3478. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.; Vinchhi, P.; Patel, M.M. Design Considerations, Formulation Approaches, and Strategic Advances of Hydrogel Dressings for Chronic Wound Management. ACS Omega 2023, 8, 8172–8189. [Google Scholar] [CrossRef]
- Kwon, J.W.; Savitri, C.; An, B.; Yang, S.W.; Park, K. Mesenchymal Stem Cell-Derived Secretomes-Enriched Alginate/Extracellular Matrix Hydrogel Patch Accelerates Skin Wound Healing. Biomater. Res. 2023, 27, 107. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Dattilo, M.; Patitucci, F.; Malivindi, R.; Pezzi, V.; Tzanov, T.; Puoci, F. Synthesis and Evaluation of Wound Healing Properties of Hydro-Diab Hydrogel Loaded with Green-Synthetized AGNPS: In Vitro and in Ex Vivo Studies. Drug Deliv. Transl. Res. 2022, 12, 1881–1894. [Google Scholar] [CrossRef]
- Li, W.; Gao, F.; Kan, J.; Deng, J.; Wang, B.; Hao, S. Synthesis and Fabrication of a Keratin-Conjugated Insulin Hydrogel for the Enhancement of Wound Healing. Colloids Surf. B Biointerfaces 2019, 175, 436–444. [Google Scholar] [CrossRef]
- Feng, Z.; Su, Q.; Zhang, C.; Huang, P.; Song, H.; Dong, A.; Kong, D.; Wang, W. Bioinspired Nanofibrous Glycopeptide Hydrogel Dressing for Accelerating Wound Healing: A Cytokine-Free, M2-Type Macrophage Polarization Approach. Adv. Funct. Mater. 2020, 30, 2006454. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound Dressing Products: A Translational Investigation from the Bench to the Market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Keast, D.H.; Janmohammad, A. The Hemostatic and Wound Healing Effect of Chitosan Following Debridement of Chronic Ulcers. Wounds 2021, 33, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yang, R. Synthesis and Characterization of Chitosan Derivatives with Dual-Antibacterial Functional Groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pitpisutkul, V.; Prachayawarakorn, J. Hydroxypropyl Methylcellulose/Carboxymethyl Starch/Zinc Oxide Porous Nanocomposite Films for Wound Dressing Application. Carbohydr. Polym. 2022, 298, 120082. [Google Scholar] [CrossRef]
- Apolinário, P.P.; Zanchetta, F.C.; Breder, J.S.C.; Adams, G.; Consonni, S.R.; Gillis, R.; Saad, M.J.A.; Lima, M.H.M. Anti-Inflammatory, Procollagen, and Wound Repair Properties of Topical Insulin Gel. Braz. J. Med. Biol. Res. 2023, 56, e12640. [Google Scholar] [CrossRef]
- Liu, Y.; Petreaca, M.; Yao, M.; Martins-Green, M. Cell and Molecular Mechanisms of Keratinocyte Function Stimulated by Insulin during Wound Healing. BMC Cell Biol. 2009, 10, 1. [Google Scholar] [CrossRef]
- Aijaz, A.; Faulknor, R.; Berthiaume, F.; Olabisi, R.M. Hydrogel Microencapsulated Insulin-Secreting Cells Increase Keratinocyte Migration, Epidermal Thickness, Collagen Fiber Density, and Wound Closure in a Diabetic Mouse Model of Wound Healing. Tissue Eng. Part A 2015, 21, 2723–2732. [Google Scholar] [CrossRef]
- Fang, W.-C.; Lan, C.-C.E. The Epidermal Keratinocyte as a Therapeutic Target for Management of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 4290. [Google Scholar] [CrossRef]
- Dawoud, M.H.S.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Insulin Mucoadhesive Liposomal Gel for Wound Healing: A Formulation with Sustained Release and Extended Stability Using Quality by Design Approach. AAPS PharmSciTech 2019, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, F.; Pessoa, A.; Moreira, G.; Dos Santos, M.; Liberti, E.; Araujo, E.; Carvalho, C.; Saad, M.; Lima, M.H. Effect of Topical Insulin on Second-Degree Burns in Diabetic Rats. Biol. Res. Nurs. 2016, 18, 181–192. [Google Scholar] [CrossRef]
- Chakraborty, T.; Gupta, S.; Nair, A.; Chauhan, S.; Saini, V. Wound Healing Potential of Insulin-Loaded Nanoemulsion with Aloe Vera Gel in Diabetic Rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102601. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid Gelation of Oxidized Hyaluronic Acid and Succinyl Chitosan for Integration with Insulin-Loaded Micelles and Epidermal Growth Factor on Diabetic Wound Healing. Mater. Sci. Eng. C 2020, 117, 111273. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, R.; Bai, L.; Cai, Y.; Lei, M.; Bao, C.; Lin, S.; Ji, S.; Liu, C.; Qu, X. Extracellular Matrix-Mimetic Immunomodulatory Hydrogel for Accelerating Wound Healing. Adv. Healthc. Mater. 2023, 12, e2301264. [Google Scholar] [CrossRef] [PubMed]
- Lungu, R.; Paun, M.-A.; Peptanariu, D.; Ailincai, D.; Marin, L.; Nichita, M.-V.; Paun, V.-A.; Paun, V.-P. Biocompatible Chitosan-Based Hydrogels for Bioabsorbable Wound Dressings. Gels 2022, 8, 107. [Google Scholar] [CrossRef]
- Bradshaw, M.; Ho, D.; Fear, M.W.; Gelain, F.; Wood, F.M.; Iyer, K.S. Designer Self-Assembling Hydrogel Scaffolds Can Impact Skin Cell Proliferation and Migration. Sci. Rep. 2014, 4, 6903. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef]
- Saco, M.; Howe, N.; Nathoo, R.; Cherpelis, B. Comparing the Efficacies of Alginate, Foam, Hydrocolloid, Hydrofiber, and Hydrogel Dressings in the Management of Diabetic Foot Ulcers and Venous Leg Ulcers: A Systematic Review and Metaanalysis Examining How to Dress for Success. J. Am. Acad. Dermatol. 2016, 74, AB293. [Google Scholar] [CrossRef]
- Walther, M.; Vestweber, P.K.; Kühn, S.; Rieger, U.; Schäfer, J.; Münch, C.; Vogel-Kindgen, S.; Planz, V.; Windbergs, M. Bioactive Insulin-Loaded Electrospun Wound Dressings for Localized Drug Delivery and Stimulation of Protein Expression Associated with Wound Healing. Mol. Pharm. 2023, 20, 241–254. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E. Effects of Insulin on Wound Healing: A Review of Animal and Human Evidences. Life Sci. 2017, 174, 59–67. [Google Scholar] [CrossRef]
- Liu, Y.; Dhall, S.; Castro, A.; Chan, A.; Alamat, R.; Martins-Green, M. Insulin Regulates Multiple Signaling Pathways Leading to Monocyte/Macrophage Chemotaxis into the Wound Tissue. Biol. Open 2018, 7, bio026187. [Google Scholar] [CrossRef]
- Besson, J.C.F.; Hernandes, L.; de Campos, J.M.; Morikawa, K.A.; Bersani-Amado, C.A.; Matioli, G. Insulin Complexed with Cyclodextrins Stimulates Epithelialization and Neovascularization of Skin Wound Healing in Rats. Injury 2017, 48, 2417–2425. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized Silk Fibroin Dressing with Topical Bioactive Insulin Release for Accelerated Chronic Wound Healing. Mater. Sci. Eng. C 2017, 72, 394–404. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X.; Wang, D.; Shi, Y.; Zhang, M.; Yu, T.; Liu, D.; Gao, M.; Zhang, X.; Liu, Y. Topical Insulin Application Accelerates Diabetic Wound Healing by Promoting Anti-Inflammatory Macrophage Polarization. J. Cell Sci. 2020, 133, jcs235838. [Google Scholar] [CrossRef] [PubMed]
- Greenway, S.E.; Filler, L.E.; Greenway, F.L. Topical Insulin in Wound Healing: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Wound Care 1999, 8, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. PH-Responsive Hydrogel Loaded with Insulin as a Bioactive Dressing for Enhancing Diabetic Wound Healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Li, C.; Yu, T.; Liu, Y.; Chen, X.; Zhang, X. Topical Application of Insulin Accelerates Vessel Maturation of Wounds by Regulating Angiopoietin-1 in Diabetic Mice. Int. J. Low. Extrem. Wounds 2015, 14, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.H.M.; Caricilli, A.M.; de Abreu, L.L.; Araújo, E.P.; Pelegrinelli, F.F.; Thirone, A.C.P.; Tsukumo, D.M.; Pessoa, A.F.M.; dos Santos, M.F.; de Moraes, M.A.; et al. Topical Insulin Accelerates Wound Healing in Diabetes by Enhancing the AKT and ERK Pathways: A Double-Blind Placebo-Controlled Clinical Trial. PLoS ONE 2012, 7, e36974. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchetta, F.C.; De Wever, P.; Morari, J.; Gaspar, R.C.; Prado, T.P.d.; De Maeseneer, T.; Cardinaels, R.; Araújo, E.P.; Lima, M.H.M.; Fardim, P. In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications. Bioengineering 2024, 11, 168. https://doi.org/10.3390/bioengineering11020168
Zanchetta FC, De Wever P, Morari J, Gaspar RC, Prado TPd, De Maeseneer T, Cardinaels R, Araújo EP, Lima MHM, Fardim P. In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications. Bioengineering. 2024; 11(2):168. https://doi.org/10.3390/bioengineering11020168
Chicago/Turabian StyleZanchetta, Flávia Cristina, Pieter De Wever, Joseane Morari, Rita Caiado Gaspar, Thaís Paulino do Prado, Tess De Maeseneer, Ruth Cardinaels, Eliana Pereira Araújo, Maria Helena Melo Lima, and Pedro Fardim. 2024. "In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications" Bioengineering 11, no. 2: 168. https://doi.org/10.3390/bioengineering11020168
APA StyleZanchetta, F. C., De Wever, P., Morari, J., Gaspar, R. C., Prado, T. P. d., De Maeseneer, T., Cardinaels, R., Araújo, E. P., Lima, M. H. M., & Fardim, P. (2024). In Vitro and In Vivo Evaluation of Chitosan/HPMC/Insulin Hydrogel for Wound Healing Applications. Bioengineering, 11(2), 168. https://doi.org/10.3390/bioengineering11020168








